Diagnosing COVID-19: A perspective from U.S. patenting activity
October 2023
Read the USPTO report
Listen to the AUTM on the Air podcast (Episode: "Behind the Numbers: Insights from U.S. Patenting on Diagnosing COVID-19")
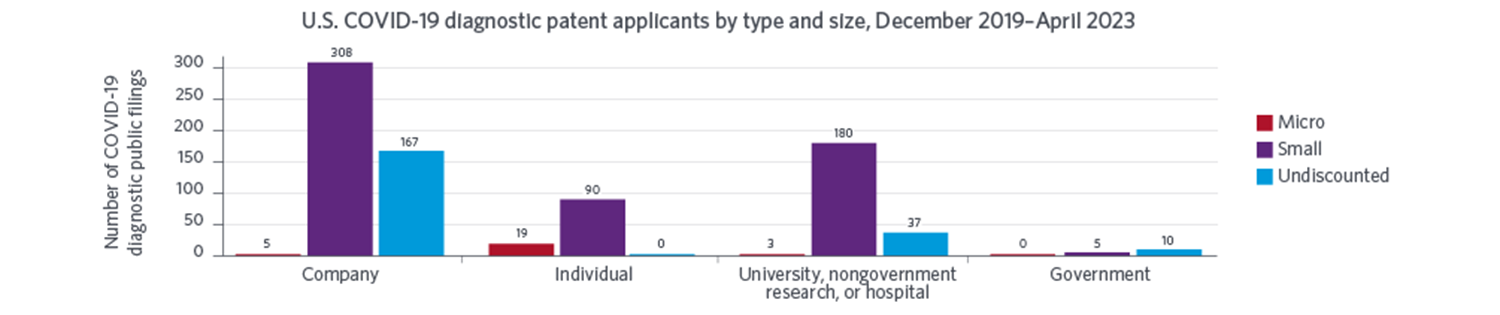
National innovation systems were surprisingly responsive to the urgent needs created by the COVID-19 pandemic. The rapid introduction of COVID-19 diagnostics shortly after the onset of the pandemic allowed individuals to better monitor their health and provided policy makers with crucial information needed to manage the public health crisis. Our study reveals that U.S. applicants, especially universities and small companies, led the way in developing methods to diagnose COVID-19, as evidenced by public U.S. patent filings through April 2023. Those organizations were also more likely to use government support, especially from the National Institutes of Health and the National Science Foundation.