CPC Definition - Subclass C04B
This place covers:
Chemical aspects of the processing of lime, magnesia or dolomite and of molten slag.
Compositional aspects of:
- inorganic binders, such as hydraulic cements ;
- mortars, concrete and artificial stone, e.g. the choice of fillers or active ingredients therefore;
- shaped ceramic products, e.g. clay-wares, refractories , non-oxides.
Physico-chemical aspects of methods for obtaining mortars, concrete, artificial stones or ceramics , e.g. for delaying the setting time of mortar compositions.
Treatment including defibrillating of materials such as fillers , agglomerated or waste materials, or refuse to enhance their filling properties in mortars, concrete or artificial stone.
Porous mortars, concrete, artificial stone or ceramic ware, and the preparation thereof.
Methods and apparatus for:
- burning or slaking lime;
- obtaining mineral binders, e.g. Portland cement or hemihydrate plaster;
- the expansion of mineral fillers , such as clay, perlite or vermiculite.
After- treatment of artificial stones, mortars, concrete and ceramics , e.g. coating or impregnation of green concrete after primary shaping.
Non-mechanical treatment of natural stone.
Processing powders of inorganic compounds in preparation to the manufacturing of ceramic products .
The joining of burned ceramics with other articles by heating.
This place does not cover:
Granulating apparatus | |
Mechanical features relating to the working of mortars, concrete, stone, clay-wares or ceramics , e.g. mixing or shaping ceramic compositions, boring natural stone | |
Chemical preparation of powders of inorganic compounds | |
Devitrified glass-ceramics | |
Compositions containing free metal bonded to carbides, diamond, oxides, borides, nitrides, silicides, e.g. cermets, or other metal compounds, such as oxynitrides or sulfides, other than as macroscopic reinforcing agents | |
Building elements or constructions; Finishing work on buildings |
Attention is drawn to the following places, which may be of interest for search:
Materials for prostheses or for coating prostheses | |
Chemical or biological purification of waste gases | |
Layered products | |
Treating inorganic non-fibrous materials to enhance their pigmenting or filling properties | |
Adhesives | |
Cementing or plastering compositions for boreholes or wells | |
Alloys based on refractory metals | |
Shaft or vertical furnaces in general | |
Hydraulic hardening materials , e.g. concretes, ceramics or refractories for protection against radiation, i.e. shielding |
In this subclass, for the parts C04B 2/00-C04B 32/00, C04B 38/00, C04B 40/00, C04B 41/00 the CIS indexing system is used. For details, see below
Combination set (C-sets)
1. Introduction
1.1 This manual relates to the rules to be applied when classifying documents using C-sets in the "cement part" of subclass C04B. With the "cement part" we mean the whole of the subclass, with the exception of the range C04B 33/00 - C04B 37/00.
However, symbols of the range C04B 33/00 - C04B 35/00 can be used as Indexing Codes (when the classification is in C04B 38/00 or C04B 41/00).
1.2 C-sets are used in three major areas:
- C04B 2/00 - C04B 32/00 and C04B 40/00: Compositions of cement/concrete mixtures or of artificial stone like materials
- C04B 38/00: porous materials
- C04B 41/00: after treatment.
1.3 Symbols that are used in the present C-set system are chosen from:
- C04B 2/00 - C04B 41/00 (with the exception of C04B 37/00):
these are symbols which can be used as classification as well as symbols in the combination sets (C-set),
these are symbols used as additional information (CCA) or within the C-set (see below).
1.4 The principles of Combination sets are based on the possibilities offered by the IPC (until IPC7) for using classification symbols also as (linked) Indexing Codes.
The C-sets are present in EPODOC:
/CCI : CPC classification symbol
/CCA: Additional information
/CLC: the combination sets (C-sets) of symbols linked to the classification (CCI) or to the additional information (CCA)
The first symbol of a C-set is referred to as the "base class". Symbols in the C-set are separated by a comma (,).
The base group can be an CCI or CCA group
2. C-sets in the range C04B 2/00 - C04B 32/00 and C04B 40/00
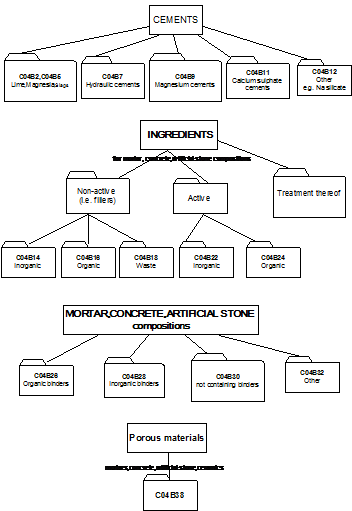
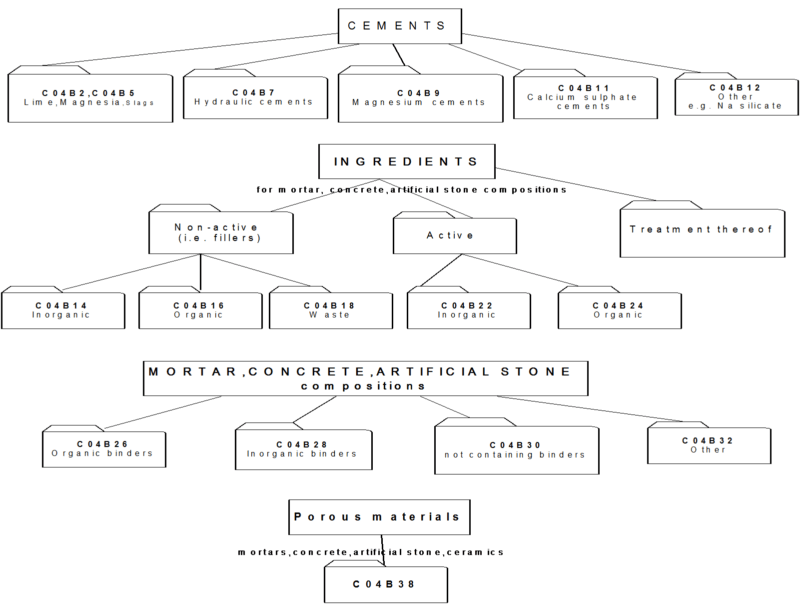
2.1 This part of C04B relates to cement-, mortar-, concrete- and artificial stone compositions or their constituents or ingredients.
As a general rule such compositions (further referred to as "mixtures") contain three types of ingredients:
- one or more binders (organic or inorganic)
- fillers (inactive ingredients)
- active ingredients, e.g. accelerators.
[Exception: main group C04B 30/00 relates to compositions not containing binders].
2.2 Overview of main groups:
- C04B 2/00 - C04B 12/00 : relate to inorganic binders as such
- C04B 14/00 - C04B 20/00 : relate to fillers
- C04B 22/00 - C04B 24/00 : relate to active ingredients
- C04B 26/00 - C04B 32/00 : relate to the mixtures
- C04B 40/00 : relates to characteristics / preparation of the mixtures
2.3 Classification rules:
2.3.1 When an invention relates to an individual ingredient, classification is made in the range C04B 2/00 - C04B 12/00 if this ingredient is a binder, in the range C04B 14/00 - C04B 20/00 if the ingredient is a filler and in the range C04B 22/00 - C04B 24/00 if it is an active ingredient.
2.3.2 When an invention relates to a mixture, classification is made in the range C04B 26/00 - C04B 32/00, according to the binder used while applying the last place rule (LPR). So if a combination of an organic and an inorganic binder is present, classification is made in C04B 28/00, not in C04B 26/00. If a combination of two inorganic binders is present, classification is done in C04B 28/00 according to the LPR for one of the binders, the others are added as symbols in the C-set and are chosen from C04B 7/00-C04B 12/00 groups. If one of the ingredients is (suspected to be) new or unusual, or special details describing this ingredient are given, classification is also made for this ingredient.
2.3.3 When the invention merely relates to the preparation or characteristics of the mixture, classification is made in C04B 40/00. If the mixture as such or one of its ingredients is considered to be new or unusual, classification is made for these aspects too. For obtaining porous materials see point 3. below.
2.3.4 When the invention relates to an active additive which is a mixture on its own, e.g. the combination of two specific polymers and a specific inorganic salt, classification is made in C04B 40/0039. If the use of one of the ingredients as such is new to the field, classification for this ingredient as such is made also.
2.4 C-set rules:
2.4.1 Primary goal of the combination set is to identify the individual constituents of the mixtures, using the classification symbols for these ingredients as part of a C-set, linked to the classification symbol which already identifies (one of) the binder(s).
Example 1:
A mixture containing a mixed binder of aluminium cement, Portland cement and a polymeric co-binder, next to diatomaceous earth and an inorganic sulfate will be classified - according to the LPR - in C04B 28/06, the other ingredients being identified by the appropriate symbols in the C-set:
CCI: C04B 28/06 C-set (CLC) : C04B 28/06, C04B 7/02, C04B 14/08, C04B 22/142, C04B 24/26
An organic co-binder next to an inorganic binder is indexed as an active organic ingredient (main group C04B 24/00).
Because in this example, all ingredients as such are known in the field, no further classification is made.
2.4.2 When for one of the ingredients alternatives are mentioned, separate C-sets are made.
Example 2:
If in the example 1, an inorganic chloride was mentioned as an alternative to the sulfate, the indexing would look like:
Set 1: C04B 28/06, C04B 7/02, C04B 14/08, C04B 22/142, C04B 24/26
Set 2: C04B 28/06, C04B 7/02, C04B 14/08, C04B 22/12, C04B 24/26
(Putting C04B 22/12 and C04B 22/142 in the same set would mean they are both present in the same mixture).
2.4.3 When classification is made for individual (active) ingredients, their function or in some cases their characteristics can be identified using the C04B 2103/00 series.
Example 3:
A new organic sulfonated plasticizer:
/CCI : C04B 24/16 /CCA : C04B 2103/30
When for a mixture, many alternatives for the same ingredient with a specific function are mentioned, instead of making a set for each alternative, only one C-set with the C04B symbol in the C-set for the function can be made. If one of the alternatives is preferred in the document a second set with the symbol for that alternative can be made too.
Example 4:
In a concrete mixture, a superplasticizer is added. This superplasticizer can be chosen from many alternatives, for each of which a C04B 24/00 entry exist. However a lignosulfonate is preferred.
/CCI : C04B 28/02 C-set 1 C04B 28/02, C04B 2103/32
C-set 2 C04B 28/02, C04B 24/18
2.4.4 In a similar way, characteristics or uses of the mixtures are identified with symbols of the C04B 2111/00 series. These symbols are always used as additional information.(CCA)
Example 5:
The composition of example 1 is used for sound insulation:
In addition to the above sets CCA: C04B 2111/52
2.4.5 When information is given about the preparation or characteristics of
the mixtures - this information not being the "main" information - additional symbols of main group C04B 40/00 can be added in the C-set.
Example 6:
The mixture of example 1 is hardened using microwaves:
C-set : C04B 28/06, C04B 7/02, C04B 14/08, C04B 22/142, C04B 24/26, C04B 40/0218
The mixture of example 1 is of the deferred action type:
C-set: C04B 28/06, C04B 7/02, C04B 14/08, C04B 22/142, C04B 24/26, C04B 40/06
2.4.6 When the process of making is the main invention a CCI in C04B 40/00 can be given. As a general rule, when classifying in C04B 40/00, symbols in the C-set are used to identify the kind of mixture, not to identify the individual ingredients. If it is important to identify these ingredients, further classification is made as mixture and the ingredients are identified by symbols linked to the classification symbol of the mixture in the C-set.
Example 7:
If only common ingredients are used:
/CCI : C04B 40/0286 C-set: C04B 40/0286, C04B 28/06
If also the composition of the mixture is of interest:
/CCI C04B 40/0286 and C04B 28/06
C-set 1: C04B 40/0286, C04B 28/06
C-set 2: C04B 28/06, C04B 14/386, C04B 22/0013
2.4.7 A special case within main group C04B 40/00 are the pre-mixtures of ingredients.
Here the same principle as for point 2.4.1 is applied, i.e. the classification symbols identifying the ingredients are linked to C04B 40/0039 (CCI) and a symbol from the range C04B 26/00 - C04B 32/00 is added to the C-set to indicate for which kind of mixture the pre-mixture is intended to be used.
Example 8:
/CCI :C04B 40/0042 C-set : C04B 40/0042, C04B 14/08, C04B 22/142, C04B 24/26, C04B 28/06
2.4.8 In the range for inorganic binders as such (C04B 2/00 - C04B 12/00) symbols can also be used in a C-set to identify aspects for which a classification symbol exists, but which aspects as such are not important enough to be classified.
Example 9:
/CCI: C04B 7/47 C-set : C04B 7/47, C04B 7/364
Example 10:
/CCI : C04B 11/26 C-set : C04B 11/26, C04B 11/024
2.4.9 For agglomerated materials (= artificial aggregates or fillers), which are classified in C04B 18/021 and subgroups, the starting materials other than the binder can be identified with further symbols in the C-set.
Example 11:
Making artificial gravel from a mixture of cement and mining refuse:
/CCI : C04B 18/021 C-set : C04B 18/021, C04B 18/12
2.4.10 Main group C04B 20/00 is a general group for fillers. When classification is made in this group, very often the specific filler involved is identified by adding the specific filler symbol in the C-set..
Example 12:
Expanding perlite in a rotary kiln:
/CCI: C04B 20/061 C-set: C04B 20/061, C04B 14/18
Example 13:
Coating alumina with metal:
/CCI : C04B 20/1062 C-set : C04B 20/1062, C04B 14/303
2.4.10a Groups C04B 20/123 and C04B 20/126 are used in the C-set only in combination with C04B 20/12 to indicate:
-in the case of C04B 20/123 that a coating is an alternative to the previous indicated coating
Example 14:
-in the case of C04B 20/126 that the coating layer is the same as a previous coating layer
Example 15:
3.Classifying in main group C04B 38/00.
3.1 This part of C04B relates to porous or lightweight cement-, mortar-, concrete-and artificial stone compositions and porous or lightweight ceramics.
More generally we could say that C04B 38/00 relates to inorganic foamed materials or bodies, with the exception of foamed metal.
Subdivision of C04B 38/00 is largely based on the methods used for obtaining the porosity or the reduction in weight, e.g. by adding lightweight filler (C04B 38/08), by adding a gas forming agent (C04B 38/02) or by burning out a burnable additive (C04B 38/06).
3.2 Classification and C-set rules:
3.2.1 Officially in main group C04B 38/00, there is no LPR. Nonetheless when porosity is obtained by a combination of methods, as a general rule, classification is made in the last appropriate place. The second method, not identified by classification (CCI), is identified by a C04B 38/00 symbol in the C-set. If of interest, documents can be even classified twice (see further point 3.2.3)
3.2.2 The central idea for classification/indexing in C04B 38/00 is:
- classification according to the method (see 3.2.1) and
- Indicating the nature of the material that is made porous or lightweight.
For identifying the nature of the material, symbols can be chosen from C04B 26/00 - C04B 35/00. In very exceptional cases also C04B 14/00 symbols can be used.
Example 16:
Obtaining a porous silicon carbide body by dissolving out a soluble salt.
/CCI : C04B 38/04 C-set: C04B 38/04, C04B 35/565
Example 17:
Obtaining porous porcelain by burning out a monolithic PUR sponge impregnated with clay slip:
/CCI : C04B 38/0615 C-set : C04B 38/0615, C04B 33/24
HOWEVER there is a fundamental difference in approach when classifying "cement type" mixtures and "ceramic type" materials or bodies: see points 3.2.6 and 3.2.7 below!
3.2.3 When a combination of methods is used, the method that is not identified by the classification is given a C04B 38/00 symbol in the C-set.
Example 18:
To the material of example 14 there is also added a gas forming agent:
/CCI : C04B 38/04 C-set: C04B 38/04, C04B 35/565, C04B 38/02
3.2.4 In the same way other aspects of interest can be identified by giving further C04B 38/00 symbols.
Example 19:
The material of example 18 is characterised by the dimensions of the nanosized pores and the overall % of porosity:
/CCI : C04B 38/04 C-set: C04B 38/04, C04B 35/565, C04B 38/0054, C04B 38/0074, C04B 38/02
3.2.5 When classifying in main group C04B 38/00, in the same way as for the indexing of mixtures as described in point 2 above, symbols of the series C04B 2111/00 can be used to indicate properties are uses, e.g. sound insulation.
Example 20:
The material of example 17 is used for electronic applications:
/CCI: C04B 38/0615 CCA : C04B 2111/00844 C-set: C04B 38/0615, C04B 33/24
3.2.6 Porous or lightweight ceramics are always classified in C04B 38/00 according to rules 3.2.1 to 3.2.5
3.2.7 Porous or lightweight cement-, concrete-, artificial stone- and like mixtures:
3.2.7a These type of mixtures are classified as such mixtures, so in the range C04B 26/00 - C04B 32/00, according to the rules of point 2 above. The appropriate C04B 38/00 symbols are added in the C-set.
Example 21:
Reinforced portland cement based concrete containing also carbon fibres and made porous by adding Al particles (Al will react with Ca(OH)2 liberated during cement hardening and thus produce H2 gas):
/CCI : C04B 28/04 C-set: C04B 28/04, C04B 14/386, C04B 22/04, C04B 32/02, C04B 38/02
Example 22:
Foaming gypsum by adding specific sulfonated foaming agent:
/CCI: C04B 28/14 C-set: C04B 28/14, C04B 24/16, C04B 38/10
3.2.7b When one or more of the other symbols give sufficient "C04B 38/00 information", no further C04B 38/00 symbols are given.
Example 23:
Expanded clay containing concrete will NOT receive symbol C04B 38/08, because C04B 14/12 already gives sufficient information:
/CCI: C04B 28/02 C-set: C04B 28/02, C04B 14/12
3.2.7c So as a general rule these kind of mixtures are not classified in main group C04B 38/00. Classification is made in this main group only when the invention relates to the process of obtaining the porosity or the reduction of weight. When the composition as such is still interesting in such a case, further classification is made for the mixture.
Example 24:
The characteristic feature of the invention of example 21 is the way in which the Al particles are handled in the context of obtaining the gas concrete:
/CCI : C04B 28/04 and C04B 38/02
C-set 1: C04B 28/04, C04B 14/386, C04B 22/04, C04B 32/02, C04B 38/02
C-set 2: C04B 38/02, C04B 28/04
3.2.8 While in general the LPR is applied in main group C04B 38/00, exception is made for obtaining porous or lightweight ceramic particles C04B 38/009. As a general rule, this group takes precedence over the other C04B 38/00 groups.
Example 25:
Obtaining porous alumina particles by burning out polymeric core:
/CCI C04B 38/009 C-set: C04B 38/009, C04B 35/10, C04B 38/0615
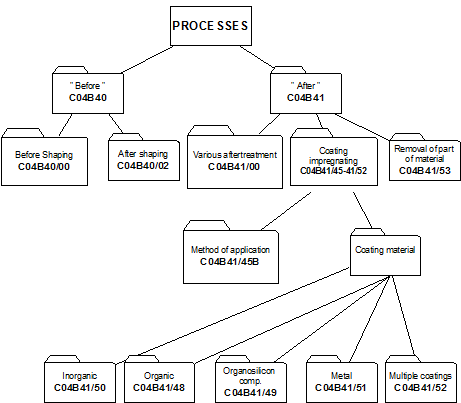
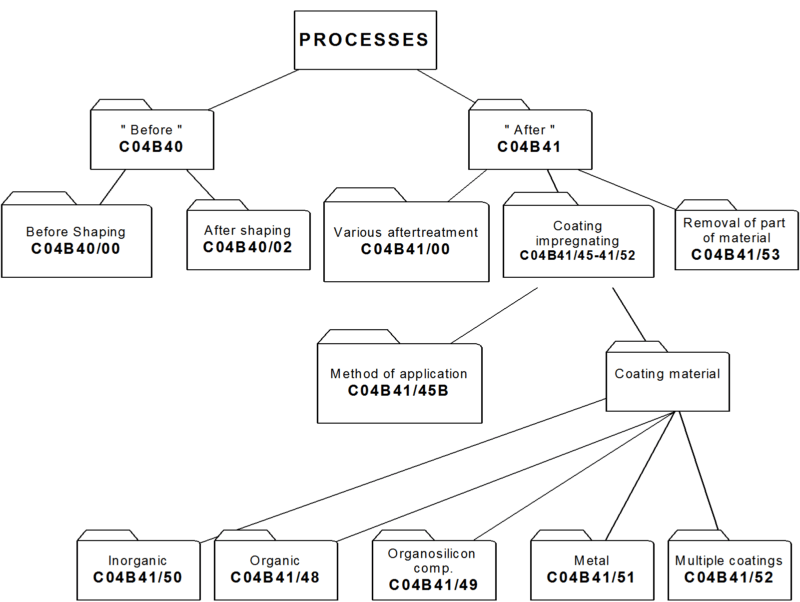
4. Classifying in main group C04B 41/00
i.e. after-treatment of cement-, mortar-, concrete- and artificial stone products as well as ceramic materials AND natural stone. Hereinafter the treated materials are referred to as "substrates".
While other kinds of after-treatment are not excluded, C04B 41/00 relates to after-treatment of substrate, mainly to :
- coating or impregnation of the substrates: C04B 41/45 and subgroups
- removing material from the substrates: C04B 41/53 and subgroups.
In main group C04B 41/00, no distinction is made between coating or impregnation. Therefore the terms coating, impregnation and layer are considered equivalent.
4.2 Classification and C-set rules:
4.2.1 As a general rule subdivision of main group C04B 41/00 is based on aspects relating to the method of after-treatment, such as the selection of the method for applying the coating material on the substrate, e.g. by CVD (C04B 41/4531) or the selection of the coating or impregnation material with which the substrate is treated, e.g. coating with carbon (C04B 41/5001).
When using C-set, only the range C04B 41/00 - C04B 41/5392 is used . Documents classified in the range C04B 41/60 - C04B 41/91 always get also a class in C04B 41/00 - C04B 41/5392, which may be combined with one or more C-sets.
4.2.2 To identify the substrate that is after-treated, the class C04B 41/009 is given and C-sets are created using complementary symbols chosen from:
- C04B 14/02 - C04B 14/36 when natural stone is treated
- C04B 26/00 - C04B 32/005 when artificial stone, e.g. concrete is treated
- C04B 33/00 - C04B 35/83 when ceramics are treated
- C04B 38/00 - C04B 38/106 when porous materials are treated
- C04B 14/38 - C04B 14/48 when ceramic fibres are treated, i.e. only when classifying in C04B 41/4584.
When the substrate is further defined e.g. a wood fiber/particle board, which in itself is information that does not require classification in the substrate class itself e.g. C04B 28/02 , then the C04B 41/009 set will be:
If a class in C04B 28/00 is also required because the mixture per se is interesting and is part of the invention information, then the C04B 41/009 set will comprise only the C04B 28/02 symbol
Example 26:
Impregnating a natural marble stone with polyester:
/CCI: C04B 41/4826 and C04B 41/009 C-set : C04B 41/009, C04B 14/285
Example 27:
Concrete based on aluminium cement is treated with waterglass (Na-silicate):
/CCI: C04B 41/5089 and C04B 41/009 C-set : C04B 41/009, C04B 28/06
Example 28:
A silicon nitride body is glazed:
/CCI: C04B 41/5022 and C04B 41/009 C-set :C04B 41/009, C04B 35/584
Example 29:
Coating alumina fibres with aluminium:
/CCI: C04B 41/4584 and C04B 41/009
C-set 1:C04B 41/009, C04B 14/4625
C-set 2: C04B 41/4584, C04B 41/5155
So for classification, C04B 41/4584 takes precedence over other C04B 41/00 groups when ceramic particles or fibres are treated!
Multiple coating of particulate or fibrous material is usually also classified in C04B 41/52 so that it is possible to attribute C-sets for each coating layer (see point 4.2.5 below).
4.2.3 In main group C04B 41/00, the LPR applies. As most subgroups relate to specific methods of applying coatings are subgroups of C04B 41/4505, while the groups identifying the nature of the coating material are further down in the scheme, this LPR in general results in a classification according to the material applied. One or more symbols identifying aspects of the method used are added in the C-set.
Example 30:
The process of example 26 is carried out under vacuum and increased temperature:
/CCI: C04B 41/4826 and C04B 41/009
C-set 1: C04B 41/009, C04B 14/285
C-set 2: C04B 41/4826, C04B 41/0072, C04B 41/4515
However, when the invention relates to the process proper, classification is made in the process group and a further symbol is used in the C-set for identifying the applied material on the substrate. If more ample information has to be given about the nature of the coating, classification is also made for this aspect in combination with a further C-set.
Example 31:
The process of example 26 is carried out under an atmosphere of very specific composition, this composition being the essential feature of the invention:
/CCI: C04B 41/4519 and C04B 41/009
C-set 1:C04B 41/009, C04B 14/285
C-set 2: C04B 41/4519, C04B 41/4826
Example 32:
In the example 31, the polyester can be mixed with other polymers:
/CCI: C04B 41/4519 and C04B 41/4826 and C04B 41/009
C-set 1: C04B 41/009, C04B 14/285
C-set 2: C04B 41/4519, C04B 41/4826
C-set 3:C04B 41/4826, C04B 41/4519, C04B 41/4811, C04B 41/4823
Exception on the LPR: for classification, C04B 41/4584 takes precedence over other groups of C04B 41/00 when treatment of ceramic fibres or particles is concerned (see example 29).
4.2.4 When alternatives are to be identified, the same procedure is applied as for concrete and like mixtures, i.e. two or more C-sets of symbols are made. There might be alternatives both for the process and the material applied to the substrate.
Example 33:
The treatment of example 27 can be carried out either under vacuum or under inert atmosphere:
/CCI: C04B 41/4826 and C04B 41/009
C-set 1: C04B 41/009, C04B 14/285
C-set 2: C04B 41/4826, C04B 41/4515
C-set 3: C04B 41/4826, C04B 41/4517
4.2.5 Multiple coating or impregnation.
When the same substrate is coated with two or more layers, classification is made in C04B 41/52. If one of the layers as such might be new in the field, classification for this layer as such is made too.
For each layer a separate C-set is made, each starting with C04B 41/52, the first set relating to the first layer, the second set relating to the second layer etc.
Example 34:
A clay ware body is first coated with an engobe and then with a glaze:
/CCI: C04B 41/52 and C04B 41/009
C-set 1: C04B 41/009, C04B 33/00
C-set 2: C04B 41/52, C04B 41/504
C-set 3: C04B 41/52, C04B 41/5022
Example 35:
The engobe used in example 34 looks new to the field:
/CCI: C04B 41/52 and C04B 41/009 and C04B 41/504
C-set 1: C04B 41/009, C04B 33/00
C-set 2: C04B 41/52, C04B 41/504
C-set 3: C04B 41/52, C04B 41/5022
Exception: when the different coatings result in layers of the same composition, classification is made according to the nature of that layer and C04B 41/52 is added to the C-set !
Example 36:
A boron carbide body is coated with two or more layers, which might slightly differ in composition, but which all result in a carbon coating:
/CCI: C04B 41/5001 and C04B 41/009
C-set 1: C04B 41/009, C04B 35/563
C-set 2: C04B 41/5001, C04B 41/52
As for single layer coatings, additional C04B 41/00 codes can be added to the C-set to identify other interesting aspects of the respective coatings.
4.2.6 When, in the case of multiple coating, alternatives are mentioned, the following procedure is followed.
If, e.g. for layer 2 an alternative is to be identified, the third C-set will represent this alternative layer, with at the end the symbol C04B 41/522. [This symbol is not to be used for classification.] So in this case, a possible third layer will be identified by the fourth C-set, because the third one refers to an alternative of the second layer (represented by the second set).
Example 37:
In the example 34, a porcelain layer can be used as an alternative to the engobe layer:
/CCI: C04B 41/52 and C04B 41/009
C-set 1: C04B 41/009, C04B 33/00
C-set 2: C04B 41/52, C04B 41/504
C-set 3: C04B 41/52, C04B 41/5038, C04B 41/522
C-set 4: C04B 41/52, C04B 41/5022
-When a coating layer is the same as a previous coating layer, a similar procedure as above is followed, adding the symbol C04B 41/524 at the end of the layer that is identical to a previously identified layer
Example 38
4.2.7 For the sake of classification/C-sets in C04B, treatment of "green" concrete or ceramics, i.e. concrete that has not hardened yet, resp. ceramic products that are not fired yet, is considered to be covered by C04B 41/00. Such documents will receive C04B 41/4578 as an extra symbol in the C-set. Only in exceptional cases, classification can be made in this group.
Example 39:
The substrate of example 25 is treated before hardening of the concrete:
/CCI: C04B 41/5089 /SI : C04B 28/06 C-set: C04B 41/5089, C04B 41/5007
4.2.8 Group C04B 41/53 relates to the removal of part of the materials of the treated article. A coating process including a step like polishing, roughening or etching is however not classified in C04B 41/53 or a subgroup (what could be expected applying the last place rule), but is classified applying the general rules for coatings above and adding C04B 41/53 or a subgroup to the C-set. If however the removal is the essential step of the invention, classification in C04B 41/53 is (also) made.
4.2.9 In the same way as when classifying/C-sets in the other parts of C04B, mentioned above, symbols of the series C04B 2111/00 can be used to identify uses or characteristics of the products obtained.
Example 40:
The material of example 36 is used for electronic applications:
/CCI: C04B 41/5001 , C04B 41/009
/Indexing Code: C04B 2111/00844
C-set 1: C04B 41/009, C04B 35/563 and
C-set 2: C04B 41/5001, C04B 41/52
In this place, the following terms or expressions are used with the meaning indicated:
Active ingredients | Ingredients having an effect on the mortar-, concrete- or artificial stone composition during processing or on the characteristics of the final product, e.g. as set accelerator, as dispersant or as gas generating agent. Other examples are processing aids or property improvers, e.g. grinding aids, used after the cement burning process or in the absence of such a burning process. |
Cement | The binder proper, i.e. excluding any additional ingredient or additive added to the finished binder as such, with the exception of mixtures of binders. |
Clinker | The unground sintered product leaving the cement kiln. In patent literature this term might be used literally, i.e. to indicate the unground sintered product leaving the cement kiln, or it might be used to indicate the ground cement without any additive, i.e. not interground with additives such as gypsum. |
Ceramics | Inorganic, non metallic products obtained by a process involving a shaping step and a sintering or comparable heat treatment step, with the exclusion of cements , cermets and glasses, glazes, vitreous enamels and devitrified glass ceramics. |
Fillers | Inactive ingredients, include pigments, aggregates and fibrous reinforcing materials. |
Fine ceramics | Ceramics having a polycrystalline fine-grained microstructure, e.g. of dimensions below 100 micrometer. |
Hydraulic binder | For the purpose of classification and search in this subclass, the terms " cement " and " hydraulic binder " are considered to be equivalent, even if in literature, an hydraulic binder might be defined as a mixture of cement and one or more inorganic additives. |
Mortar- , concrete- and artificial stone compositions | They are considered as a single group of materials, are mixtures of one or more binders with fillers or other ingredients. In the context of such compositions, the terms " cement " and "binder" are considered equivalent. |
Resin mortar or resin concrete | Mortar or concrete containing resin as a binder instead of cement , i.e. excluding any inorganic binder and containing a considerable amount of inorganic filler compared with the amount of the organic binder. |
Refractories | Ceramics or mortars withstanding high temperatures of at least about 1500 degrees C. For classification and search in this subclass no substantial distinction is made between the terms " refractories " and " ceramics ". |
Porous materials | Materials which are deliberately made porous, e.g. by adding gas-forming, foaming, burnable or lightweight additives to the composition they are made of. |
This place covers:
Lime binders as such; Preparation thereof;
C-set is used only incidentally in this class. If so, symbols are chosen from other C04B 2/00 groups and C04B. A C04B 2/00 symbol in a C-set set having a C04B 28/00 CPC class indicates the presence of a second binder.
This place does not cover:
Hydraulic lime cements | |
Mixtures containing lime as a binder |
This place does not cover:
Obtaining Ca(OH)2 otherwise than by simple slaking of quick lime |
This place covers:
Slaking, with water including air slaking, filtering after slaking
This place does not cover:
Devices for filtering after slaking | |
Simultaneous dehydrating of gypsum and slaking of lime | |
Warming up food and the like, e. g. by slaking lime | |
Hydration of MgO | |
Chemical heat sources |
This place does not cover:
Hydrating cement clinker | |
Quenching coke |
This place covers:
Devices for slaking lime, e.g. devices for preparing milk of lime or for purifying slaked lime e.g. by filtering
This place does not cover:
Mechanical aspects | |
Manufacture of slag wool | |
Other cast stone | |
Treatment of slag in, or for the production of metals |
In this place, the following terms or expressions are used with the meaning indicated:
Metallurgical slag | Slag from metallurgy processes |
This place covers:
Hydraulic cements as such and their manufacturing methods.
This place does not cover:
Porsal cement |
CIS is used only incidentally in this class. If so, index codes are chosen from other C04B 7/00 groups and C04B. A C04B 7/00 index code in a CIS set having a C04B 28/00 EC class indicates the presence of a second binder.
In this place, the following terms or expressions are used with the meaning indicated:
Hydraulic cement | - like cements setting under the influence of water and - cements hardening in the air and under water |
This place covers:
Portland cement (PC),
i.e. hydraulic cement produced by firing limestone or chalk and clay (or other silica, alumina, iron bearing materials) so that Ca-silicate sand aluminates are formed. Average composition: 45% C3S, 25%C2S, rest C3A, C4AF;
i.e. average oxide composition: SiO2 17-24, Al2O3 3-7, Fe2O3 1-5, CaO60-65, MgO 1-5, alkali 1, SO3 1-3;
i.e. average water : cement ratio is 0.4 - 0.6;
i.e. during hydration Ca(OH)2 is formed, given thus an alkaline reaction;
i.e. PC clinker mostly coground with gypsum to retard setting;
e.g. WHITE PC: low proportion of iron oxide by the choice of raw materials or by firing in reducing flame;
e.g. MEDUSA CEMENT = white PC ;
e.g. LOW HEAT CEMENT : high % of C2S and C4AF, low % of C3S and C3A;
e.g. MASONRY CEMENT for more plastic mortar, often produced by grinding more finely than ordinary PC , a mixture of PC and limestone (or colloidal clay, diatomaceous earth);
e.g. RAPID HARDENING PC = ground finer than PC, slightly altered, setting time similar but strength developed more rapidly;
e.g. SULPHATE RESISTANT PC = high % of C3S and C2S, low % of C3A and C4AF. Should not contain C3A to avoid formation of ettringite (="cement bacillus")
This place covers:
Portland cement using raw materials containing gypsum, e.g. using CaSO4 instead of chalk or limestone as raw material in the combined production of cement and H2SO4 (actually production of SO2)
This place does not cover:
Ca-aluminosulfate cements |
This place does not cover:
Slag cements | |
Cements based on fly ash | |
Cements based on combustion residues, e.g. from coal | |
Pozzuolans as fillers | |
Compositions based on (fly) ash, without addition of lime (producing) compound | |
Lime-pozzuolana based compositions |
Attention is drawn to the following places, which may be of interest for search:
Artificial pozzuolana cements | C04B 7/24 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
Pozzuolana | a material that, ground and mixed with lime and water, produces at ordinary temperatures compounds with hydraulic properties; |
Pozzuolana cement | obtained by grinding together a pozzuolana with cement clinker or (hydraulic) lime; |
In patent documents, the following words/expressions are often used as synonyms:
- " Natural pozzuolanas "," Santorini earth "," Trass "," Volcanic ash "and " Diatomaceous earth "
This place covers:
Hydraulic cements comprising slags as raw material, e.g. cements having low heat of hydration, cements with higher glass content (improved hydraulic characteristics)
This place does not cover:
Mâchefer (= slag from coal combustion) | |
Slags from combustion of coal, or waste incineration | |
Silicates added as active ingredients before/during the burning process |
This place covers:
Hydraulic cement containing metallurgical slag,
Examples of metallurgical slag :
blast furnace slag .;
STEELMAKING SLAGS.
L.D. slags, (as such not suited as hydraulic cement because of high content of CaO and MgO (lime and magnesia are sprayed on the bath during the oxygen injection for decarburizing and refining the steel)
This place does not cover:
Treatment of slag |
In this place, the following terms or expressions are used with the meaning indicated:
Scorie | metallurgical byproduct based on silicates |
In patent documents, the following abbreviations are often used:
Laitier = scorie de haut fourneau = blast furnace slag
Laitier d'aciéries = steelmaking slags
LD slags = scorie d'aciéries
This place covers:
Hydraulic cement containing metallurgical slag with other inorganic cementitious materials or other activators,
e.g. basic slags + PC clinker or anhydrite;
This place does not cover:
Ingredients added to the slag in the molten state |
In patent documents, the following abbreviations are often used:
METAAL CEMENTEN = basic slags + PC clinker or anhydrite;
when the alkali activated slag results in a polymeric - Davidovits type - cement, additional classification in C04B 12/005 should be given
In this place, the following terms or expressions are used with the meaning indicated:
waterglass | Sodium silicate |
This place covers:
Hydraulic cement containing metallurgical slag mixed with calcium oxide containing activators,
e.g. SLAG CEMENT = "cold process slag cement" = obtained by cogrinding granulated B.F. slag
In patent documents, the following abbreviations are often used:
BF slag = ciment de laitier
slakkencementen (BE) = ciment de laitier à la chaux (FR)
This place covers:
Hydraulic cement containing metallurgical slag mixed with Portland cements,
e.g. PORTLAND BLAST FURNACE CEMENT (GB) = cogrinding 65% B.F. slag +PC clinker (no gypsum);
e.g. PORTLAND BLAST FURNACE SLAG CEMENT (US) = 25-65% granulated B.F.slag;
e.g. CIMENTS DE HAUT-FOURNEAU (BE) = 30-70% granulated B.F. slag;
e.g. CIMENTS PERMETALLIQUES (BE) = more than 70% B.F. slag;
e.g. CIMENTS PORTLAND DE FER (FR) = 25-35% B.F. slag;
e.g. CIMENTS METALLURGIQUES MIXTES (FR) = 45-55% B.F. slag;
e.g. CIMENTS DE HAUT-FOURNEAU (FR) = 65-75% B.F. slag;
e.g. CIMENTS DE LAITIER AU CLINKER (FR) = more than 80% B.F. slag;
e.g. EISENPORTLANDZEMENT (DE) = less than (35 or) 40% B.F. slag;
e.g. HOCHOFENZEMENT (DE) = 36 (or 41) -85% B.F. slag;
In patent documents, the following abbreviations are often used:
Portland cements = ciments de haut-fourneau = ciment permétallique,= ciments Portland de fer = ciments métallurgiques mixtes = ciments de laitier au clinker = eisenportalndzement = hochofenzement
This place covers:
Hydraulic cement containing metallurgical slagmixed with calcium sulfate containing activators,
e.g. SUPERSULPHATED CEMENT = granulated B.F. slag + CaSO4 + small % PC or lime
This place does not cover:
Cement containing metallurgical slag mixed with alkali metal containing activators |
In patent documents, the following abbreviations are often used:
Supersulphated cement = ciment métallurgique sursulfaté = ciment sursulfaté = sulfathüttenzement
This place covers:
Hydraulic cement using as raw materials oil shales, residues or waste resulting from different processes, e.g. combustion waste, demolition waste, household, not being slag
This place does not cover:
Waste as additive to the raw material | |
Waste as fillers for concrete compositions |
This place covers:
Hydraulic cements produced from oil shales, residues or wastes mixed with activators or composition-correcting additives
Attention is drawn to the following places, which may be of interest for search:
when the alkali activated waste results in a polymeric - Davidovits type - cement, additional classification in C04B 12/005 should be given
In this place, the following terms or expressions are used with the meaning indicated:
Activator | Material used to enhance the hydraulic activity of (waste) raw materials |
In patent documents, the following abbreviations are often used:
Composition correcting additives = activators
This place does not cover:
Hydraulic cements from oil shales, residues or wastes other than slag mixed with activators or composition-correcting additives |
This place covers:
Hydraulic cements produced from combustion residues,
e.g. artificial pozzuollans other than slags or fly ashes.
This place does not cover:
Hydraulic cements from oil shales, residues or waste other than slag mixed with activators or composition-correcting additives | |
Hydraulic cements from raw materials containing flue dust | |
Concrete compositions containing artificial pozzuollans |
This place covers:
Hydraulic cements produced from oils shale, from oil shale residues, from lignite processing,
e.g. simultaneous production of cement and combustion gas from coal
In patent documents, the following abbreviations are often used:
Schistes houillers = bitumineus kalksteen
This place covers:
Hydraulic aluminous cements, obtained by melting (or sintering) a mixture of bauxite and chalk; cooling; grinding.
Composed of :3CaO.Al2O3 (tricalcium dialuminate), CaO.2Al2O3 (calciumtetraaluminate), CaO.Al2O3 (calcium metaaluminate).
Comp.: CaO 37.7 ,Al2O3 38.5 , Fe2O3 12.7 , FeO 3.9 , SiO2 5.3 , SO3 0.1 .
Hydration: -->mostly 3CaO.Al2O.6H2O + Al(OH)3 formed;
i.e. characteristics: less aggressive to the skin than PC,
* very rapid strength development (24h = 28 days for PC)
*setting time = similar to PC
* sulphate/seewater resistant
* colour =black ,
* to be used to -10 C
used for castable refractories;
e.g. high alumina cement;
e.g. 11CaO.7Al2O3.CaX2
This place covers:
Hydraulic calcium aluminosulfate cements
e.g. 4CaO.3Al2O3.SO3;
This place covers:
i.e. HYDRAULIC LIME : obtained from limestone containing clay, burnt at 1000 - 1200 C ..>; beta-C2S, C2AS, C4AF .
WATERKALK. The more hydraulic the closer to cement;
i.e. ROMAN CEMENT = ROCK CEMENT = obtained by calcining a natural mixture of clay and limestone;
i.e. NATURAL CEMENT = idem (below sintering);
e.g. SELENITIC CEMENT = lime + 5 - 10% plaster of lime;
e.g. HYDRAULIC HYDRATED LIME = hydrated dry cement. Product obtained by calcining limestone containing silica and alumina to a temper. short of incipient fusion --->; sufficient free CaO formed to permit hydration and leaving unhydrated suffic. calc. silicate;
e.g. HIGH CALCIUM HYDRAULIC HYDRATED LIME = hydraulic hydrated lime containing <= 5% MgO;
e.g. HIGH MAGNESIUM HYDRAULIC HYDRATED LIME = hydraulic hydrated lime containing >= 5% MgO;
This place covers:
Controlling, monitoring hydraulic cement manufacturing processes,, e.g.. automation
This place covers:
Manufacture of hydraulic cements preventing environmental pollution during the process e.g. desulfuration
This place covers:
Manufacture of hydraulic cements by treating raw materials with active ingredients added before or during the burning processes e.g. additives for obtaining white cement
This place does not cover:
Blended cements with slags | |
Adding ingredients after the burning process |
This place covers:
Manufacture of hydraulic cements by treating raw materials with acids or salts added before or during the burning processes e.g. vanadates
This place does not cover:
Automatisation aspects | |
Desulfuration | |
Aspects only relating to the installation | |
Furnaces, kilns, ovens and details thereof |
This place covers:
Manufacture of hydraulic cement by preheating without addition of fuel during the preheating step, for example by using exhaust gases, e.g. RSP = reinforced suspension preheater
This place covers:
Manufacture of hydraulic cement by preheating with addition of fuel , e.g. with addition of fuel in the calcining step, besides the addition of fuel in the kiln itself
This place covers:
Treatment or selection of the fuel for the burning during the manufacture of hydraulic cement e.g. fuel for burning other raw material; waste hot gases
e.g. heavy fuel oil (S-content >1%)
This place does not cover:
Refuse consuming furnaces |
This place does not cover:
Calcination in fluidised beds |
This place covers:
Electric burning or melting during the manufacture of hydraulic cement
This place does not cover:
Non-electric melting |
This place covers:
Clinker hydration during manufacture of hydraulic cement, i.e. in principle for the hydration of the lime content of the clinker;
e.g. hydrating ground clinker
This place does not cover:
Hydrating ground clinker | |
Still contains grinding aids | |
Grinding aids | are classified as active ingredients, e.g. in C04B 24/00, and receive C04B 2103/52 as an Indexing Code |
Grinding aids in general |
This place does not cover:
Obtaining spherical cement particles in the manufacture of hydraulic cement |
This place covers:
Cements characterised by fineness obtained by the clinker grinding e.g. "microcement": particles with diameter smaller than 15 micrometer
This place does not cover:
Unground clinker |
This place does not cover:
Avoiding environmental pollution |
Attention is drawn to the following places, which may be of interest for search:
For aspects relating to cement kiln dust |
This place covers:
Cements are based on magnesium, e.g. Mg oxychloride, Mg oxysulfate; Preparation thereof;
CIS is used only incidentally in this class. If so, index codes are chosen from other C04B 9/00 groups and C04B. A C04B 9/00 index code in a CIS set having a C04B 28/00 EC class indicates the presence of a second binder.
This place covers:
Process of manufacturing magnesium cements or similar cements, e.g. burning, calcining
This place does not cover:
Preheating, burning, calcining or cooling lime stone, magnesite or dolomite |
This place covers:
Calcium sulfate cements,
e.g. Natural forms of CaSO4.2H2O: SELENITE, MARIAGLAS, TERRA ALBA,SATINITE, ALABASTER;
e.g. MORMOR CEMENT = Ca-sulphate;
e.g. MACK'S CEMENT = plaster of Paris + K2SO4 or Na2SO4;
e.g. LANDPLASTER = CaSO4.2H2O ;
e.g. aging of calcined gypsum
CIS is used only incidentally in this class. If so, index codes are chosen from other C04B 11/00 groups and C04B.
This place covers:
Methods and apparatus for dehydrating gypsum,
e.g. PLASTER = mostly alpha + beta.
e.g. regeneration of gypsum molds: (classification being //( C04B 11/02; C04B 11/262) or other way around)
This place does not cover:
Drying alpha-hemihydrate | |
Calcining in general | |
For other purposes than cement manufacture |
This place covers:
e.g. drying of alpha-gypsum;
e.g. quick setting alpha-plaster + K2CO3;
used for making moulds for the ceramic industry.
This place does not cover:
Attention | |
Take precedence |
The presence of C04B 11/032 in a c-set indicates that alpha-hemihydrate form is used
This place covers:
Devices for the dry process of dehydrating gypsum
This place does not cover:
C04B 11/0281 - C04B 11/0288 take precedence
The presence of C04B 11/036 in a c-set indicates that beta-hemihydrate form is used
Attention is drawn to the following places, which may be of interest for search:
Alkali metal silicates per se and their preparation | |
Ammonium silicates per se and their preparation |
Attention is drawn to the following places, which may be of interest for search:
In or for the manufacturing of ceramics |
This place covers:
Inorganic materials used ad fillers for mortars, concrete or artificial stone, and their treatment to enhance their filling properties e.g. inorganic pigments other than oxides;
C04B 14/00 and subgroups are used as substrate codes for coatings of natural stone
This place does not cover:
Oxide pigments | C04B 14/30 and subgroups |
Expanding or defibrillating materials |
This place covers:
Carbon used as fillers for mortar, concrete or artificial stone, elemental carbon, e.g. COKE, KOKS (=90%C), LIGNITE, COCKES, "TEERKOKS";
In patent documents, the following abbreviations are often used:
"MINERAL NOIR" = shale (70% SiO2, 30%C) = coal black = " NOIRMINER" = "NOIR D´ IVOIRE"
This place covers:
Silica-rich materials, silicates used as fillers for mortars, concrete or artificial stone
e.g. "LOESS";
e.g. GREYWACKE, GRAYWACKE = conglomerate rock (round pebbles + sand, cemented together.)
Attention is drawn to the following places, which may be of interest for search:
Clay |
This place covers:
Magnesium silicates used as fillers for mortars, concrete or artificial stone,
e.g. SEPIOLITE; ASBESTINE
This place covers:
Alkali-metal containing silicates, Al-alkali metal silicates used as fillers for mortars, concrete or artificial stone,
e.g. PETALITE Li2O.Al2O3.8SiO2;
e.g. SPODUMENE LiO2.Al2O3.4SiO2;
e.g. EUCRYPTITE LiO2.Al2O3.2SiO2;
e.g. NEPHELINE SYENITE;
e.g. MAGADIITE
This place does not cover:
Waterglass |
This place covers:
Quartz, sand used as filler for mortar, concrete or artificial stone,
e.g. SILICA (SiO2) is polymorphic i.e. capable of existing in two or more crystal forms. Main forms of crystalline silica : QUARTZ,TRIDYMITE, CRISTOBALITE.
e.g. vitreous silica, amorphous silica;
e.g. precipitated silica; pyrogenic silica;
e.g. SILT = fine sand;
e.g. CHERT; FLINT; MOLDING SAND;
This place covers:
microsilica used as filler for mortar, concrete or artificial stone, e.g. colloidal silica 0.001-0.2 microns
This place does not cover:
C04B 18/146, C04B 12/04 take precedence
Preparing microsilica slurries or suspensions |
In this place, the following terms or expressions are used with the meaning indicated:
Microsilica | Silica having micro- or nanosize particles |
This place covers:
Silica aerogel used as filler for mortar, concrete or artificial stone,
e.g. silica aerogel being obtained by forming a SiO2 gel impregnated with a solvent, and evaporating the solvent under hypercritical conditions
This place covers:
Specific natural sands used as filler for mortar, concrete or artificial stone, e.g. BARKHAN SAND, BARHAN SAND
This place covers:
Diatomaceous earth used as filler for mortar, concrete or artificial stone,
e.g. hydrated amorphous silica, skeletons of Diatomacea which are related to brown algae
e.g. INFUSORIAL EARTH;
e.g. TRIPOLITE;
e.g. FOSSIL FLOUR,, MOUNTAIN FLOUR;
In patent documents, the following abbreviations are often used:
FOSSIL FLOUR= FARINE FOSSILE= MOUNTAIN FLOUR
KIESELGUHR, KIESELMEHL
"DIATOMEEN PELITE";
"MOLERERDE"
BERGHMEHL
This place covers:
Clay used as filler for mortar, concrete or artificial stone,
e.g. Al silicates;
e.g. BALL CLAY;;FULLERS EARTH;;
e.g. MARL;
e.g. PORCELANITE
This place does not cover:
Sepiolite | C04B 14/042, some older document are in C04B 14/10 |
Chamotte, fireclay, fired clay, grog | C04B 18/025 (older documents have a C-set C04B 14/10; C04B 18/023) |
In patent documents, the following abbreviations are often used:
BALL CLAY= GLAISE = FULLERS EARTH= LEEM;
MERGEL" = "MARNE" = MARL
OCRE
This place covers:
Bentonite, e.g. montmorillonite used as filler for mortar, concrete or artificial stone,
e.g. HECTORITE, synthetic hectorite;
e.g. BLEACHING EARTH, "BLEICHERDE" = Al-Mg-Silikate
This place does not cover:
Waste bleaching earth |
This place covers:
Kaolin used as filler for mortar, concrete or artificial stone,
Kaolin is not sintered
e.g. METAKAOLIN, KAOLINITE;
e.g. SMECTITE [9212];
This place covers:
Shale, slate used as filler for mortar, concrete or artificial stone
This place does not cover:
Shale residues, colliery shale |
In patent documents, the following abbreviations are often used:
SHALE = SLATE = "SCHISTE" = "SCHIEFER" = "LEISTEEN" (harde klei) =ARDOISE
This place covers:
Expanded clay used as filler for mortar, concrete or artificial stone
e.g. porous clay; expanded shale;
This place covers:
Mineral of volcanic origin used as filler for mortar, concrete or artificial stone
e.g. natural pozzuolanes (pozzolana, puzzolan) other than diatomaceous earth;
e.g. IGNEOUS ROCK
e.g. ANDESITE, RHYOLITE, PORFIER;
e.g. OBSIDIAN = vulcanic glass with little or no crystal water;
e.g. TRASS = TUFF = unconsolidated vulcanic ashes;
This place does not cover:
Granite |
In this place, the following terms or expressions are used with the meaning indicated:
TUF | porous rock formed from cemented volcanic ashes or from calcareous deposits in lakes or springs |
In patent documents, the following abbreviations are often used:
IGNEOUS ROCK = "ERGUSSGESTEIN " = "ROCHE EFFUCIVE
UITVLOEIINGS GESTEENTE" = "GESTOLDE LAVA"
"PECHSTEIN" = acidic vulcanic glass;
This place covers:
Porous minerals of volcanic origin used as filler for mortar, concrete or artificial stone
e.g. PUMICE = porous volcanic rock;
e.g. LIPARITE =(Ca pegmatite) e.g. SHIRAZU, SHIRASU = volcanic ash;
e.g. PUMIZITE = volcanic glass
e.g. SCORIA, CINDER
In patent documents, the following abbreviations are often used:
PUMICE = "PIERRE PONCE " = "PUIMSTEEN" = "BIMS"(12mm)
FLUGSAND = like bims but finer (7-10mm);
This place covers:
Perlite being mineral of volcanic origin used as filler for mortar, concrete or artificial stone e.g. volcanic glass
This place covers:
Expanded perlite (mineral of volcanic origin) used as filler for mortar, concrete or artificial stone
e.g. expanded by evaporation of crystal water
This place covers:
Mica, vermulite used as filler for mortar, concrete or artificial stone
e.g. "GLIMMER", KAl3Si3O10(OH)2;
e.g. BIOTITE = dark or magnesia mica (rich in Mg and Fe);
e.g. MUSCOVITE = (Na,K)2O.3Al2O3.6SiO2.2H2O;
e.g. TRACHYLIPARITE;
e.g. sericite
This place does not cover:
Punching of mica | |
Mechanical splitting | |
Mica treatment | |
Pulp or paper comprising mica or vermiculite |
This place covers:
Vermiculite used as filler for mortar, concrete or artificial stone
e.g. = hydrated biotite mica (OH)2(Mg Fe)3(SiAlFe)4O10.4H2O;
This place does not cover:
Delamination of mica | |
Chemical delamination |
This place does not cover:
Ion exchanged silicates |
This place does not cover:
Mechanical delamination |
This place covers:
Any type of glass used as filler for mortar, concrete or artificial stone
e.g. recuperated, waste glass;
e.g. frits, email
This place does not cover:
Vitreous SiO2 |
This place does not cover:
Lightweight materials |
This place covers:
Carbonates used as filler for mortar, concrete or artificial stone
e.g. MAGNESITE, DOLOMITE, "DOLOMIE"
This place covers:
Carbonates of calcium used as filler for mortar, concrete or artificial stone
e.g. TRIPOLI;
e.g. SHELLS, CORAL, MOTHER OF PEARLS,
e.g. CHALK,
e.g. ICELAND SPAR = pure crystalline calcite (CaCO3)
In patent documents, the following abbreviations are often used:
"ARDUIN" = blue stone
Chalk = "NEUBERGER KREIDE", "KIESELKREIDE
This place covers:
Oxides other than silica used as filler for mortar, concrete or artificial stone
e.g. simple oxides;
e.g. oxides used as pigments;
This place does not cover:
Ferrites | |
Oxides as active ingredients |
used as CIS codes for refractory filler in concrete
This place covers:
Alumina used as filler for mortar, concrete or artificial stone
e.g. Al(OH)3;
e.g. BAUXITE;
This place does not cover:
Gelatinous Al(OH)3 |
This place covers:
Iron oxide used as filler for mortar, concrete or artificial stone
e.g. CAPUT MORTUUM = ENGLISH RED = Fe2O3
This place covers:
Inorganic materials not classified in groups C04B 14/022, C04B 14/04 - C04B 14/34 used as filler for mortar, concrete or artificial stone
e.g. mineral salt (NaCl);
e.g. LITHOPONE = BaSO4 + ZnS (=pigment);
e.g. SPINEL = MgAl2O4
This place covers:
Soil used as filler for mortar, concrete or artificial stone
e.g. mud, sapronel, laterite
This place does not cover:
Harbour/river sludge |
In patent documents, the following abbreviations are often used:
mud, sapronel, = "Faulschlamm
This place does not cover:
Fibres in general |
This place does not cover:
Carbon nanotubes | |
Fabrication of carbon fibres |
This place does not cover:
- treating asbestos fibres see D06M 7/005, D02G 3/20;
- coating of asbestos in general see C03C 25/00;
- coating of asbestos CIS example C04B 20/10, C04B 14/40;
- disposal of asbestos see B09B 3/00;
- asbestos from old buildings CIS example ( C04B 14/40, C04B 18/16)
In this place, the following terms or expressions are used with the meaning indicated:
CHRYSOTILE (serpentine family) | 3MgO.2SiO2.2H2O |
CROCIDOLITE (amphibole family) | BLUE ASBESTOS3Na2O.6FeO.2Fe2O3.16SiO2.H2O |
AMOSITE (amphibole family) | 2Ca.5MgO.8SiO2.H2O |
TREMOLITE | amphibole family |
ANTHOPHYLLITE | (Mg,Fe)7Si8O22(OH)2 |
ASBESTINE | fibrous variety of talc +tremolite |
This place covers:
Glass fibers, glass whiskers used as filler for mortar, concrete or artificial stone
This place does not cover:
Composition of (alkali-resistant) glass fibres | |
Coating glass fibres used for cement reinforcement | |
Glass fibres for resin matrix |
This place does not cover:
Composition of alkali resistant glass fibres | |
Coating of glass fibres |
This place does not cover:
Take precedence | |
Ceramic fibres as such |
This place covers:
Oxides , hydroxides of rock wool used as filler for mortar, concrete or artificial stone
This place does not cover:
TiO2 |
This place covers:
Titanates, TiO2 of rock wool used as filler for mortar, concrete or artificial stone
This place does not cover:
Cellulosic fibres | |
Cellulosic waste materials, e.g. sawdust, rice husks |
This place covers:
Macromolecular organic compounds used as filler for mortar, concrete or artificial stone e.g. glass-clear thermoplastic MBS resin (methacrylate-butadiene-styrene) for packaging , medical applications
This place does not cover:
Cellulosic materials as fillers for mortars, concrete or artificial stone |
This place covers:
Fibrous organic macromolecular compounds used as filler for mortar, concrete or artificial stone
e.g. regenerated cellulose fibers;
e.g. textile waste,
In patent documents, the following abbreviations are often used:
textile waste="Textilschnitzel"
This place covers:
Fibrilles used as filler for mortar, concrete or artificial stone
e.g. polyalkanes;
e.g. stretched films,
e.g. "pulp" aramid fibers = very short , highly fibrillated with very fine fibrils or subfibers attached to core fibre
This place does not cover:
Fibrillated films in general |
In patent documents, the following abbreviations are often used:
stretched films= films "etirés"
This place covers:
Fibrilles of polyamaide, of polyaramides used as filler for mortar, concrete or artificial stone e.g. aromatic polyetheramide fibers;
e.g. ARAMID fibers = poly (p-phenylene terephtalamide)
e.g. NYLON = aliphatic polyamide
This place covers:
Porous organic macromolecular compounds used as filler for mortar, concrete or artificial stone
e.g. PS, EPS;
e.g. expanded PS
This place does not cover:
Working up macromolecular substances to porous or cellular articles or materials |
This place does not cover:
Takes precedence |
This place covers:
Organic materials used as fillers for mortar, concrete or artificial stone characterised by their shape
This place does not cover:
Fibrous macromolecular compounds | |
Porous macromolecular compounds | |
Only characterised by the form |
This place does not cover:
Use of waste materials for the manufacture of cement | |
Granulating materials in general | |
Making microcapsules or microballoons |
Attention is drawn to the following places, which may be of interest for search:
Temporary compacting of cement | |
Temporary compacting of gypsum | |
Conditioning silica fume |
In this place, the following terms or expressions are used with the meaning indicated:
Artificial aggregates, synthetic aggregates | Aggregates which are not "natural" in the way that crushed rocks or sands and gravels are. These are implied to be human-made materials, whether they are by-products of some other industry or even if they are deliberately manufactured. |
Attention is drawn to the following places, which may be of interest for search:
Lightweight agglomerated materials, e.g. artificial aggregates | |
Waste materials or refuse from building or ceramic industry |
For agglomerated materials (artificial aggregates or fillers) which are classified in C04B 18/021 or other equivalent subgroups of C04B 18/00, the starting materials, other than the binder, can be identified in the C-set with Indexing Symbols. Symbols are chosen from C04B 14/00, C04B 16/00, C04B 18/00 and less often from C04B 22/00 or C04B 24/00.
Attention is drawn to the following places, which may be of interest for search:
Lightweight agglomerated materials, e.g. artificial aggregates |
See the special rules under C04B 18/021.
This place covers:
Agglomerated materials wherein a melting or firing step takes place during the agglomeration.
Attention is drawn to the following places, which may be of interest for search:
Expanded clay | |
Porous fired material | |
Pelletizing fly ash | |
Expanding clay, perlite, vermiculite or like granular materials, which are (a) used as fillers for mortars, concrete or artificial stone, (b) specially adapted to enhance their filling properties in mortars, concrete or artificial stone, or (c) expanding or defibrillating materials | |
Porous or hollow ceramic granular material |
See the special rules under C04B 18/021.
A lightweight material (C04B 18/027) which is fired or melted (C04B 18/023) is classified as (C04B 18/027, C04B 18/023).
This place covers:
Grog used as fillers for mortars, concrete or artificial stone
e.g. crushed refactory materials added to ceramic mixes to reduce lamination in clays and shrinkage on drying,
e.g. crushed pottery, firekrick, quartz quartzite, burned ware, saggers;
e.g. CHAMOTTE, FIRED CLAY, FIRECLAY as filler for concrete
Before [9105] documents were classified in C04B 14/10 and received C04B 20/04 index code in the C-set
This place covers:
Agglomerated materials wherein a melting step takes place during the agglomeration
This place does not cover:
Glass, devitrified glass used as filler for mortar, concrete or artificial stone |
This place covers:
Low density or porous agglomerated material used as filler for mortar, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Expanded clay | |
Porous glass | |
Expanding clay, perlite, vermiculite or like granular materials, which are (a) used as fillers for mortars, concrete or artificial stone, (b) specially adapted to enhance their filling properties in mortars, concrete or artificial stone, or (c) used as expanding or defibrillating materials | |
Porous or hollow ceramic granular material for porous mortars, concrete, artificial stone or ceramic ware; Preparation thereof |
For crushed porous concrete aggregate, one set of symbols is used with C04B 18/027 or C04B 38/00 and an alternative classification in C04B 18/16.
A lightweight material (C04B 18/027) which is fired or melted (C04B 18/023) is classified as (C04B 18/027, C04B 18/023).
This place covers:
Waste materials or refuse, e.g. from industrial or other processes or Si-Stoff, or a waste from alumina production. This place also covers mixtures of wastes.
Attention is drawn to the following places, which may be of interest for search:
Waste added during the cement production, i.e. in the kiln raw materials | |
Waste glass | |
Making harmful chemical agents harmless | |
Destroying solid waste or transforming solid waste into something useful or harmless, e.g. disposal of asbestos | |
Solidification of sludges | |
Solidification of liquid or solid radioactive waste |
This place covers:
Waste from the purification of bauxite used as filler for mortar, concrete or artificial stone
Attention is drawn to the following places, which may be of interest for search:
treatment of waste from aluminium production |
In this place, the following terms or expressions are used with the meaning indicated:
Red mud, red sludge | solid waste product of the Bayer process, composed of a mixture of solid and metallic oxide-bearing impurities (high content of oxidised iron) |
This place covers:
Dry waste materials used as fillers for mortars, concrete or artificial stone,
Wet waste materials that is dried before use as fillers for mortars, concrete or artificial stone
This place does not cover:
Other slurries or sludges |
Attention is drawn to the following places, which may be of interest for search:
Soil | |
Waste materials in general |
This place covers:
Synthetic gypsum form waste material used as filler for mortars, concrete or artificial stone.
This place does not cover:
Gypsum from smoke purification |
In this place, the following terms or expressions are used with the meaning indicated:
Synthetic gypsum, flue-gas desulfurisation gypsum | byproduct of coal-fired power plants (coal burned with flue gas desulfurisation) |
In this place, the following terms or expressions are used with the meaning indicated:
Bleaching earth | (waste) bleaching earth is used as absorbent material for cleaning/bleaching of mineral, natural oils, fats and waxes. After the use it becomes "fat" bleaching earth. The fats/oils are removed as much as possible by boiling with soda and salt giving a low fat ("mager") bleaching earth |
This place covers:
Other specific industrial waste materials not provided in the other subgroups of C04B 18/00 used as fillers for mortars, concrete or artificial stone
e.g. PYRITE CINDER Fe2O3;
In patent documents, the following abbreviations are often used:
kiesabbraende
This place covers:
Combustion residues used as fillers for mortars, concrete or artificial stone, e.g. purification products of smoke, fumes or exhaust gases, bottom ash, coal ash or cinders.
Attention is drawn to the following places, which may be of interest for search:
Collecting residues from parts of furnace plants |
This place covers:
Ashes from fluidised bed furnaces used as fillers for mortars, concrete or artificial stone, e.g. AFBC ashes = atmospheric fluidised bed combustion ashes
fly ashes from fluidised bed furnaces take two alternative sets of codes one with C04B 18/061 and one with C04B 18/08
This place covers:
Residues from coal gasification used as fillers for mortars, concrete or artificial stone
e.g. residues from the partial oxidation of coal
This place covers:
Flue dust, fly ash; which is used as fillers for mortars, concrete or artificial stone.
Examples include:
- Class N fly ash;
- Class F fly ash;
- Class C fly ash;
Attention is drawn to the following places, which may be of interest for search:
Slaking of lime in the presence of fly ash |
In this place, the following terms or expressions are used with the meaning indicated:
Flue dust | by-product of the burning of pulverised coal |
In patent documents, the following abbreviations are often used:
PFA | Pulverised Fuel Ash |
EFA | Electrofilter Ash |
HVFA | High Volume Fly Ash |
Class F Fly Ash | pozzolanic, usually from bituminous coal, low Ca, glass part is reactive with cement |
Class C Fly Ash | pozzolanic and cementitious, normally from lignite or sub-bituminous coal |
This place covers:
Flue dust from brown coal or lignite used as filler for mortars, concrete or artificial stone,
In patent documents, the following abbreviations are often used:
Lignite fly ash = Braunkohlefilterasche"
This place covers:
Cenospheres used as fillers for mortars, concrete or artificial stone,
e.g. = floating fraction of PFA = hollow spheres (20-200micron) of aluminosilicate glass;
e.g. ARMOSPHERES, FILLITE, EXTENDOSPHERES
This place covers:
Pelletizing fuel dust used as filler for mortars, concrete or artificial stone,
Melting fuel dust to form aggregate;
e.g. sintered PFA
Attention is drawn to the following places, which may be of interest for search:
For LYTAG | |
Agglomerated fired materials | |
Expanding clay, vermiculite, perlite and the like |
This place covers:
Burned or pyrolised refuse used as filler for mortars, concrete or artificial stone, e.g. municipal solid waste, slags from waste incineration or burned paper processing waste.
Attention is drawn to the following places, which may be of interest for search:
In patent documents, the following abbreviations are often used:
MSW | Municipal solid waste |
This place covers:
Burned rice husks or other burned vegetable material used as filler for mortars, concrete or artificial stone
e.g. expanded rice hull ash, reburned rice hull ash
This place covers:
Burned or pyrolised sludges used as filler for mortars, concrete or artificial stone,
e.g. SSA = SEWAGE SLUDGE ASH / SLAG
This place covers:
Gaseous combustion products or dusts collected from waste incineration used as filler for mortars, concrete or artificial stone,
e.g. AQCS =Air quality combustion system = fly ash + desulfurisation products
This place covers:
Waste materials form quarries, mining or the like used as filler for mortars, concrete or artificial stone.
In patent documents, the following abbreviations are often used:
Tailings = BERGE", "BERGEMATERIAL", "GRUBENBERGE", "WASCHBERGE
This place covers:
Waste materials from metallurgical processes used as filler for mortars, concrete or artificial stone, such as nephelin slurry from Al production.
This place does not cover:
Treatment of molten slag |
Attention is drawn to the following places, which may be of interest for search:
Cements containing slag |
This place covers:
Slags from metallurgical processes used as filler for mortars, concrete or artificial stone, e.g. blast furnace slag;
e.g. cupola slag, "
In patent documents, the following abbreviations are often used:
cupola slag = KUPOLOFENSCHLACKE
This place covers:
Filter dust from silicon metal or ferrosilicon alloy production;
(non-thixotropic)
Attention is drawn to the following places, which may be of interest for search:
Thixotropic silica fume e.g. CAR-BO-SIL |
In patent documents, the following words/expressions are often used as synonyms:
- "MICROSILICA", "ferrosilicon dust", "silica flue dust" and "amorphous silica"
This place covers:
Waste materials from metallurgical processes other than silica fume or slag used as filler for mortars, concrete or artificial stone,
e.g. EAFD, electric arc furnace dust
This place covers:
Waste materials or refuse from building or ceramic industry used as filler for mortars, concrete or artificial stone, e.g. reclaiming cement slurry or broken ceramic tiles.
Attention is drawn to the following places, which may be of interest for search:
Materials agglomerated by a mineral binder | |
Lightweight materials | |
Separating of concrete slurry as refuse |
Foamed concrete as aggregate: two C-Sets are given, one with C04B 18/16 and one with C04B 18/027.
This place covers:
Cement kiln dust or lime kiln dust used as filler for mortars, concrete or artificial stone.
Dust resulting from cement production.
Attention is drawn to the following places, which may be of interest for search:
Recuperation of cement kiln dust during cement fabrication |
This place covers:
Organic waste materials used as filler for mortars, concrete or artificial stone, such as hair, feathers, leather, manure, mest or wool fibers.
This place does not cover:
Burned or pyrolised refuse |
Attention is drawn to the following places, which may be of interest for search:
Recycled expanded polystyrene | |
Recovery from working up of polymers |
This place covers:
Vegetable refuse, cellulosic materials used as fillers for mortar, concrete or artificial stone
e.g. CORK, SISAL, PEAT, KAPOK;
e.g. VEGETABLE IVORY = CORAJO = TAGUA;
e.g. COMPOST;
e.g. expanded cellulosic material i.e. puffed rice, popcorn is classified in this group and received the C-set containing C04B 20/06
This place does not cover:
Regenerated cellulose fibers | C04B 16/06 ( C04B 18/24 still to be cleaned) |
Processing, machining of boards fabricated from pressed wood fibers | |
"Spaanplaten" |
This place covers:
Paper products used as fillers for mortar, concrete or artificial stone,
e.g. pulp from bark;
e.g. waste paper
Attention is drawn to the following places, which may be of interest for search:
Still contains older documents | |
Wood pulp |
This place does not cover:
Burned paper processing waste |
Attention is drawn to the following places, which may be of interest for search:
Waste paper itself |
This place covers:
Cork, bark used as filler for mortars, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Mechanical working of cork |
Wood and bark are not synonymous, wood used as filler being classified in C04B 18/26.
Attention is drawn to the following places, which may be of interest for search:
Vegetable refuse, e.g. rice husks, maize-ear refuse, peat or algae; Cellulosic materials, e.g. paper or cork |
This place covers:
Wood used as fillers for mortars, concrete or artificial stone,
e.g. BAMBOO;
e.g. ARBOLITE = wood chips/waste cement boards;
e.g. FIBROLITE = wood wool cement boards
This place covers:
Mineralising vegetable refuse, compositions therefor used as filler for mortars, concrete or artificial stone
Attention is drawn to the following places, which may be of interest for search:
With organic materials | C04B 20/1018, older documents still present in C04B 18/28. |
In this place, the following terms or expressions are used with the meaning indicated:
Mineralising | Treatment with a mineral substance |
This place does not cover:
Takes precedence |
Attention is drawn to the following places, which may be of interest for search:
Nanotechnology for materials or surface science | |
Manufacture or treatment of nanostructures | |
Reinforcing elements for concrete |
Attention is drawn to the following places, which may be of interest for search:
Lightweight agglomerated material | |
Hollow or porous ceramic granular material |
This place covers:
Mixtures of different fibres. Fibrous materials wherein the fibre type is important but not a singular type.
In this place, the following terms or expressions are used with the meaning indicated:
fibre | relatively short, elongated member of natural or artificial material |
filament | endless or quasi-endless, elongated member of natural or man-made material |
yarn | unitary assembly of fibres or filaments, usually produced by spinning |
thread | assembly of yarns or filaments usually produced by twisting |
This place covers:
Mixtures of fibres of different physical characteristics used as materials for mortars, concrete or artificial stone, e.g. obtained by twisting.
This place covers:
Materials used as fillers for mortars, concrete or artificial stone according to more than one of groups C04B 14/00 - C04B 18/00 and characterised by the grain distribution.
In this place, the following terms or expressions are used with the meaning indicated:
fine aggregate | material less than 5 mm |
coarse aggregate | material at or more than 5 mm |
concrete | material with more than 50% coarse aggregate |
granules 0-3 | material less than or equal to 3mm |
mortar | material with no coarse aggregate |
This place covers:
Treatment specially adapted to enhance filling properties of materials used as fillers for mortars, concrete or artificial stone according to more than one of the groups C04B 14/00 - C04B 18/00, e.g. removing dust from particles or forming circular particles from scrap glass.
Attention is drawn to the following places, which may be of interest for search:
Cation exchange of vermiculite | |
Temporarily agglomerated materials |
This place covers:
Heat treatment of materials according to more than one of the groups C04B 14/00 - C04B 18/00 specially adapted to enhance their filling properties in mortars, concrete or artificial stone, e.g. drying.
This place covers:
Expanding clay, perlite, vermiculite or like granular materials by grate sintering to enhance their filling properties in mortars, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Grate sintering of ores or scrap | |
Endless-strand sintering apparatus |
In this place, the following terms or expressions are used with the meaning indicated:
Dwight-Lloyd process | blast roasting |
This place covers:
Expanding clay, perlite, vermiculite or like granular materials in fluidised beds to enhance their filling properties in mortars, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Expanded clay | |
Expanded aggregates |
This place covers:
Defibrillating asbestos to enhance its filling properties in mortars, concrete or artificial stone, e.g. dispersing or flocculating asbestos, or separating asbestos from bearing material or ores.
This place does not cover:
Defibrillating other fibres |
Attention is drawn to the following places, which may be of interest for search:
Opening fibres in general | |
Carding machines |
This place does not cover:
Roofing granules |
Attention is drawn to the following places, which may be of interest for search:
Mineralising wood | |
Coating glass fibres, asbestos or other mineral fibres |
If a mixture is used for the coating: last place rule applies, and C04B 20/10 symbols are added for other ingredients in the C-Sets.
This place covers:
Coating or impregnation of materials used as fillers for mortars, concrete or artificial stone according to more than one of groups C04B 14/00 - C04B 18/00, with macromolecular compounds, to enhance their filling properties, e.g. coating filler with polymer and pigments.
This place covers:
Coating or impregnating with organo-metallic compounds, organo-silicon compounds, materials according to more than one of the groups C04B 14/00 - C04B 18/00 to enhance their filling properties in mortars, concrete or artificial stone.
This place covers:
Coating or impregnating materials according to more than one of the groups C04B 14/00 - C04B 18/00 with silicates to enhance their filling properties in mortars, concrete or artificial stone, e.g. sand, silica fume or glass.
Attention is drawn to the following places, which may be of interest for search:
Waterglass |
C04B 20/123 and C04B 20/126 are only used as subsequent symbols in C-Sets along with C04B 20/12 and should not be used as single symbols.
In the case of a composition with two coatings, wherein the first coating has two alternatives, C04B 20/123 is added to a C-Sets to indicate the second alternative coating:
In the case of a composition with multiple coatings, the presence of a layer of the same material as in the previous coating layer is indicated by adding C04B 20/126 as a subsequent symbol in the C-Sets:
This place covers:
Inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. hydrazine (NH2NH2), silicates or hydroalumite 3CaO.Al2O3.CaX2.nH2O, X = (NO3, NO2, OH, CH3COO, CO3, SO4).
Attention is drawn to the following places, which may be of interest for search:
Alkali metal or ammonium silicates | |
Zeolites |
Classification in C04B 22/00 and subgroups is made when these ingredients are (or suspected to be) new or unusual or special details describing this ingredient are given.
Attention is drawn to the following places, which may be of interest for search:
Nitre cake |
Inorganic materials used as active ingredients for mortars, concrete or artificial stone are further classified in C04B 22/0006. Classification is provided in C04B 22/0006 only when another C04B 22/00 symbol is already applied to indicate that the ingredient(s) is a waste product.
This place covers:
Boron compounds, e.g. fluoro-boron compounds used as active ingredients for mortars, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Organic boron compounds |
This place covers:
Water, e.g. magnetised water, ionized water or alkali-ion water.
This symbol is given as a subsequent symbol in C-Sets or as an invention symbol only when very particular aspects of the mixing water are given.
This place covers:
Aluminates used as active ingredient for mortars, concrete or artificial stone.
Attention is drawn to the following places, which may be of interest for search:
Calcium sulphoaluminates | |
Cement or like inorganic materials added as expanding or shrinkage compensating ingredients in mortars or concrete compositions |
This place covers:
Elements used as active ingredients for mortars, concrete or artificial stone, e.g. Si or O3.
This place covers:
Metals used as active ingredient for mortars, concrete or artificial stone.
This place covers:
Oxides or hydroxides used as active ingredients for mortars, concrete or artificial stone, e.g. gelatinous Al(OH)3.
This place does not cover:
Boron compounds |
Attention is drawn to the following places, which may be of interest for search:
Microsilica, e.g. colloidal silica | |
Alumina | |
Acids or salts containing carbon in the anion |
This place covers:
Oxides or hydroxides of the alkali or alkaline-earth metals used as active ingredients for mortars, concrete or artificial stone, e.g. NH4OH, hydroxylamine (NH2OH), NaOH with soda impurities or KOH.
In patent documents, the following words/expressions are often used with the meaning indicated:
Natron lye | Aqueous solution of NaOH |
Potash lye | Aqueous solution of KOH |
This place covers:
Peroxides used as active ingredients for mortars, concrete or artificial stone, e.g. H2O2.
This place covers:
Acids or salts of inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. molybdates or permanganates.
This place does not cover:
Boron compounds used as active ingredients for mortars, concrete or artificial stone |
This place covers:
Acids or salts of inorganic materials containing carbon in the anion, used as active ingredients for mortars, concrete or artificial stone, e.g. soda, (NH4)2CO3, K4(Fe(CN)6).3H2O yellow, K3(Fe(CN)6) red, cyanates, KCN or CO2.
In patent documents, the following words/expressions are often used with the meaning indicated:
Salmiac | (NH4)2CO3 |
This place covers:
The addition of CO2 in the cementitious mixture is as an additive.
CO2 may be added in gaseous or liquid form.
This place covers:
Acids or salts of inorganic materials containing halogen in the anion used as active ingredients for mortars, concrete or artificial stone, e.g. CaCl(OCl).CaO.H2O, Hg chloride or HCl.
In patent documents, the following words/expressions are often used with the meaning indicated:
Bleaching powder | CaOCl2, calcium hypochlorite, Ca(OCl)2 or calcium oxychloride |
Muriatic acid | Aqueous solution of hydrochloric acid [HCl] |
This place covers:
Chlorides of ammonium or of alkali or alkaline earth metals used as active ingredients for mortars, concrete or artificial stone, e.g. NH4Cl.
In patent documents, the following words/expressions are often used with the meaning indicated:
Ammonium muriate | NH4Cl |
This place covers:
Fluorine compounds of inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. silicofluorides or fluorosilicates.
This place covers:
Acids or salts thereof of inorganic materials containing sulfur in the anion used as active ingredients for mortars, concrete or artificial stone, e.g. sulfamic acid (NH2SO3H), sodium thiosulfate (Na2S2O3, x 5H2O) or calcium thiocyanate.
This place covers:
Sulfates of inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. MgSO4, KFe(SO4)2, x 12H2O or mixtures of sulfates covered by C04B 22/142 and its subgroups.
Attention is drawn to the following places, which may be of interest for search:
Acids or salts containing sulfur in the anion, e.g. Jarosite or bisulfates | |
Aluminium-sulfate, e.g. alums |
In patent documents, the following words/expressions are often used with the meaning indicated:
Alums | hydrate double sulfate salt of aluminium: XAl(SO4)2.12 H2O |
Epsom salt | MgSO4 |
Jarosite | basic hydrous sulfate of potassium and ferric iron: KFe3(SO4)2(OH)6 |
Potassium bisulfates | KHSO4 |
This place covers:
Alkali-metal sulfates or ammonium sulfate of inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. glauberite (Na2Ca(SO4)2).
Attention is drawn to the following places, which may be of interest for search:
Sulfates other than alums | |
Aluminium-sulfate, e.g. alums or alunite |
In patent documents, the following words/expressions are often used with the meaning indicated:
Alums | hydrate double sulfate salt of aluminium: XAl(SO4)2.12 H2O |
Alunite | (KAl3(SO4)2(OH)6 |
This place covers:
Aluminium-sulfate of inorganic materials used as active ingredients for mortars, concrete or artificial stone, e.g. alunite (KAl3(SO4)2(OH)6) or alums XAl(SO4)2.12 H2O.
This place covers:
Acids or salts thereof of inorganic materials containing phosphorus in the anion, e.g. Na(NH4)HPO4.4H2O.
In patent documents, the following words/expressions are often used with the meaning indicated:
Microcosmic salt | Na(NH4)HPO4.4H2O |
This place covers:
Organic materials used as active ingredients for mortars, concrete or artificial stone,
e.g. LIGROIN (benzine);
e.g. RUTIN;
e.g. WHITE SPIRIT = mainly heptane, octane;
e.g. impure NAPHTALENE e.g. esters of inorganic acids, ethylene carbonate;
e.g. PERFUME;
e.g. YEAST
e.g. norbornene and its derivatives (e.g. as modifiers for sulfur cements)
e.g. (di) cyclopentadiene (e.g. as modifiers for sulfur cements)
Organic active ingredients as components of a cementitious mixture are indexed in the C-set of the CIS database using entries from C04B 24/00. Classification in C04B 24/00 and subgroups is done when these ingredients are (or suspected to be) new or unusual or special details describing such an ingredient are given.
In this place, the following terms or expressions are used with the meaning indicated:
Vinasse | residual liquid from the distillation of liquid alcohol; |
Fusel oil | mixture of alcohols, fatty acids and esters obtained during distillation of fermentation alcohol |
This place does not cover:
Phosphorus containing polymers |
In patent documents, the following abbreviations are often used:
PHOSPHONOCARBOXYLIC ACID | H2O3P-R-COOH |
PHOSPHONIC ACID | RPO3H2 |
PHOSPHINIC ACID | R2PO2H |
PHOSPHORIC ESTER | (HO)2PO-OR |
PHYTIC ACID = PHYTINIC ACID = meso-inositol hexaphosphoric acid | C6H6(OPO(OH2))6 |
This place covers:
all halogenated compounds except chlorosilanes
This place does not cover:
Pesticides | |
Halogenated polymers of the type corresponding to groups C04B 24/28- C04B 24/383 | receive two classes, one in C04B 24/005 and one in the polymer group |
Halogen containing polymers obtained by reactions only involving carbon to carbon unsaturated bonds | |
Chlorosilanes |
In this place, the following terms or expressions are used with the meaning indicated:
PERFLUOR compounds | CnF2n-1 (all H exchanged for F) |
PENTA CHLORO PHENOLATE = PENTA CHLORO PHENATE | C6Cl5ONa |
This place covers:
Aldehydes, ketones used as active ingredients for mortars, concrete or artificial stone,
e.g. GLYOXAL OHCCHO;
e.g. FURFURAL;
e.g. FORMALIN (40% aq. sln of formaldehyde);
e.g. ACETYL ACETONE CH3COCH2COCH3;
e.g. DIOXAN
This place does not cover:
Paraformaldehyde |
This place covers:
All Alcohols, phenols, ethers used as active ingredients for mortars, concrete or artificial stone,
e.g. ALCOHOL;
e.g. PHENOL;
e.g. POLYHYDRIC ALCOHOLS i.e. DIOLS: GLYCOL, TRIOLS: GLYCEROL =GLYCERINE = 1,2,3-PROPANETRIOL, ETHYLENE GLYCOL (HOCH2CH2OH),TRIMETHYLENE GLYCOL = 1,3-PROPANEDIOL (HO(CH2)3OH);
e.g. "KRESOL" CRESOL = CH3C6H4OH, o-, m-, p-;
e.g. ETHYLALCOHOL = SPIRITUS;
e.g. SORBIT = SORBITOL C6H14O6 (6 OH groups), SORBITAN =MONOANHYDROSORBIT C6H4O(OH)4;
e.g. FUCUSOL = FUCOSOL = furfurol + methylfurfurol;
e.g. 2,3-DIHYDROXY-1,4-DIOXAN = glyoxal trimer;
e.g. CATECHOL = 1,2-C6H4(OH)2;
e.g. RESORCINOL = 1,3-C6H4(OH)2;
e.g. HYDROQUINONE = 1,4-C6H4(OH)2;
e.g. PHLOROGLUCINOL = 1,3,5-C6H3(OH)3;
e.g. PYROGALLOL = 1,2,3-trihydroxybenzene;
e.g. NAPHTHOL;
e.g. PENTAERYTHRITOL;
e.g. ALKOXIDE = ALCOOLATE i.e. Al(OC2H5)3;
e.g. EUGENOL;
e.g. TERPINEOL, TERPINENOL
In patent documents, the following abbreviations are often used:
PHENOL = "CARBOLSAEURE"
RESORCINOL = "RESORZIN"
This place covers:
All ethers used as active ingredients for mortars, concrete or artificial stone,
e.g. ETHER R-O-R';
e.g. DIETHYLENE GLYCOL HOCH2CH2OCH2CH2OH;
e.g. TRIOXYMETHYLENE = TRIOXANE (CH2O)3 = PARAFORMALDEHYDE;
e.g. epoxide
This place does not cover:
Glycidylether: appears as terminal group of epoxy resin structures |
This place covers:
All fatty alcohols used as active ingredients for mortars, concrete or artificial stone
e.g. ethoxylated fatty alcohols
This place covers:
Carboxylic acids, Salts, anhydrides thereof used as active ingredients for mortars, concrete or artificial stone
e.g. CARBOXYLIC ACIDS R-COOH (carboxy-, -oic acid);
e.g. CARBOXYLIC ACID SALTS R-COOM (M carboxylate, -oate);
e.g. ANHYDRIDES;
e.g. GLYOXYLIC ACID HOOCHO;
e.g. BENZOIC ACID, BENZOATES;
e.g. AGATHIC ACID;
e.g. NAPHTHENIC ACID, NAPHTHENATES;
e.g. RESIN ACID, RESINATES e.g. complex mixture of monocarboxylic acids derived from pine tree extrudate, tree stumps, or tall oil manufacturing. Major components : ABIETIC ACID (=SYLVIC ACID)and PIMARIC ACID;
e.g. KETOCARBOXYLIC ACIDS HO2CCH2CH2COCOOH= a-ketoglutaric acid;
e.g. COAL ACIDS;
e.g. ERYTHORBIC ACID, ERYTHORBATES;
e.g. RESIN SOAPS, SAPONIFIED RESINS
This place does not cover:
ROSIN = mainly resin acids |
In patent documents, the following abbreviations are often used:
"HARZSEIFEN"=RESIN SOAPS, SAPONIFIED RESINS
This place covers:
Esters of carboxylic acids used as active ingredients for mortars, concrete or artificial stone,
e.g. organic carbonates e.g. ETHYLENE CARBONATE;
e.g. ESTERS R-COOR' (R-carboxylate, R- oxycarbonyl, R-oate);
e.g. GLYCERIDES = glycerine esters;
e.g. ACETINS = acetates (ethanoates) of glycerol, MONOACETIN,DIACETIN,TRIACETIN;
e.g. ETHYLENE GLYCOL DIACETATE = ethylidene diacetate = acetaldehydediacetate H3CCO-O-C2H4-O-OCCH3;
e.g. LACTONES = cyclic esters;
e.g. CAPROLACTONE
This place covers:
Carboxylic acids, salts, anhydrides containing hydroxy groups, used as active ingredients for mortars, concreter or artificial stone
e.g. OXYCARBOXYLIC ACIDS;
e.g. LACTIC ACID CH3-CH(OH)-COOH;
e.g. CREAM OF TARTAR = potassium hydrogen tartrate C4H5O6K;
e.g. Na a- and b- GLUCOHEPTONATE;
e.g. CITRIC ACID;
e.g. GLUCONIC ACID;
e.g. GALLIC ACID;
e.g. ALDONIC ACID;
e.g. SACCHARIC ACID = TETRAHYDROXYADIPIC ACID;
e.g. CRESYLIC ACID;
e.g. FLUORESCEIN = RESORCINOLPHTHALEIN = URANINE (Na salt) =DIOXYLFLUORANE C20H12O5;
e.g. BILE ACID (cholic acid)
This place covers:
Fats, fatty oils, ester type waxes, oxidised oils or fats used as active ingredients for mortars, concrete or artificial stone,
e.g. FATS = esters of long chain fatty acids and glycerol;
e.g. FATTY OILS = idem e.g. SOJA OIL, OLIVE OIL,RICINUS OIL = CASTOROIL,LINSEED OIL, PALM OIL;
e.g. ESTER TYPE WAXES = "CIRE" = monoesters of long chain unbranched fatty acids and alcohols e.g. MONTAN WAX, CHIN-SAP WAX, SPERMACETI WAX=WALRAT;
e.g. LIPIDES = esters of long chain carboxylic acids e.g. FATS;
e.g. RAPESEED OIL (COLZA OIL);
e.g. CHINA WOOD OIL;
e.g. ETHOXYLATED FATTY ACID
In patent documents, the following abbreviations are often used:
"RUEBOEL" = RAPESEED OIL ("REPSOEL", "RAPSOEOL","RUEBSENOEL", "RUEBOEL", COLZA OIL);
e.g. "HOLZOEL" = "TUNGOEL" = CHINA WOOD OIL
This place covers:
Higher fatty acids, i.e. having at least seven carbon atoms in an unbroken chain bound to a carboxyl group used as active ingredients for mortars, concrete or artificial stone,
e.g. SOAPS = salts of higher fatty acids;
e.g. UNSATURATED HIGHER FATTY ACIDS : PALMITOLEIC, OLEIC, RICINOLEIC,LIONLEIC, LINOLENIC, ELEOSTEARIC;
e.g. SATURATED HIGHER FATTY ACIDS: CAPROIC, CAPRYLIC, CAPRIC, LAURIC,MYRISTIC, PALMITIC, STEARIC, ARACHIDIC, BEHEMIC, LIGNOCERIC, CEROTIC;
e.g. OLEIN;
e.g. OLEIC ACID
This place does not cover:
Rosin (= mainly resin acids) |
In patent documents, the following abbreviations are often used:
OLEIC ACID = "ÖLSAÜRE" = "OLIENSAÜRE" = "OCTADECENSAÜRE"= "ACIDUMOLEINICUM"
This place covers:
Carbohydrates or derivatives thereof used as active ingredients for mortars, concrete or artificial stone
e.g. historically Cx(H2O)y = polyhydroxylated compounds;
e.g. OLIGOSACCHARIDES : DISACCHARIDES, TRISACCHARIDES, TETRASACCHARIDES;
e.g. MOLASSE, MELASSE, SUCROSE;
e.g. MANNITOL= MANNITE;
e.g. WHEY (lactose is the most important ingredient after water);
e.g. SKIMMED MILK;
e.g. SAPONIN = plant glycosides, forming soapy lathers on shaking with water;
e.g. GLYCOSIDES (GLUCOSIDES) , hydrolyse into sugars and other organic substances;
e.g. LEVULOSE = d-FRUCTOSE= FRUIT SUGAR, DIABETIN, LEVOGLUCOSE,SUCROLEVULOSE;
e.g. ALDOSE, KETOSE
In patent documents, the following abbreviations are often used:
MANNITOL= MANNITE= "MANNAZUCKER";
MANNITOL= MANNITE;
e.g. WHEY = "MOLKE" = "WEI" = "BOTERMELK"
This place covers:
Nitrogen containing compounds used as active ingredients for mortars, concrete or artificial stone
e.g. PENAZOLINE e.g. CA93:136836, CA92:63547, CA93:172622, CA90:141458;
e.g. CHLOROPHYLL;
e.g. AMINE OXIDE R1-R2-R3-N=O
This place does not cover:
Hydrazine |
This place covers:
Amines, polyamines used as active ingredients for mortars, concrete or artificial stone
e.g. and derivatives e.g. salts;
e.g. TM UROTROPINE = HEXAMETHYLENE TETRAMINE;
e.g. ANILINE C6H5-NH2 = PHENYLAMINE = AMINOBENZENE;
e.g. FATTY AMINES
This place covers:
Hydroxy amines and derivatives, e.g. salts used as active ingredients for mortars, concrete or artificial stone;
e.g. NH2 + OH;
e.g. ETHANOLAMINES: MONOETHANOLAMINE, DIETHANOLAMINE, TRIETHANOLAMINE;
e.g. ADRENALINE
This place covers:
Amino-carboxylic acids and derivatives used as active ingredients for mortars, concrete or artificial stone
e.g. NH2 + COOH;
e.g. NITRILOTRIACETIC ACID N(CH2COOH)3;
e.g. GLUTAMIC ACID = a-AMINOGLUTARIC ACID = 2-AMINOPENTANEDIOIC ACID =amino acid derived from hydrolysis of vegetable protein;
e.g. GLYCINE = AMINOACETIC ACID H2N-CH2-COOH;
e.g. EDTA = ETHYLENEDIAMINETETRAACETIC ACID
e.g. betaine
This place covers:
Amides, acid amides and derivatives used as active ingredients for mortars, concrete or artificial stone
e.g. e.g. RCONH2, (RCO)2NH, (RCO)3N;
e.g. FATTY AMIDES e.g. COCOAMIDE
This place does not cover:
Carbamide = urea | |
Isocyanuric acid | |
Lactams = cyclic amides, caprolactam | |
Glycylglycine = diglycine = dipeptide H2N-CH2-CO-NH-CH2-COOH |
This place covers:
Compounds containing one or more carbon-to nitrogen double or triple bonds and derivatives used as active ingredients for mortars, concrete or artificial stone
e.g. CYANATES R-N=C=O;
e.g. CYANAMIDE NH2CN;
e.g. DICYANDIAMIDE H2N-CNH-NH-CN;
e.g. NITRILES RCN;
e.g. IMINES R-CH=NH
This place covers:
Urea and derivatives used as active ingredients for mortars, concrete or artificial stone
e.g. = CARBAMIDE NH2-CO-NH2;
e.g. THIOUREA = THIOCARBAMIDE NH2-SC-NH2
This place does not cover:
N,N-dimethyloldihydroxyethylene urea |
This place covers:
All heterocyclic nitrogen compounds , even if they fall under one of the categories covered by the previous subgroups used as active ingredients for mortars, concrete or artificial stone
e.g. LACTAMS = cyclic amides , CAPROLACTAM;
e.g. CYANURATES, ISOCYANURIC ACID;
e.g. HYDANTOIN = GLYCOLYLUREA = GLYCOLUSIL;
e.g. IMIDAZOLE
e.g. DINITROPENTAMETHYLENE TETRAMINE
This place covers:
PEPTIDES (much smaller number of amino units per molecule than proteins); ENZYMES;
PROTEINS (polymers of a-amino acids) and derivatives thereof used as active ingredients for mortars, concrete or artificial stone
e.g. GELATIN, COLLAGEN, KERATIN;
e.g. CASEIN,
e.g. BLOOD, HEMOGLOBINE;
e.g. GLUTEN;
e.g. ZEIN = corn protein
e.g. BLACK GRAM = polysaccharide-protein
e.g. PROTALBINIC ACID, LYSALBINIC ACID;
e.g. soluble proteins: ALBUMINS, GLOBULINS, GLUTELINS, HISTONES,PROTAMINES;
e.g. GLYCYLGLYCINE = dipeptide
In patent documents, the following abbreviations are often used:
CASEIN,=COLLE D´OS
"KOELNER LEIM
This place covers:
Sulfur-containing compounds used as active ingredients for mortars, concrete or artificial stone,
e.g. SULfONIC COMPOUNDS = -SO3H, sulfonated;
e.g. SULFURIC COMPOUNDS = -OSO3H, sulfated;
e.g. BUNTE SALTS = water soluble organic thiosulphate compounds;
e.g. TURKEY RED OIL = SULFORICINATE;
e.g. ISOPROPYL THIOCYANATE (CH3)2CHSCN
This place does not cover:
Sulfonated polystyrene | |
Sulfonated ketone resins |
In patent documents, the following abbreviations are often used:
TURKEY RED OIL = SULFORICINATE = "TURKISCH ROTÖL"
This place covers:
Lignin sulfonic acid or derivatives thereof used as active ingredients for mortars, concrete or artificial stone,
e.g. LIGNIN SULFONIC ACID = substituted phenylpropane R-CH(SO3H)-CH(OH)-R
e.g. modified lignosulfonate
This place covers:
Sulfonated aromatic compounds used as active ingredients for mortars, concrete or artificial stone
e.g. SULFANOL;
e.g. FOKS = fuel oil cracking sulfonated = salt of sulfonated andoxidised product resulting from the reaction of SO3 with fuel oils from steam cracking of oil products
This place covers:
Condensation or polymerisation products thereof of sulfonated aromatic compounds used as active ingredients for mortars, concrete or artificial stone
Condensation or polymerisation products containing aromatic nucleus;
e.g. sulphonated polystyrene;
e.g. sulphonated amino-s-triazine
This place covers:
Sulfonated melamine-formaldehyde condensation products used as active ingredients for mortars, concrete or artificial stone,
e.g. sulphonated melamine resins;
e.g. triazine-HCHO condensation product
This place covers:
Macromolecular compounds used as active ingredients for mortars, concrete or artificial stone,
e.g. LIGNIN;
e.g. HUMIC ACID;
e.g. LIGNATE
This place does not cover:
Peptides, proteins, derivatives thereof | |
Sulfur-containing compounds |
Attention is drawn to the following places, which may be of interest for search:
petroleum resins | C04B 24/26 ( C04B 24/24 still to be cleaned) |
This place covers:
Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone,
e.g. PETROLEUM RESIN (C4/C6 alkene fraction, C8/C10 aromatic fraction or dicyclopentadiene fraction);
e.g. VERSATATES;
e.g. LATEX RESIN;
e.g. COUMARONE RESIN = CUMAR RESIN or GUM = PARACOUMARONE RESIN =BENZOFURAN = synthetic rein from coal tar destillates;
e.g. DIALLYL POLYMERS
Resins as such equivalent to those are classified in C08F
This place does not cover:
Phosphorous-containing polymers used as active ingredients for mortars, concrete or artificial stone, |
This place covers:
Polyalkenes obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone,
e.g. POLYTERPENES i.e. NATURAL RUBBER
This place covers:
Coumarone polymers used as active ingredients for mortars, concrete or artificial stone, e.g. INDENE-CUMARONE RESIN
This place covers:
Polyvinylalcohols, polyvinylacetates obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone,
e.g. ETHYLENE VINYLACETATE;
e.g. VINYLLAURATE-VINYLACETATE;
e.g. POLYVINYL PROPIONATE
This place covers:
Polyacrylates, polymethyacrylates obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone,
e.g. COPOLYMERS of POLY(METH)ACRYLATES with other VINYL MONOMERS (e.g. styrene, vinylacetate);
e.g. mixture of acrylic monomers
Attention is drawn to the following places, which may be of interest for search:
copolymers having three different monomers |
This place covers:
Nitrogen containing polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. ACRYLNITRIL-STYROL COPOLYMER;
e.g. ACRYLNITRIL-BUTADIENE COPOLYMER;
e.g. Polyvinylamide
This place covers:
Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds of ethylenically unsaturated dicarboxylic acid polymers and used as active ingredients for mortars, concrete or artificial stone
e.g. STYROL-MALEIC ACID COPOLYMER;
e.g. VINYLACETATE-MALEIC ACID COPOLYMER;
e.g. ISOBUTYLENE-MALEIC ACID COPOLYMER;
e.g. POLYALKYLENE SUCCENIC ANHYDRIDE;
e.g. VINYL ACETATE-DIBUTYL MALEATE COPOLYMER
This place covers:
Polystyrenes compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. STYRENE-BUTADIENE;
e.g. STYRENE-BUTADIENE-PVA
This place does not cover:
Styrene-acryl copolymers | |
Styrene-maleic anhydride copolymers |
C04B 24/2641 takes precedence.
This place covers:
Halogen containing polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. PTFE (RN 9002-84-0);
e.g. CHLOROPRENE
Attention is drawn to the following places, which may be of interest for search:
Polyacrylates |
C04B 24/2682 takes precedence
This place covers:
Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. POLYETHYLENE TEREPHTHTALATE;
e.g. POLYIMIDES; POLYESTER IMIDE; POLYAMIDIMINE;
e.g. POLYTHIOCARBONATE;
e.g. POLYSULFONES; POLYSULPHONAMIDE;
e.g. POLYTHIOETHER;
e.g. POLYSULPHIDE;
e.g.. ETHOXYLINE RESIN;
e.g. FURAN RESIN (deriv. from furfuryl alcohol);
e.g. POLYALKYLENE POLYAMINES;
e.g. POLYAMINES; POLYESTERAMIDES
e.g. POLYETHYLENE IMINE (CH2CH2NH)x
resins as such equivalent to those classified in C08G
This place does not cover:
Phosphorous-containing polymers used as active ingredients for mortars, concrete or artificial stone, |
This place covers:
Polyepoxides compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. a-EPICHLORHYDRINE-1 CHLORO 2,3 EPOXY PROPANE
This place covers:
Polyesters compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
e.g. ALKYD resins;
e.g. polylactic acid, polylactide
This place covers:
The use for instance of phenol resin as binder, novolac resin, resol resin.
Phenol-formaldehyde resins, as a group, are formed by a step-growth polymerization reaction that can be either acid- or base-catalysed. Since formaldehyde exists predominantly in solution as a dynamic equilibrium of methylene glycol oligomers, the concentration of the reactive form of formaldehyde depends on temperature and pH.
Phenol is reactive towards formaldehyde at the ortho and para sites (sites 2, 4 and 6) allowing up to 3 units of formaldehyde to attach to the ring. The initial reaction in all cases involves the formation of a hydroxymethyl phenol:
HOC6H5 + CH2O → HOC6H4CH2OH
Novolacs (originally Novolak, the name given by Leo Baekeland), are phenol-formaldehyde resins made where the molar ratio of formaldehyde to phenol of less than one. The polymerization is brought to completion using acid-catalysis. The phenol units are mainly linked by methylene groups
Base-catalysed phenol-formaldehyde resins are made with a formaldehyde to phenol ratio of greater than one (usually around 1.5). These resins are called resols. Phenol, formaldehyde, water and catalyst are mixed in the desired amount, depending on the resin to be formed, and are then heated

Phenol - the simplest of the phenols
Formaldehyde
This place does not cover:
Using phenol resin for joining ceramic with ceramic | |
Using phenol resin for joining ceramic with metal | |
Using phenol resin for joining ceramic with glass | |
The use of phenol-formaldehyde condensation products in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Condensation polymers of aldehydes or ketones added as active ingredient to cement, concrete, mortar or artificial stone: phenol-formaldehyde condensation polymers | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: condensation polymers of aldehydes and ketones: with phenols |
This place covers:
Melamine resin or melamine formaldehyde (also shortened to melamine) is a hard, thermosetting plastic material made from melamine and formaldehyde by polymerization.
This place does not cover:
The use of condensation polymers of aldehydes or ketones in coatings of ceramic substrates: melamine-formaldehyde condensation products |
Attention is drawn to the following places, which may be of interest for search:
Condensation polymers of aldehydes or ketones added as active ingredient to cement, concrete, mortar or artificial stone: melamine-formaldehyde condensation polymers |
This place covers:
Urea-formaldehyde, also known as urea-methanal, named so for its common synthesis pathway and overall structure, [1] is a non-transparent thermosetting resin or plastic, made from urea and formaldehyde heated in the presence of a mild base such as ammonia or pyridine
This place does not cover:
The use of condensation polymers of aldehydes or ketones in coatings of ceramic substrates: urea-formaldehyde condensation products |
Attention is drawn to the following places, which may be of interest for search:
Condensation polymers of aldehydes or ketones added as active ingredient to cement, concrete, mortar or artificial stone: urea-formaldehyde condensation polymers |
This place covers:
Polyethers compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds and used as active ingredients for mortars, concrete or artificial stone
also polyethylene glycol esters
e.g. POLYETHYLENE OXIDE;
e.g. POLYETHYLENEPOLYAMINE, POLYETHYLENE IMINE;
e.g. POLYETHYLENE GLYCOL,
e.g. POLYACETAL GLYCOL;
e.g. ALKYLPHENOLPOLYGLYCOL ETHER;
e.g. PARAFORMALDEHYDE;
e.g. ALKYLAMINO POLY(OXYMETHYLENE)GLYCOL
This place covers:
Natural resin used as active ingredients for mortars, concrete or artificial stone
e.g. natural (animal, vegetable) waxes: CANDELLILA WAX, CARNAUBA WAX,KANNUTILLA WAX;
e.g. TURPENTINE OIL (PINENES);
e.g. COLOPHONIUM = COLOPHANE;
e.g. CREOSOTE;
e.g. ROSIN is obtained from various species of pine = ABIETIC ACID;
e.g. SHELLAC
e.g. LACQUER = shellac dissolved in alcohol, coloured with saffron or dragon's blood;
e.g. EUCALIPTUS OIL; TALL OIL;
e.g. GUTTA-PERCHA;
e.g. v g tale;
e.g. VINSOL
This place does not cover:
Phosphorous-containing polymers used as active ingredients for mortars, concrete or artificial stone, | |
Polysaccharides |
In patent documents, the following abbreviations are often used:
CREOSOTE ="HOLZTEER"
SHELLAC = "SCHELLACK" = "SCHOLLENLACK" = "BLATTLACK" = "GUMMILACK"= "LACCA IN TABULIS";
"MUCILAGE";
"GOUDRON"
This place covers:
Bituminous materials used as active ingredients for mortars, concrete or artificial stone
e.g. ASPHALT, ASPHALTITE;
e.g. KEROSINE, PARAFFIN;
e.g. "PARAFFIN OIL, VASELINE OIL;
e.g. CARBOLINEUM;
e.g. PETROLATUM;
e.g. mineral, paraffin waxes
This place does not cover:
Phosphorous-containing polymers used as active ingredients for mortars, concrete or artificial stone, |
In patent documents, the following abbreviations are often used:
ASPHALT= "KOOLTEER"= ASPHALTITE;
PARAFINE= PARAFFIN OIL
"CERESIN" = "ZERESIN" = "HART PARAFIN" = "GEREINIGTES ERDWACHS" ="CERA MINERALIS ALBA";
"BRAI de HOUILLE"
This place covers:
Polysaccharides or derivatives thereof used as active ingredients for mortars, concrete or artificial stone. Examples include:
- ARABIC GUM, GUM ACACIA;
- GALACTO-MANNAN, GUAR GUM, GLUCO-MANNAN;
- AGAR AGAR;
- TANNIN = TANNIC ACID;
- GUM GHATTI, LOCUST BEAN GUM = CAROB BEAN GUM;
- CAROB FRUIT;
- INULIN;
- WELAN GUM;
- FRENCH CEMENT = gum arabic + powdered starch;
- ALGINATES, GLYCOGEN, PECTINE; CHITOSAN; DEXTRAN;
- XANTHOMANOS GUM = XANTHAN GUM;
- SCLEROGLUCAN, CURDLAN, PULLULAN;
- STARCH;
- DEXTRIN
This place does not cover:
Phosphorous-containing polymers used as active ingredients for mortars, concrete or artificial stone, |
Attention is drawn to the following places, which may be of interest for search:
Polyethers |
In patent documents, the following words/expressions are often used as synonyms:
- Johannesbrood, carob fruit
- Inulin, polyvructhensuiker
This place covers:
Cellulose or derivatives thereof used as active ingredients for mortars, concrete or artificial stone. Examples include:
- CELLULOSE; CARBOXY METHYL CELLULOSE CMC;
- HYDROXY ETHYL CELLULOSE HEC;
- METHYL HYDROXY ETHYL CELLULOSE MHEC;
- HEMICELLULOSE = KARAYA GUM = PENTASANE = GALACTON-GELOSE;
- REGENERATED CELLULOSE;
- CELLULOSE ETHERS;
- VISCOSE; TRAGANTH
In patent documents, the following abbreviations are often used
STAERKE"; DEXTRIN = starch derivative
This place covers:
Any organo-inorganic complexes used as active ingredients for mortars, concrete or artificial stone
e.g. metal carbonyls
This place covers:
Organo-silicon compounds used as active ingredients for mortars, concrete or artificial stone
e.g. POLYSILANES -Si-Si-Si-Si-;
e.g. SILICONES -Si-O-Si-O-Si-
This place covers:
Organic or polymeric concretes or mortars i.e. compositions bearing an organic or polymeric binder.
This group covers organic or polymeric concrete compositions comprising at least 50% inorganic filler.
e.g. organic (e.g. polymer) P or B compounds as binder;
e.g. waterproof lacquer, benzol, acetone, aluminium powder and camphor e.g. LIGNIN derivatives;
e.g. MONTAN WAX
This place does not cover:
A combination of an organic and inorganic binder | C- set : (C04B 28/00; C04B 24/00) |
Polymer modified concrete (PMC) or polymer concrete (PC) or polymer mortar (PM ) | C-set: (C04B 28/00; C04B 24/00) |
Mechanical aspects, moulding polymer or resin concrete |
Attention is drawn to the following places, which may be of interest for search:
Oil well cements containing organic binders | are classified in C04B 26/00 according to the composition and receive also a C09K 8/44 class |
Organic or polymeric compositions with filler content less than 50% | |
Polyester compositions | |
Bituminous compositions | |
Grouting with organic compounds | E02D20/02 |
Classification is made according to the binder used, applying last place rule (LPR). Fillers and active ingredients are classified using the C-set symbols chosen from C04B 14/00, C04B 18/00, C04B 22/00 or C04B 24/00. If one of these ingredients is (or suspected to be) new or unusual or special details describing this ingredient are given, classification is also made for this ingredient.
When a list of possible organic binders is given, classification is made to the more general entry e.g. C04B 26/04 or C04B 26/10 or even C04B 26/02. If specific examples are given of one binder out of a list, a second more specific class relating to the exemplified binder is given.
This place covers:
Compositions of mortars, concrete or artificial stone containing oil-based binders
e.g. DRYING OILS, linseed oil
This place does not cover:
Attention P compounds | |
Lignin derivatives | |
Montan wax | |
Petroleum resins |
This place covers:
Compositions of mortars, concrete or artificial stone containing proteins or derivatives thereof
e.g. MILK
This place covers:
Compositions of mortars, concrete or artificial stone containing macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds
e.g. PETROLEUM RESINS
This place covers:
Compositions of mortars, concrete or artificial stone containing macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds being polyalkylenes
e.g. polybutadiene
This place covers:
Compositions of mortars, concrete or artificial stone containing macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds being acrylates
e.g. thermoplastic polymethylmethacrylate type polymer
This place does not cover:
Grouting with organic components |
This place covers:
Compositions of mortars, concrete or artificial stone containing phenol-formaldehyde condensation polymers
e.g. CRESOL -FORMALDEHYDE
This place covers:
Compositions of mortars, concrete or artificial stone containing polyepoxides
e.g. POLYETHYLENE OXIDE
This place covers:
Compositions of mortars, concrete or artificial stone containing polyurethanes
e.g. castor oil, polymerises with isocyanates
This place does not cover:
polyester compositions | C08L 67/00 + F (inorganic filler); C08L 67/02 (saturated); C08L 67/06 (unsaturated) |
This place covers:
Compositions of mortars, concrete or artificial stone containing natural resins as organic binders
e.g. ARAUCARIA RESIN
This place covers:
Compositions of mortars, concrete or artificial stone containing bituminous materials as organic binders
e.g. PARAFFIN;
e.g. tar + asphalt + sulphur
Construction of, or surfaces for roads E01C
This place does not cover:
Compositions of bituminous materials |
In patent documents, the following abbreviations are often used
"HOLZZEMENT" = tar + asphalt + sulphur
This place covers:
Compositions of mortars, concrete or artificial stone containing cellulose or derivatives thereof as organic binders
e.g. "ZELLIN"
This place does not cover:
Compositions of mortars, concrete or artificial stone containing cellulosic waste liquor as organic binder |
In patent documents, the following abbreviations are often used
"ZELLIN"
This place covers:
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder
e.g. (water soluble) fluorosilicate as binder
This place does not cover:
Dental cements | |
Surgical cements |
Attention is drawn to the following places, which may be of interest for search:
Oil well cements containing inorganic binders | |
According to the composition and receive also |
Classification is made according to the binder used, applying last place rule (LPR). Fillers and active ingredients are classified using the C-set symbols chosen from C04B 14/00, C04B 16/00, C04B 18/00, C04B 22/00 or C04B 24/00. If one of these ingredients is (or suspected to be) new or unusual or special details describing this ingredient are given, classification is also made for this ingredient.
When more than one inorganic binders are used, classification is made in C04B 28/00 according to LPR and the second or third binder are indicated with entries chosen from C04B 7/00 or C04B 11/00 (or if one of these binders can be seen as an active ingredient e.g. lime, from C04B 22/00).
This place covers:
Compositions of mortars, concrete or artificial stone, containing unburned clay as inorganic binders
e.g. clay + NAOH mixtures as binder;
Attention is drawn to the following places, which may be of interest for search:
Polymer binder - clay mixtures used in well cementing |
In this place, the following terms or expressions are used with the meaning indicated:
TORCHIS" = COB = DAUB | clay + straw |
This place covers:
Compositions of mortars, concrete or artificial stone, containing gelatineous or gel forming binders
e.g. Al(OH)3;
e.g. Al2(OH)xXy
This place covers:
Compositions of mortars, concrete or artificial stone, containing mineral polymers as inorganic binders
e.g. TECTOALUMINOSILICATE;
e.g. POLY(SIALATE) (-Si-O-Al-O-) (PS);
e.g. POLY(SIALATE-SILOXO) (-Si-O-Al-O-Si-O-) (PSS);
e.g. POLY(SIALATE-DISILOXO) (-SI-O-Al-O-Si-O-Si-O-) (PSDS)
Attention is drawn to the following places, which may be of interest for search:
Alkali-activated cements |
This place does not cover:
Alkali-activated combustion residues | |
Fly ash as filler | |
Mixtures of the lime-pozzuolane type |
when the alkali activated waste results in a polymeric - Davidovits type - cement, additional classification in C04B 28/006 should be given
In this place, the following terms or expressions are used with the meaning indicated:
Portland cement | Hydraulic cement produced by pulverizing clinkers consisting essentially of hydraulic calcium silicates, usually containing one or more of the forms of calcium sulfate as an inter ground addition |
This place does not cover:
Monolithic refractories or refractory mortars |
This place covers:
Compositions of mortars, concrete or artificial stone, containing calcium aluminosulfate cements as inorganic binders
e.g. aluminous cement + portland cement + gypsum;
This place covers:
Compositions of mortars, concrete or artificial stone, containing slag cements as inorganic binders
e.g. slag + water --> hydraulic reaction with formation of GEHLENITE or ETTRINGITE according to the base present
Attention is drawn to the following places, which may be of interest for search:
Water glass as activator |
In patent documents, the following abbreviations are often used:
"Laitiers granules
This place does not cover:
Lime paints / varnishes |
In patent documents, the following words/expressions are often used as synonyms:
- "slaked lime", "calcium hydroxide" and "hydrated lime"
This place covers:
Compositions of mortars, concrete or artificial stone, based on magnesium oxide or magnesium carbonate binders
In this place, the following terms or expressions are used with the meaning indicated:
Hydraulic lime | a lime mainly consisting of calcium silicates, calcium aluminates and calcium hydroxide. Produced by burning argilaceous limestones |
This place does not cover:
Dentistry preparations based on gypsum | |
Gypsum bandages | |
Gypsum-paper boards |
In this place, the following terms or expressions are used with the meaning indicated:
VERGOLDER-GUSSMASSE | gypsum + chalk + glue |
This place covers:
Compositions of mortars, concrete or artificial stone, containing dehydrated gypsum before the final hardening step, blocks of natural gypsum as inorganic binders
- the "silica" used has to be indicated (possible entries C04B 14/06+, C04B 18/08 etc. ) but the lime is not classified separately, as it is considered inherent to the composition.
Rules of classification:
- Ca silicate --> final product : C04B 28/188,...; - Ca silicate + CaO + "SiO2" --> final product : C04B 28/188 , "C04B 14/00:06", ... ;
- CaO + "SiO2" --> Ca silicate --> final product : C04B 28/186,"C04B 14/00:06", ... ;
- CaO + "SiO2" --> Ca silicate + CaO + "SiO2" --> final product: (C04B 28/186,"C04B 14/00:06",..)(C04B 28/186, "C04B 14/00:06", C04B 28/188);
- C04B 28/188 as a symbol means : Ca silicate as an intermediate product, mixed with a second CaO-"SiO2" mixture.
see rules in C04B 28/18
See rules in C04B 28/18
This place does not cover:
Colloidal silica as filler | |
(water soluble) fluorosilicates as binder | |
Reaction mixtures resulting in mineral polymers | |
Fibres + colloidal silica | |
Polymeric reaction products of alkali metal silicates with isocyanates |
This place does not cover:
Reaction mixtures resulting in mineral polymers | |
Foundry moulds based on alkali metal silicates | |
Alkali metal silicates as such | |
Paints based on alkali metal silicates | |
Adhesives based on alkali metal silicates | |
For soil stabilisation | |
Use of waterglass in road making |
This place does not cover:
Dental cements | |
Surgical ionomer cements |
This place does not cover:
Magnesium oxide cements |
This place does not cover:
Dental cements |
Preparations for dental purposes | |
Surgical cements | |
Materials for prostheses |
This place does not cover:
Coating of metals in general |
- since 1/4/92 classification is done according to the note following C04B 28/34;
- ammonium compounds are considered to be alkali metal compounds;
- phosphate compositions for coating metallic surfaces (for passivating purposes) are classified in C04B 28/34 and subgroups and receive C04B 2111/00525 as symbol
- starting mixture of oxide + phosphate C04B 28/34;
- starting mixture of acid + oxide C04B 28/342;
- starting mixture containing phosphate only C04B 28/344;
- starting mixture of acid + phosphate C04B 28/346;
- starting mixture of acid + oxide + phosphate C04B 28/348
This place does not cover:
Insulation for cavity walls |
In this place, the following terms or expressions are used with the meaning indicated:
Artificial stone, i.e. cast stone | Synthetic stone compounds |
This place covers:
- in principle as defined in the title- no binder;
- but also e.g. fibers held together with a minor amount of binder can receive C04B 30/02 as additional class (in which case the binder is indexed from C04B 7/00 if inorganic, or C04B 24/00 if organic);
- also fibers held together by minor amounts of e.g. refractory oxides- these oxides are then indexed as filler from C04B 14/00
This place does not cover:
Cast stone from molten slag | |
Artificial stone obtained by melting the polymeric ingredient of the composition | |
Compositions of mortars, concrete or artificial stone containing sulphur, sulphides or selenium, as inorganic binder | |
Glass compositions containing a non-glass component |
This place covers:
This group is only used as symbol in the C-set to indicate the presence of reinforcements (in the sense of E04C 5/00). The group itself does not contain any documents
This place does not cover:
Cathodic protection of reinforced concrete | |
Reinforcing elements for concrete |
This place covers:
All ceramic products based on clay materials, the processing of clay materials preparatory to the making of clay products, the following shaping methods for clay materials: slip-casting (C04B 33/28) and dry-pressing (C04B 33/20).
working by grinding or polishing B24
Processes for the shaping of clay materials, except for slip-casting (C04B 33/28) and dry-pressing (C04B 33/20) B28B
Preparing clay; producing mixtures containing clay B28C
Working stone or stone-like materials, e.g. brick, concrete or glass , not provided for elsewhere; machines, devices, tools therefore B28D
This place does not cover:
Granular clay used as filler in cement, concrete or artificial stone | |
Heat treating clay to expand it for use as filler in cement, concrete or artificial stone | |
Clay used as active ingredient in cement, concrete or artificial stone | |
Unburned clays used as filler in cement, concrete or artificial stone | |
Ceramic materials based on silicates other than clays | |
Creating porosity in a ceramic, cement, concrete, mortar or artificial stone by using expanding clay | |
Coating or impregnating a ceramic substrate with clay | |
Aspects relating to ceramic starting mixtures or sintered ceramic products | C04B 2235/00 and subgroups |
Aspects relating to ceramic laminates or to joining of ceramic articles with other articles by heating | C04B 2237/00 and subgroups |
Clays used in catalysts | |
Clays used in molecular sieves | |
Clay used as binding agent in refractory moulds | |
Clay moulds for slip-casting metals | |
Devitrified glass-ceramics | C03C 10/00 and subgroups |
Use of clays as compounding ingredient for polymers | |
Treatment of clay materials to enhance pigmenting or filling properties for non-clay and non-ceramic products (usually for polymer products) | |
Interference pigments characterized by the core material, the core consisting of glass or silicate material like mica or clays, e.g. kaolin | |
Coverings or linings, e.g. for walls or ceilings with an outer layer of ceramics or clay | |
Rigid pipes of glass or ceramics, e.g. clay, clay tile, porcelain |
Attention is drawn to the following places, which may be of interest for search:
Monolithic refractories or refractory mortars | |
Joining of a ceramic or clay layer to another layer | C04B 37/00 and subclasses |
Porous ceramic products | C04B 38/00 and subclasses |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. water glass (Na2SiO3) | C04B 2235/3427 and subgroups |
Clays as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bentonites/smectites such as montmorillonite, kaolines such as halloysite, illite, talc, sepiolite and attapulgite, vermiculite | |
Clays added to foodstuffs | |
Clay used in cosmetics | |
Clay used in medicines | |
Clays used for solid sorbents or as filter aid | |
Catalysts comprising clays or other mineral silicates | |
Proportioning the ingredients for mixing clay or cement with other substances | |
Resin-bonded materials containing containing mineral aggregates, e.g. sand, clay or the like | |
Laminated products composed mainly of ceramics, e.g. clay ceramics | |
Using clay fillers in resin laminates | |
Clay used as pigment in printing ink | |
Clay used in the coating of printing paper | |
Modelling clay for creating decorative effects | |
Making or composition of clay compounds (powders) | |
Preparation of acyclic or carbocyclic hydrocarbons, using clay catalysts | C07C 2521/16, C07C 2529/04 and subgroups |
Clay used in aqueous well drilling compositions | C09K 8/04 and subclasses |
Chemical nature of materials in mouldable or extrudable form for sealing or packing joints or covers, clays | C09K 2200/0252 and subgroups |
Clay used in lubricant compositions | C10M 113/10 , C10M 125/30, C10M 2201/103 and subgroups |
Clays used in detergent compositions | |
Clay used as carrier in detergent compositions | |
Clay used in pulp compositions | |
Clay pigments used for coating paper | |
Clays used in foundations, excavations, embankments, underground or underwater structure | E02D 2300/0037 and subgroups |
Machines for obtaining or the removal of materials in open-pit mines, for quarrying stone, sand, gravel, or clay | |
Rigid pipes, of glass or ceramics, e.g. clay, clay tile, porcelain | |
Apparatus for preheating charges or arrangements for preheating charges: drying of green clay prior to baking | |
Target discs characterised by their material, structure or surface, e.g. clay pigeon targets characterised by their material | |
Clay-pigeon targets; clay-disc targets | F41J 9/16 and subclasses |
Treating radioactively contaminated material by fixation in stable solid media in an inorganic matrix, e.g. clays, zeolites |
The indexing scheme C04B 2235/00-C04B 2235/9692 is used in C04B 33/00, with the exception of a few symbols that overlap with classes in C04B 33/00. The following symbols are not used in C04B 33/00:
C04B 2235/349: clay additives
C04B 2235/5472: ceramic or refractory mixtures of materials with different sizes
C04B 2235/6027: slip-casting of ceramic or refractory mixtures
C04B 2235/604: pressing at non-sintering temperatures of ceramic or refractory mixtures
C04B 2235/606: drying of green ceramic or refractory bodies
C04B 2235/9661: colouring of ceramic or refractory materials
If the phase composition of the sintered clay material is specified, C04B 35/14, C04B 35/16 or one of its subgroups might be given to indicate the main phase of the sintered clay product.
The processing classes C04B 35/624-C04B 35/62695 are also used in the clay field, just as powder and fiber coating classes from C04B 35/628. The inorganic binder classes C04B 35/6306-C04B 35/6316 and the organic binder classes C04B 35/6325-C04B 35/638 are also used in the clay field.
In this place, the following terms or expressions are used with the meaning indicated:
Clay | Clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays, but there is some overlap in both particle size and other physical properties, and there are many naturally occurring deposits which include silts and also clay. The distinction between silt and clay varies by discipline. Geologists and soil scientists usually consider the separation to occur at a particle size of 2 µm (clays being finer than silts), sedimentologists often use 4-5 μm, and colloid chemists use 1 μm. Geotechnical engineers distinguish between silts and clays based on the plasticity properties of the soil, as measured by the soils' Atterberg Limits. ISO 14688 grades clay particles as being smaller than 2 μm and silts larger. Clay minerals are hydrous aluminum phyllosilicates, sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations. Clays have structures similar to the micas and therefore form flat hexagonal sheets Clays are commonly referred to as 1:1 or 2:1. Clays are fundamentally built of tetrahedral sheets and octahedral sheets, as described in the structure section below. A 1:1 clay would consist of one tetrahedral sheet and one octahedral sheet, and examples would be kaolinite and serpentine. A 2:1 clay consists of an octahedral sheet sandwiched between two tetrahedral sheets, and examples are illite, smectite, attapulgite, and chlorite (although chlorite has an external octahedral sheet often referred to as "brucite"). Clay minerals include the following groups:Kaolin group which includes the minerals kaolinite, dickite, halloysite, and nacrite (polymorphs of Al2Si2O5(OH)4). Some sources include the kaolinite-serpentine group due to structural similarities. Smectite group which includes dioctahedral smectites such as montmorillonite and nontronite and trioctahedral smectites for example saponite. Illite group which includes the clay-micas. Illite is the only common mineral. Chlorite group includes a wide variety of similar minerals with considerable chemical variation. Other 2:1 clay types exist such as sepiolite or attapulgite, clays with long water channels internal to their structure. Clay mineral group Halloysite – Al2Si2O5(OH)4 Kaolinite – Al2Si2O5(OH)4 Illite – (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2 O)] Montmorillonite – (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2 O Vermiculite – (MgFe,Al)3(Al,Si)4O10(OH)2·4H2 O Talc – Mg3Si4O10(OH)2 Palygorskite – (Mg,Al)2Si4O10(OH)·4(H2 O) Pyrophyllite – Al2Si4O10(OH)2 |
clay means any clay or ceramic material |
This place covers:
The powders are treated either as a powder or in shaped form
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: expanding clay, perlite, vermiculite or like granular materials | C04B 20/06 and subgroups |
Apparatus or methods for mixing clay with other substances | B28C 3/00 and subgroups |
Controlling the operation of apparatus for producing mixtures of clay, ceramic or cement with other substances; supplying or proportioning the ingredients for mixing clay or cement with other substances; discharging the mixture | B28C 7/00 and subgroups |
This place covers:
The document mentions that the inorganic starting materials deliberately have different mesh sizes, such as a fraction of < 400 mesh, a fraction of 200-400 mesh and a fraction > 200 mesh, or the document mentions different particle sizes, e.g. two fractions, one with sizes below and one with size above 0,1 mm. A certain constituent is added with two different particle sizes, by adding for instance kaolin with a size of 1 micron and kaolin with a size of 10 micron. A powder is added that contains one fraction, but this fraction has a bimodal particle size distribution.
This place does not cover:
Clay mixtures in which the organic additives have different size fractions |
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: characterised by the grain distribution: fillers with bimodal grain size distribution | |
Ceramic or refractory mixtures of materials with different sizes | |
Separation of particles of different sizes through sedimentation | B01D 21/00 and subgroups |
Inorganic particles per se with a bimodal particle size distribution |
This class is not complete. The years 1981-2005 are missing. Some documents with clay-mixtures of materials with different sizes might have the symbol C04B 2235/5472.
This place covers:
Mixtures contain clay or kaolin additives
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with clay/kaolin | C04B 41/5037 and subgroups |
Clays as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bentonites/smectites such as montmorillonite, kaolines such as halloysite, illite, talc, sepiolite and attapulgite, vermiculite |
This place covers:
The lime in or for the clay material is reacted to form calcium alumino-silicate phases
Attention is drawn to the following places, which may be of interest for search:
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime |
In this place, the following terms or expressions are used with the meaning indicated:
Lime | calcium oxide or calcium hydroxide |
This place covers:
Any method that prevents the efflorescence (or salting out) of salts present in the clay mixture or present in the starting materials to be used for forming a clay mixture
Attention is drawn to the following places, which may be of interest for search:
Metal salts chosen for the nature of the anions as starting material for making ceramics, e.g. phosphides, hydrides, acetylacetonate, hydroxides, or present as secondary phase in the sintered ceramic | C04B 2235/44 and subgroups |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
Efflorescence | the loss of water (or a solvent) of crystallization from a hydrated or solvated salt to the atmosphere on exposure to air. |
This place covers:
Removing lime or iron salts from the clay mixture
Attention is drawn to the following places, which may be of interest for search:
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime | |
Iron oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. hematite (Fe2O3) or magnetite (Fe3O4) | C04B 2235/3272 and subgroup |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Separation of particles of different sizes through sedimentation | B01D 21/00 and subgroups |
This place covers:
Preparing mixtures for making clay materials, e.g. adding waste glass to a clay mixture.
This place does not cover:
Reinforced clay wares | |
Reinforced ceramics | C04B 35/71 and subgroups |
Apparatus or methods for producing or N:processing clay suspensions, e.g. slip | B28C 1/02 and subgroups |
Apparatus or methods for processing clay-containing substances in non-fluid condition | B28C 1/10 and subgroups |
Supplying or proportioning the ingredients | B28C 7/04 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Glass starting materials for making ceramics, e.g. silica glass | C04B 2235/36 and subgroup |
Pigments for ceramics |
If waste glass is used, the symbol C04B 2235/36 is added as well.
This place covers:
All organic additives added to form the product
This place does not cover:
Organic additives added to ceramic or refractory mixtures | C04B 35/632 and subclasses |
Organic additives that are added to the clay material to create porosity after a heat treatment | C04B 38/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Alkoxides as starting material for making ceramics, e.g. methoxide, tert-butoxide | |
Organic acids as starting material for making ceramics, e.g., EDTA, citrate, acetate, oxalate | |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
Organic fibers used as starting material for making ceramics |
If a certain polymer is specified as additive, one of the subclasses of C04B 35/634 can be given, to indicate the specific polymer. C04B 35/6325, C04B 35/636, C04B 35/6365 and C04B 35/638 can be used as well. A certain amount of documents of clay mixtures with organic additives might have received C04B 35/632 or one of the subclasses, such as one of the polymer additive classes (C04B 35/634 and subclasses) without having received the class C04B 33/1305. If the organic additive is a binder, C04B 33/1315 is given as well.
This place covers:
All inorganic additives added to form the product
This place does not cover:
Inorganic additives to ceramic or refractory mixtures | C04B 35/6303 and subclasses |
Inorganic additives that are added to the clay material to create porosity after a heat treatment | C04B 38/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Metal oxides, mixed metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/32 and subgroups |
Non-metal oxides, mixed non-metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/34 and subgroups |
Glass starting materials for making ceramics, e.g. silica glass | C04B 2235/36 and subgroup |
Non-oxides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/38 and subgroups |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Non metallic elements as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. sulphur, phosphor, selenium or tellurium | C04B 2235/42 and subgroups |
If a certain inorganic is specified as additive, C04B 35/6306 or one of its subclasses, or C04B 35/6316 can, if appropriate be given. A certain amount of documents of clay mixtures with inorganic additives might have received C04B 35/6303 or one of the subclasses without having received the class C04B 33/131. The class has not been used in the years 1983-2005. If the inorganic additive is a binder, C04B 33/1315 is given as well. If none of the subclasses of C04B 35/6303 is appropriate, symbol from the range C04B 2235/32-C04B 2235/549 can be given.
This place covers:
Binders for clay mixtures that are neither clay materials themselves nor ceramic materials as classified in C04B 35/01-C04B 35/597.
Attention is drawn to the following places, which may be of interest for search:
Binders for ceramic products | |
Binders for refractory moulds | B22C 1/16 and subgroups |
The binder is normally also classified in either C04B 33/1305 or C04B 33/131. If the binder is one of the materials classified in C04B 35/6306 (and subclasses), C04B 35/6316, C04B 35/634 (and subclasses) or C04B 35/636 (and subclasses), the respective additive class from C04B 35/00 is given as well. If none of the subclasses of C04B 35/6303 or C04B 35/632 is appropriate, symbol from the range C04B 2235/32-C04B 2235/549 can be given.
This place covers:
The use of waste materials to make clay objects, not covered by any of the subclasses, such as silica fume, except for waste glass.
Disposal of solid waste B09B
Removing ash, clinker, or slag from combustion chamber F21J
This place does not cover:
The addition of waste glass to clay materials | |
Adding lean materials, e.g. grog quartz | |
The addition of waste materials to ceramic or refractory mixtures | C04B 35/62204 and subgroups |
Waste materials that are added to the clay material to create porosity after a heat treatment |
Attention is drawn to the following places, which may be of interest for search:
Cements containing slag | C04B 7/14 and subgroups |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone | C04B 18/04 and subgroups |
Use of agglomerated or waste materials or refuse as fillers for mortars, concrete or artificial stone, or treatment of agglomerated or waste materials or refuse, specially adapted to enhance their filling properties in mortars, concrete or artificial stone: waste material from metallurgical processes being silica fume | C04B 18/146 and subgroups |
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: waste inorganic materials | |
Coating or impregnating of mortars, concrete, artificial stone or ceramics with waste materials | |
Phosphates or phosphites (calcium phosphates C04B 2235/3212) as starting material for making ceramics, e.g. orthophosphate (PO43-), pyrophosphate (P2O74-), hypophosphite (H2PO2-), or present as secondary phase in the sintered ceramic | |
Manufacture of articles from scrap or waste metal particles | |
Active carbon from waste materials, e.g. tyres, spent sulphite pulp liquor | |
Preparation of alkali metal aluminates; aluminium oxide or hydroxide there from by treating aluminous minerals or waste-like raw materials with alkali hydroxide, | |
Melting in furnaces of glass-forming waste materials | |
Use of waste materials, e.g. slags as ingredients generally applicable to manufacture of glasses, glazes, or vitreous enamels | |
Devitrified glass ceramics containing waste materials, e.g. slags | |
Foundations for pavings characterised by material or composition used, e.g. waste or recycled material |
If more than one type of waste is used as additive for making one and the same clay object, all types of wastes are indicated with the appropriate class. Also when only small amounts are added, e.g. less than 5 wt% of the respective waste material, it is still being classified.
In this place, the following terms or expressions are used with the meaning indicated:
Devitrified glass ceramics | glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition |
This place covers:
The use of waste slurries such as unburned sewage sludge for making clay objects
Treatment of water, waste water, sewage, or sludge C02F
This place does not cover:
Slurries of specific well-defined waste streams, e.g. phosphate muds, other than red mud | |
The use of burned sewage sludge for making clay objects |
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: wet materials, e.g. slurries | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: dredged harbour or river sludge | |
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: waste slurries or solutions used as gauging water | |
Use of inorganic or non-macromolecular organic substances as compounding ingredients in polymers: waste materials, e.g. treated or untreated sewage sludge | |
Incineration of waste adapted for burning two or more kinds, e.g. liquid and solid, of waste being fed through separate inlets | |
Incinerators or other apparatus for consuming industrial waste for sludges or waste products from water treatment installations |
This place covers:
The use of unburned red mud for making clay objects
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone, e.g. waste from the purification of bauxite, e.g. red mud | |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
Iron oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. hematite (Fe2O3) or magnetite (Fe3O4) | C04B 2235/3272 and subgroup |
Preparation of alkali metal aluminates; aluminium oxide or hydroxide there from by treating aluminous minerals or waste-like raw materials with alkali hydroxide: separation of the insoluble residue, e.g. red mud | C01F 7/0646 and subgroup |
In this place, the following terms or expressions are used with the meaning indicated:
Red mud | Is a solid waste product of the Bayer process, the principal industrial means of refining bauxite in order to provide alumina as raw material for the electrolysis of aluminium by the Hall–Héroult process. A typical plant produces one to two times as much red mud as alumina. This ratio is dependent on the type of bauxite used in the refining process. Red mud is composed of a mixture of solid and metallic oxide-bearing impurities, and presents one of the aluminium industry's most important disposal problems. The red colour is caused by the oxidised iron present, which can make up to 60% of the mass of the red mud. In addition to iron, the other dominant particles include silica, unleached residual aluminium, and titanium oxide. |
This place covers:
Residues from sawing stones or ceramics, left refractory material, etc. is used for making a clay product
Attention is drawn to the following places, which may be of interest for search:
Hydraulic cements from waste building materials, e.g. waste asbestos-cement products, demolition waste | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: waste from quarries, mining or the like | C04B 18/12 and subgroup |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: waste from building or ceramic industry | C04B 18/16 and subgroup |
Grain-sized magnesia-based refractories | C04B 35/043 and subgroups |
Grain-sized alumina-based refractories | C04B 35/101 and subgroups |
Grain-sized titania-based refractories | C04B 35/46 and C04B 35/66 |
Grain-sized zirconia-based refractories | |
Grain-sized silicon carbide-based refractories | C04B 35/565 and subgroups, and C04B45/66 |
Monolithic refractories or refractory mortars | |
Compositions of refractory mould or core materials; grain structures thereof | B22C 1/00 and subgroups |
This place covers:
For instance waste containing halogens.
This place does not cover:
Treating radioactively contaminated material; decontamination arrangements therefore; treating liquids by fixation in an inorganic matrix, e.g. clays, zeolite | |
Treating radioactively contaminated material; decontamination arrangements therefore; treating solids by fixation in an inorganic matrix |
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: hazardous waste | |
Waste asbestos fibers added as filler to concrete, cement, mortar or artificial stone | |
Dredging sludge waste used for making clay wares | |
Halide containing anions as starting material for making ceramics, e.g. chlorate (ClO3-), bromide (Br-), iodate (IO3-), chlorite (ClO2-), or present as secondary phase in the sintered ceramic | |
Processes for making harmful chemical substances harmless or less harmful, by effecting a chemical change in the substances | A62D 3/00 and subgroups |
Treating radioactively contaminated material; decontamination arrangements therefore | G21F 9/00 and subgroups |
This place covers:
Waste containing metals or metal salts such as V, Cr, Mo, W, Mn, Co, Ni, Cd, Hg, Sn, Pb, Sb, Bi, etc. being used as additive for making clay products.
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: hazardous waste contaminated by heavy metals | |
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Vanadium oxides, vanadates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. magnesium vanadate (Mg2V2O7). | |
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
Niobium or tantalum oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Nb2O5 or Ta2O5 | C04B 2235/3251 and subgroup |
Molybdenum oxides, molybdates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. cadmium molybdate (CdMoO4) | |
Tungsten oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. scheelite (CaWO4) | C04B 2235/3258 and subgroup |
Manganese or rhenium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MnO | C04B 2235/3262 and subgroups |
Cobalt oxides, cobaltites or cobaltates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc cobaltite (ZnCo2O4) or bismuth cobaltate (BiCoO3) | C04B 2235/3275 and subgroup |
Nickel oxides, nickelates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. NiO | |
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroup |
Zinc oxides, zincates, cadmium oxides, cadmiates, mercury oxides, mercurates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. ZnO | |
Gallium oxides, gallates, indium oxides, indates, thallium oxides, thallates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc gallate (ZnGa2O4) | |
Germanium oxides, N:antimonite or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. copper N:antimonite (CuGeO3) | |
Noble metal oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. IrO2, PdO, RhO2 | C04B 2235/3289 and subgroup |
Tin oxides, stannates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g., indium tin oxide (ITO) | |
Antimony oxides, antimonates, antimonites or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, indium antimonite (InSbO4) | |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
Bismuth oxides, bismuthates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc bismuthate (Zn(BiO3)2) |
This place covers:
Alumino-silicate products made by sintering waste materials, without adding any clay material.
Normally a document that is classified in this class will also receive one of the other waste classes from C04B 33/00, to indicate the type of waste material.
This place covers:
The residues of the combustion of all wastes not covered by one of the subgroups, e.g. residues of the combustion of hazardous waste, refuse
Removal or treatment of combustion products or combustion residues F23J
This place does not cover:
Fly ash used in cement | |
Silica fume added as ingredient for clay mixtures |
Attention is drawn to the following places, which may be of interest for search:
Hydraulic cements from combustion residues, e.g. ashes or slags from waste incineration | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues, e.g. purification products of smoke, fumes or exhaust gases | C04B 18/06 and subgroups |
Hydraulic ash cements, e.g. fly ash cements, cements based on incineration residues, kiln dust cements | |
Porous mortars, concrete, artificial stone or ceramic ware, by burning-out added substances: waste material; refuse other than vegetable refuse | |
Chemical or biological purification of waste gases |
This place covers:
All fuel ashes, usually coal ashes from the burning of coal, which results in a light fraction, the fly ash or flue dust and the heavy fraction the (coal) bottom ash.
This place does not cover:
Ashes, such as fly ashes, from the burning of household waste, municipal waste, industrial waste, general garbage and sewage sludge |
Attention is drawn to the following places, which may be of interest for search:
Slaking of impure quick lime, e.g. contained in fly ash | |
Hydraulic cements with activators or composition-correcting additives, e.g. mixtures of fly ash and alkali activators | |
Hydraulic cements from raw materials containing flue dust, i.e. fly ash | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: residues from coal gasification | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues: flue dust, i.e. fly ash | C04B 18/08 and subgroups |
Ash cements, e.g. fly ash cements | |
Preparation of alkali metal aluminates; aluminium oxide or hydroxide there from by treating aluminous minerals from waste-like raw materials, e.g. fly ash, Bayer calcination dust with alkali hydroxide, | |
Working up raw materials other than ores, e.g. scrap, to produce non-ferrous metals and compounds thereof: working-up flue dust | |
Devices for conducting smoke or fumes, e.g. flues | F23J 11/00 and subgroups |
Fittings for chimneys or flues | F23J 13/00 and subgroups |
Arrangement of devices for treating smoke or fumes | F23J 15/00 and subgroups |
This place covers:
The residues from the incineration of household waste, municipal waste, industrial waste, general garbage
This place does not cover:
All ashes from fuel burning, such as fly ash or bottom ash from coal combustion |
Attention is drawn to the following places, which may be of interest for search:
Cements containing slags from waste incineration | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues: burned or pyrolised refuse | C04B 18/10 and subgroups |
Incineration of waste, incinerator constructions; details, accessories or control therefore | F23G 5/00 and subgroups |
Incinerators or other apparatus for consuming industrial waste, e.g. chemicals | F23G 7/00 and subgroups |
Treating radioactively contaminated material; decontamination arrangements therefore; treating liquids by incineration; by calcination, e.g. desiccation | |
Treating radioactively contaminated material; decontamination arrangements therefore; treating solids by incineration |
This place covers:
Sewage sludge that has been burned/incinerated is used as additive for making clay objects
This place does not cover:
Sewage sludge that not has been burned/incinerated is used as additive for making clay objects |
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues: burned or pyrolised sludges | |
Incinerators or other apparatus for consuming industrial waste, e.g. chemicals for sludges or waste products from water treatment installations |
This place covers:
Waste materials resulting from metallurgical processes that are used for making clay products.
This place does not cover:
Use of silica fume from metallurgical processes for making clay materials | |
Combusted metallurgical waste products used for making clay products |
Attention is drawn to the following places, which may be of interest for search:
Treatment of metallurgical slag. Artificial stone from molten metallurgical slag | C04B 5/00 and subgroups |
Hydraulic cements containing metallurgical slag | C04B 7/147 and subgroups |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: waste from metallurgical processes | C04B 18/14 and subgroup |
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder containing hydraulic cements other than calcium sulphates: slag cements | C04B 28/08 and subgroups |
Coating or impregnating e.g. injection in masonry, partial coating of green or fired ceramics with masses bonded by inorganic slag cements | |
Manufacture of articles from scrap or waste metal particles | |
Shaping clay or other ceramic compositions, slag or mixtures containing cementitious material e.g. plaster: specially adapted for producing articles from molten material, e.g. slag | |
Manufacture or treatment of flakes, fibres, or filaments from softened glass, minerals, or slags | C03B 37/00 and subgroups |
Ingredients generally applicable to manufacture of glasses, glazes, or vitreous enamels: use of waste materials, e.g. slags | |
Devitrified glass ceramics containing waste materials, e.g. slags | |
General features in the manufacture of pig-iron: recovery of by-products, e.g. slag | C21B 3/04 and subgroups |
Making pig-iron in the blast furnace: making slag of special composition | |
Manufacture of carbon-steel: processes yielding slags of special composition | |
Working up raw materials other than ores, e.g. scrap, to produce non-ferrous metals and compounds thereof: working-up slag | |
Equipment for removing or retaining slag | F27D 3/1545 and subgroup |
Devices or methods for removing incrustations, e.g. slag, metal deposits, dust; Devices or methods for preventing the adherence of slag | F27D 25/00 and subgroup |
In this place, the following terms or expressions are used with the meaning indicated:
Devitrified glass ceramics | glass ceramics having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition |
Slag | A partially vitreous by-product of smelting ore to separate the metal fraction from the unwanted fraction. It can usually be considered to be a mixture of metal oxides and silicon dioxide. However, slags can contain metal sulfides (see also matte) and metal atoms in the elemental form. |
This place covers:
Clays products of which the colour is specified or to which a colouring additive is added.
This place does not cover:
The colouring of glazes |
Attention is drawn to the following places, which may be of interest for search:
Iron oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. hematite (Fe2O3) or magnetite (Fe3O4) | C04B 2235/3272 and subgroup |
Colouring of ceramics or refractories | |
Pigments for ceramics | C09C 1/0009 and subgroup |
Pigments exhibiting interference colours | C09C 1/0015, C09C 2200/00 and subgroups |
Pigments consisting of flaky, non-metallic substrates, characterised by a surface-region containing free metal | |
Composite particulate pigments or fillers, i.e. containing at least two solid phases, except those consisting of coated particles of one compound | C09C 1/0081 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: metallic pigments or fillers | C09C 1/62 and subgroups |
The colouring additives that are added, e.g. iron oxide or cobalt oxide, are normally classified with a symbol from the C04B 2235/00-scheme.
This place covers:
The addition of lean materials such as grog, quartz, alumina to the clay mixture.
This place does not cover:
Materials consisting mainly out of grog/chamotte | |
Ceramic silica based materials | |
Ceramic silicate based materials | C04B 35/16 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: granular materials: quartz; sand | C04B 14/06 and subgroups |
Use of agglomerated or waste materials or refuse as fillers for mortars, concrete or artificial stone, treatment of agglomerated or waste materials or refuse, specially adapted to enhance their filling properties in mortars, concrete or artificial stone: grog | |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint, e.g. silicic acid H2Si2O5 |
The lean materials that are added, e.g. quartz or alumina, are normally classified with a symbol from the C04B 2235/00-scheme.
If the phase composition of the sintered clay material is specified, C04B 35/16 or one of its subgroup might be given to indicate the main phase of the sintered clay product.
In this place, the following terms or expressions are used with the meaning indicated:
Grog | Also known as firesand and chamotte, is a ceramic raw material. It has high percentage of silica and alumina. It can be produced by firing selected fire clays to high temperature before grinding and screening to specific particle sizes. It can also be produced from pitchers. The particle size distribution is generally coarser in size than the other raw materials used to prepare clay bodies. It tends to be porous and have low density. It is available as a powder, mortar, or in the form of fire bricks. Grog is composed of: 40% minimum alumina (Al2O3), 30% minimum silica (SiO2), 4% maximum Iron(III) oxide (Fe2O3), and 2% maximum of calcium oxide (CaO) and magnesium oxide (MgO) combined.[1] |
Lean materials | materials having a high percentage in silica and/or alumina, containing little alkali metal oxides, alkaline earth metal oxides, iron oxides, etc. |
This place covers:
Making slurries of clay materials. Adding additives to facilitate the formation and/or stability of clay material slurries
The additives used to liquefy the batches are indicated with the classes C04B 33/1305, C04B 33/131 and C04B 33/1315, the classes C04B 35/6306-C04B 35/6316, C04B 35/6325 and C04B 35/63404-C04B 35/638. Symbols from the range C04B 2235/00 and subgroups can be used as well.
This place covers:
The preparation of the powder to improve the pressing properties and methods of dry-pressing the powder.
This place does not cover:
Compounding ingredients of clay mixtures | C04B 33/13 and subgroups |
Dry-pressing clay at sintering temperatures |
Attention is drawn to the following places, which may be of interest for search:
Pressing at sintering temperatures of ceramic or refractory mixtures | C04B 35/645 and subgroup |
Pressing at non-sintering temperatures of ceramic or refractory mixtures | |
Making metallic articles by compacting | B22F 3/02 and subgroups |
Mechanical aspects of hot-pressing ceramic materials | |
Press moulds and press-ram assemblies for shaping clay or other ceramic compositions |
This place covers:
Materials consisting mainly out of grog/chamotte
This place does not cover:
Clay products or clay compositions to which grog/chamotte is added as a minority additive |
If the phase composition of the sintered clay material is specified, C04B 35/14, C04B 35/16 or one of its subgroups might be given to indicate the main phase of the sintered product.
In this place, the following terms or expressions are used with the meaning indicated:
Grog | Also known as firesand and chamotte, is a ceramic raw material. It has high percentage of silica and alumina. It can be produced by firing selected fire clays to high temperature before grinding and screening to specific particle sizes. It can also be produced from pitchers. The particle size distribution is generally coarser in size than the other raw materials used to prepare clay bodies. It tends to be porous and have low density. It is available as a powder, mortar, or in the form of fire bricks. Grog is composed of: 40% minimum alumina (Al2O3), 30% minimum silica (SiO2), 4% maximum Iron(III) oxide (Fe2O3), and 2% maximum of calcium oxide (CaO) and magnesium oxide (MgO) combined.[1] |
This place covers:
Compositions that lead to porcelain, e.g. containing high amount of china clay, are being used
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating of green or fired ceramics with porcelain | |
Porcelain or ceramic teeth | |
Porcelain materials for prosthesis | |
Coating or prosthesis-covering structure made of porcelain |
If the phase composition of the sintered clay material is specified, C04B 35/14, C04B 35/16 or one of its subgroups might be given to indicate the main phase of the sintered clay product.
In this place, the following terms or expressions are used with the meaning indicated:
Porcelain | ceramic material made by heating raw materials, generally including clay in the form of kaolin, in a kiln to temperatures between 1,200 °C (2,192 °F) and 1,400 °C (2,552 °F). The toughness, strength, and translucence of porcelain arise mainly from the formation of glass and the mineral mullite within the fired body at these high temperatures |
This place covers:
Porcelain used in the electric industry
This place covers:
Slip casting of clay/porcelain mixtures
This place does not cover:
Mechanical features of slip-casting clay materials | B28B 1/26 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Slip casting of ceramic or refractory mixtures | |
Semi-permeable inorganic membranes for separation processes made by slurry techniques, e.g. die or slip-casting | |
Slip casting metallic articles | |
Making clay or ceramic tubular articles by slip casting and moulds therefore | |
Slip casting plastics |
This place covers:
Drying methods for clay-based powder slurries or clay-based green bodies
Drying solid materials or objects by removing liquid therefrom F26B
This place does not cover:
Mechanical aspects of drying clay objects |
Attention is drawn to the following places, which may be of interest for search:
Drying ceramic or refractory powder mixtures | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: Selection of the hardening environment | C04B 40/02 and subgroups |
Removal of physically bonded water from cement or ceramics, e.g. drying of hardened concrete | |
Drying of green ceramic or refractory bodies | |
Processing clay- or ceramic containing substances in non-fluid condition by heating, drying | |
Surface treatment of glass not in the form of fibres or filaments: drying; dehydroxylation] |
This place covers:
All specific burning and sintering methods used for shaped clay materials, e.g. using a specific heating or cooling rate, a specific furnace, a specific atmosphere
This place does not cover:
Heat treatments of clay powders | C04B 35/62645 and subgroups |
Superficial sintering of clay objects with the goal of creating a porous object | C04B 38/0038 and subgroup |
Mechanical aspects of sintering clay objects |
Attention is drawn to the following places, which may be of interest for search:
Heat treatment, e.g. precalcining, burning, melting; cooling of hydraulic cements | C04B 7/43 and subgroups |
Burning or sintering processes of ceramic or refractory products | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: making use of a rise in temperature, e.g. caused by an exothermic reaction | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: heating up to sintering temperatures | |
After-treatment of mortars, concrete, artificial stone or ceramics: heat treatment | |
Aspects relating to heat treatment of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes | C04B 2235/65 and subgroups |
Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; apparatus specially adapted therefore; Presses and furnaces | B22F 3/00 and subgroups |
Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression | B22F 7/00 and subgroups |
Sintering glass | C03B 19/06 and subgroups |
Shaft or like vertical or substantially vertical furnaces wherein no smelting of the charge occurs, e.g. calcining or sintering furnaces |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well.
This place covers:
Complete melting of the clay material or at least to a large extent
This place does not cover:
Heat treatments such as] calcining; fusing pyrolysis in general | B01J 6/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Artificial stone from molten metallurgical slag | C04B 5/00 and subgroups |
Artificial stone obtained by melting at least part of the composition, e.g. metal | |
Melting of material to make a ceramic powder | |
Melting of ceramic or refractory material to make a bulk ceramic | C04B 35/653 and subgroup |
Porous clay ceramics obtained by generating pores in the ceramic material while in the molten state | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: involving melting of at least part of the composition | |
Thermally activated mortars, e.g. by melting ingredients | |
Coating or impregnating "in situ", e.g. impregnating of artificial stone by subsequent melting of a compound added to the artificial stone composition | |
Coating or impregnating applied from the molten state; thermal spraying, e.g. plasma spraying | C04B 41/4523 and subgroup |
Superficial melting of the ceramic substrate before or during the coating or impregnating step | |
Shaping methods specially adapted for producing clay or ceramic articles from molten material, e.g. slag refractory ceramic materials |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well.
This place covers:
Methods such as sinterforging, HIP (Hot Isostatic Pressing), SPS (spark plasma sintering).
Presses in general B30B
This place does not cover:
Pressing and heating of the clay green compact at the same time at temperatures lower than the sintering temperature | |
Processes using ultra high pressure, e.g. for the formation of diamonds; apparatus therefore, e.g. moulds, dies | B01J 3/06 and subgroups |
Mechanical aspects of hot-pressing clay materials |
Attention is drawn to the following places, which may be of interest for search:
Pressure sintering of ceramics and refractories | C04B 35/645 and subgroup |
Pressing at non-sintering temperatures of ceramic or refractory mixtures | |
Using constraining layers before or during sintering of ceramic laminates or ceramic substrates that are joined with other substrates | C04B 2237/56 and subgroups |
Both compacting and sintering of metallic articles | |
Both compacting and sintering of metallic articles by forging | |
Hot-pressing glass powder |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well. If SPS is used, C04B 2235/666 should be given as well.
This place covers:
Applying a glaze, engobe or enamel before sintering and then sinter.
This place does not cover:
Method of applying the glaze and/or choice of the substrate for glazing | C04B 41/5022 and subgroup, C04B 41/86 |
Coating or impregnating ceramic substrates with engobes | |
Mechanical aspects of glazing clay objects | |
Composition of enamels and glazes | C03C 8/00 and subgroups |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well.
This place covers:
Clay materials containing macro-additives such as fibers and/or whiskers, that give strength to the compact
This place does not cover:
Clay materials having additives such as binders, waste material, colouring additives | C04B 33/1315, C04B 33/132 and subgroups, C04B 33/14 (respectively) |
Mechanical aspects of shaping clay objects containing fibers | |
Arrangements specially adapted for the production of shaped ceramic articles with elements wholly or partly embedded in the moulding material; production of reinforced objects | B28B 23/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Fibrous materials and whiskers added to cement, concrete, mortar or artificial stone | C04B 14/38 and subgroups, C04B 20/0048 and subgroups |
Compositions for artificial stone, not containing binders, containing fibrous materials | |
Making ceramic fibers per se | |
Coating ceramic and carbon fibers | C04B 35/62844 and subgroups |
Ceramic material reinforced with fibers | C04B 35/71 and subclasses, e.g. C04B 35/83, C/C composites |
Fibers used in ceramic composition | C04B 2235/5208 and subgroups |
Fiber or whisker reinforced substrate joined with another substrate or being part of a ceramic laminate | |
Making metallic fibers per se | |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Glass fibre or filament compositions | C03C 13/00 and subgroups |
Glass compositions containing a non-glass component, e.g. compositions containing fibres, filaments, whiskers, platelets, or the like, dispersed in a glass matrix | |
Making fibers of inorganic material, not being glass, metallic or ceramic, e.g. carbon |
The classes C04B 35/71-C04B 35/83 are not used in combination with C04B 33/36. The reinforcements are indicated with symbol from the scheme C04B 2235/00-C04B 2235/549, and mainly from the range C04B 2235/5208-C04B 2235/5296 (different types of fiber additives).
This place covers:
Ceramic compositions or refractories based on oxides or oxide mixtures or solid solutions of two or more oxides; processes for their manufacture.
Ceramic compositions based on rare earth compounds or on compounds of actinides; processes for their manufacture.
Ceramic compositions or refractories based on non-oxides, e.g. on carbon, sulphides, selenides, fluorides, carbides, borides, nitrides or silicides; processes for their manufacture.
Monolithic refractories or refractory mortars, including those whether or not containing clay; processes for their manufacture.
Ceramic products containing macroscopic reinforcing agents, e.g. shaped metallic or non-metallic materials; processes for their manufacture.
Shaped ceramic products or refractories characterised by their composition; processes for manufacturing these shaped ceramic products or refractories:
- Shaped products obtained by a ceramic-forming technique;
- Shaped products obtained from polymer precursors;
- Shaped products obtained by Sol-Gel processing;
- Shaped products obtained by Rapid Prototyping techniques;
- Processing powders of inorganic compounds preparatory to the manufacturing of the shaped products ;
- Additives specially adapted for forming the shaped products , e.g. binders;
Processes characterised by the burning or sintering step.
Shaped products obtained by processes involving a melting step.
Filters, membranes for separation processes B01D
Catalysts B01J
Working by grinding or polishing B24
Mechanical features relating to the working of mortars, concrete, stone, clay-wares or ceramics , e.g. mixing or shaping ceramic compositions, boring natural stone B28B
Chemical preparation of powders of inorganic compounds C01
Chemical composition of glasses, glazes, or vitreous enamels C03C
Treating inorganic non-fibrous materials to enhance their pigmenting or filling properties C09C, C09C
Compositions containing free metal bonded to carbides, diamond, oxides, borides, nitrides, silicides, e.g. cermets, or other metal compounds, such as oxynitrides or sulphides, other than as macroscopic reinforcing agents C22C
Furnaces, kilns, ovens, or retorts F27
Basic electric elements H01
This place does not cover:
Clay-wares | C04B 33/00 and subgroups |
Devitrified glass-ceramics | C03C 10/00 and subgroups |
Manufacture of carbon fibres | D01F 9/12 and subgroups |
Casings, linings, walls, roofs of furnaces, kilns, ovens, or retorts | F27D 1/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Joining of a ceramic layer to another layer | C04B 37/00 and subgroups |
Obtaining porous ceramic products | C04B 38/00 and subgroups |
Coating or impregnating ceramic substrates with ceramic material | C04B 41/5025 and subgroups, C04B 41/87 |
Infiltration of sintered ceramic preforms with molten metal | |
Aspects relating to ceramic starting mixtures or sintered ceramic products | C04B 2235/00 and subgroups |
Ceramic interlayer used for joining a ceramic with another substrate | C04B 2237/04 and subgroups |
Ceramic substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/32 and subgroups |
Dental prostheses, e.g. porcelain or ceramic teeth | A61C 13/08 and subgroups |
Ceramic materials for prostheses or for coating prostheses | A61L 27/10 and subgroup |
Ceramic material for prosthesis | |
Materials for catheters or for coating catheters | A61L 29/00 and subgroups |
Materials for other surgical articles | A61L 31/00 and subgroups |
Inorganic membranes | B01D 71/02 and subgroups |
Articles characterised by particular shape, see the relevant classes, e.g. linings for casting ladles, tundishes, cups or the like | B22D 41/02 and subgroups |
Producing shaped articles from the material , e.g. by slip-casting | B28B 1/00 and subgroups |
Apparatus or methods for mixing clay or ceramic with other substances | B28C 3/00 and subgroups |
Proportioning the ingredients for mixing clay or cement with other substances | B28C 7/00 and subgroups |
Layered products essentially comprising ceramics , e.g. refractory products | |
Printing plates or foils; Materials therefore made entirely of inorganic materials other than natural stone or metals, e.g. ceramics, carbide materials, ferroelectric materials] | |
Luminescent materials | C09K 11/00 and subgroups |
Fireproofing materials | C09K 21/00 and subgroups |
Alloys based on carbides, oxides, borides, nitrides or silicides, e.g. cermets | C22C 29/00 and subgroups |
Materials for coating by flame or plasma spraying | C23C 4/10 and subgroups |
Materials for coating by sputtering, e.g. ceramic targets | C23C 14/06 and subgroups |
Single crystals or homogeneous polycrystalline material with defined structure | C30B 29/00 and subgroups |
Ceramics; oxides in machines or engines in general (F01) or machines for liquids ( F04) | F05C 2203/08 and subgroups |
Materials for parts of bearings, e.g. sliding-contact bearings | F16C 33/00 and subgroups |
Materials for friction linings | F16D 69/02 and subgroups |
Materials for pistons, trunk pistons, plungers | F16J 1/01 and subgroups |
Materials for piston-rings or seats therefore | F16J 9/26 and subgroups |
Materials for rigid pipes, of glass or ceramics, e.g. clay, clay tile, porcelain | F16L 9/10 and subgroups |
Materials for protection of pipes or pipe fittings against corrosion or incrustation | F16L 58/00 and subgroups |
Shades containing photoluminescent material | |
Refractors containing photoluminescent material | |
Reflectors containing photoluminescent material | |
Elements containing photoluminescent material distinct from or spaced from the light source | |
Elements with provision for controlling the spectral properties or intensity containing photoluminescent material | |
Casings, linings, walls of combustion chambers characterised by the shape of the bricks or blocks | F23M 5/02 and subgroups |
Arrangement or mounting of linings for fire-boxes, e.g. fire-back | F24B 13/02 and subgroups |
Shaft or vertical furnaces in general | F27B 1/00 and subgroups |
Measuring steady or quasi-steady pressure of a fluid or a fluent solid material by electric or magnetic pressure-sensitive elements. Transmitting or indicating the displacement of mechanical pressure-sensitive elements, used to measure the steady or quasi-steady pressure of a fluid or fluent solid material by electric or magnetic means using a ceramic diaphragm, e.g. alumina, fused quartz, glass | |
Ceramics; Glasses; Refractories as protection against x-radiation, gamma radiation, corpuscular radiation or particle bombardment | |
Materials for conductors or conductive bodies | H01B 1/00 and subgroups |
Materials for insulators or insulating or dielectric bodies | H01B 3/00 and subgroups |
Superconductive or hyperconductive conductors, cables, or transmission lines | H01B 12/00 and subgroups |
Materials for varistor cores | H01C 7/105 and subgroups |
Materials for magnets or magnetic bodies | H01F 1/00 and subgroups |
Superconducting magnets or coils | H01F 6/00 and subgroups |
Materials for fixed capacitors, e.g. ceramic dielectrics | H01G 4/12 and subgroups |
Materials for inert electrodes with catalytic activity for electrochemical generators, e.g. for fuel cells | H01M 4/86 and subgroups |
Fuel cells containing glass or ceramic materials | H01M 8/0215 and subgroups |
Materials for solid electrolytes of fuel cells | H01M 8/10 and subgroups |
Dielectric resonators of the waveguide type | H01P 7/10 and subgroups |
Diaphragms comprising ceramic-like materials, e.g. pure ceramic, glass, boride, nitride, carbide, mica and carbon materials | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
Materials for piezoelectric or electrostrictive elements | H10N 30/00 and subgroups |
Materials for superconductive or hyperconductive devices | H10N 60/00 and subgroups |
Ceramic substrates for microelectronic semi-conductors | |
Details of semiconductor or other solid state devices characterised by the material , encapsulations, e.g. encapsulating layers, coatings, characterised by the material, Oxides or nitrides or carbides, e.g. ceramics, glass | |
Details of semiconductor or other solid state devices characterised by the material , e.g. ceramic substrates | H10W 99/00 and subgroups |
In this group, in the absence of an indication to the contrary, compositions are classified according to the constituent present in the highest proportion by weight.
In this group, magnesium is considered as an alkaline earth metal.
In this group, a composite is considered as a sintered mixture of different powdered materials, other than sintering aids, the materials being present as separate phases in the sintered product.
In this group, fine ceramics are considered as products having a polycrystalline fine-grained microstructure, e.g. of dimensions below 100 micrometers.
The production of ceramic powder is classified in this group in so far as it relates to the preparation of powder with specific characteristics. If the powder is used for making a sintered ceramic, it is classified in C04B 35/00, e.g. making alumina powder that is used for a sintered alumina ceramic. If the composition of powder is new, the preparation of the powder is classified as well, irrespective of whether a sintered ceramic is made, e.g. the preparation of a barium titanate powder with a new composition that is used as filler in polymers is still classified in C04B 35/00. A new method for making an already known ceramic powder that is not used for making a sintered ceramic is not classified in C04B 35/00, but in C01 or C09, e.g. a new method for making alumina powder that is used for abrasives or as polymer filler is not classified in C0B35.
Any ingredient of a refractory mortar composition containing a hydraulic cement , e.g. aluminous cement , classified in C04B 35/66, which is considered to represent information of interest for search, may also be classified according to the Last Place Rule of note (2) after the subclass title of C04B, in groups C04B 7/00 - C04B 24/00. This can for example be the case when it is considered of interest to enable searching of compositions using a combination of classification symbols. Such non-obligatory classification should be given as "additional information". For example, such an additional classification in group C04B 24/00 may be given for an organic retarder added to the refractory mortar composition.
The symbols from C04B 2235/00 are usable for all documents classified in C04B 35/00 (as well as for C04B 33/00, C04B 37/00 and B32B 18/00). The symbols from C04B 2235/00 indicate additional information regarding additives used in the starting mixture, methods for making green bodies, aspects relating to the heat treatments that are given, secondary phases present in the final product, physical aspects of the final product and properties of the final product.
In this place, the following terms or expressions are used with the meaning indicated:
Ceramics | Inorganic, non metallic products obtained by a process involving a shaping step and a sintering or comparable heat treatment step, with the exclusion of cements, cermets and glasses, glazes, vitreous enamels and devitrified glass ceramics. |
Fine ceramics | Ceramics having a polycrystalline fine-grained microstructure, e.g. of dimensions below 100 micrometer. |
Glass-ceramic | having a crystalline phase dispersed in a glassy phase and constituting at least 50% by weight of the total composition |
Refractories | Ceramics or mortars withstanding high temperatures of at least about 1500 degrees C. For classification and search in this subclass no substantial distinction is made between the terms "refractories" and "ceramics ". |
Carbon-carbon composites | Products consisting of carbon fibres in a carbon matrix are usually referred to as "carbon-carbon composites ". |
Porous materials | Materials which are deliberately made porous, e.g. by adding gas- forming, foaming, burnable or lightweight additives to the composition they are made of. |
This place covers:
All oxide ceramics that are not classified in one of the sub-groups. These are for instance oxides based on gallium, indium, thallium, cobalt, nickel, noble metals, antimony, germanium, e.g. cobaltates, germanates, antimonates.
This place does not cover:
Oxide ceramics containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with oxide ceramic material | |
Metal oxides, mixed metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/32 and subgroups |
Gallium oxides, gallates, indium oxides, indates, thallium oxides, thallates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc gallate (ZnGa2O4) | |
Germanium oxides, germanates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. copper germanate (CuGeO3) | |
Noble metal oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. IrO2, PdO, RhO2 | C04B 2235/3289 and subgroup |
Antimony oxides, antimonates, antimonites or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, indium antimonate (InSbO4) | |
Bismuth oxides, bismuthates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc bismuthate (Zn(BiO3)2) | |
Non-metal oxides, mixed non-metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/34 and subgroups |
Boron oxide or borate as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Oxide interlayer used for joining a ceramic with another substrate | C04B 2237/06 and subgroups |
Oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/34 and subgroups |
Materials for prostheses based on metal oxides | A61L 27/10 and subgroups |
Oxide ceramic membranes | B01D 71/024 and subgroups |
The preparation of gallium, indium or thallium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 15/00 and subgroups |
The preparation of antimony compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 30/00 and subgroups |
The preparation of cobalt compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 51/00 and subgroups |
The preparation of nickel compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 53/00 and subgroups |
The preparation of ruthenium, rhodium, palladium, osmium, iridium, or platinum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 55/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of antimony | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing antimonates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt | C09K 11/60 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing gallium, indium or thallium | C09K 11/62 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead | C09K 11/66 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth | C09K 11/74 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth germanates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth germinates | C09K 11/7707, C09K 11/7735, C09K 11/775, C09K 11/7775, C09K 11/7793 |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth antimonates; arsenates | |
Oxide single crystals | C30B 29/16 and subgroups |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting oxides | H01C 7/043 and subgroups |
Light-sensitive devices comprising an oxide semiconductor electrode | |
Electrodes for accumulators with non-aqueous electrolyte, e.g. for lithium-accumulators; Processes of manufacture thereof: based on mixed oxides or hydroxides, or on mixtures of oxides or hydroxides, e.g. LiCoOx | |
Fuel cells operating at high temperature, e.g. with stabilised ZrO2 electrolyte, Electrode material consisting of oxides | H01M 4/9025, and subgroup |
Fuel cells operating at high temperature, e.g. with stabilised ZrO2 electrolyte, the electrolyte consisting of oxides | H01M 8/1246 and subgroups |
Forming inorganic semiconducting materials on a substrate, the substrate being an oxide |
Classification occurs by identifying which phase of the final product is present in the largest amount. This does not necessarily need to be more than 50%, you could also have 40% A, 35% B and 25% C. If there are two or more phases present in the same amount as the largest amount, all phases are classified, thus with 30% A, 30% B, 30% C and 10% D the phases A, B and C are all three classified. For example, a final product containing 50% zirconia and 50% alumina receives the classes C04B 35/119 (alumina reinforced with zirconia) and C04B 35/4885 (zirconia reinforced with alumina).
The alkali metal oxides, alkaline earth metal oxides and rare earth oxides form many different mixed oxides with other metal oxides. If alkali metal oxides, alkaline earth metal oxides and rare earth oxides are present in a mixed oxide with another metal oxide, the other metal oxide is almost always determining the classification.
The symbols from C04B 2235/00 are usable for all documents classified in C04B 35/00 (as well as for C04B 33/00, C04B 37/00 and B32B 18/00). The symbols from C04B 2235/00 indicate additional information regarding additives used in the starting mixture, methods for making green bodies, aspects relating to the heat treatments that are given, secondary phases present in the final product, physical aspects of the final product and properties of the final product.
This place covers:
Oxide ceramics containing carbon products, e.g. oxide refractories containing a carbon binder such as pitch, tar, bitumen (materials which are classified in C04B 35/63496), or oxide materials containing graphite, diamond or carbon black additives.
This place does not cover:
Alumina-based refractories containing carbon |
Attention is drawn to the following places, which may be of interest for search:
Bituminous additives for ceramic materials, e.g. tar, pitch | |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
If the carbon additive is tar or pitch, C04B 35/63496 is given as well. The carbonaceous additives are further indicated with the symbols C04B 2235/424 (carbon black), C04B 2235/425 (graphite) and C04B 2235/427 (diamond). In the case polymeric additives from the classes C04B 35/63404 and subgroups, C04B 35/63448 and subgroups and C04B 35/63492 are added to an oxide ceramic mixture and are carbonised, C04B 2235/48 is given, but C04B 35/013 not.
This place covers:
All ceramics or ceramic mixtures based on manganese oxide and all manganites and manganates, e.g. perovskites such as lanthanum manganate LaMnO3
This place does not cover:
Mixed oxides containing more of other transition metal oxides, e.g. LaCo0.6Mn0.4O3 | C04B 35/01 (for the cobaltate), C04B 2235/3227 (for the La), C04B 2235/3262 (for the Mn), C04B 2235/768 (for the perovskite structure) |
Mixed oxides containing more of group 13-15 metal oxides, e.g. BaAl0.6Mn0.4O3 | C04B 35/44 (for the aluminate), C04B 2235/3215 (for the Ba), C04B 2235/3262 (for the Mn), C04B 2235/768 (for the perovskite structure) |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with manganates | |
Manganese or rhenium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MnO | C04B 2235/3262 and subgroups |
The preparation of manganese compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 45/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing manganese or rhenium | C09K 11/57 and subgroups |
Electrolytic production of manganese oxides | |
Electrolytic production of electrodes based on manganese dioxide or lead dioxide | |
Manganite magnets | |
Diluted non-magnetic ions in a magnetic cation-sublattice, e.g. La1-x(Ba,Sr)xMnO3 | |
Electrodes for fuel cells and batteries composed of or comprising active material of manganese oxides or hydroxides | H01M 4/50 and subgroups |
Fuel cells applied on a support operating at high temperature, the electrode being of complexed oxides, optionally doped, of the type M1MeO3, M1 being an alkaline earth metal or a rare earth, Me being a metal, e.g. perovskites, with the anode and the cathode in the form of gas diffusion electrodes |
This place covers:
Oxides based on single oxide phases of MgO or CaO or mixed oxides of MgO and CaO, or mixed oxides of alkaline earth oxides with either alkali metal oxides and/or rare earth oxides, in which the alkaline earth metal oxide forms the largest fraction. Mixed oxides of magnesia/calcia with zirconium oxide, in which the amount of magnesia/calcia is larger than the amount of zirconia, e.g. Mg0.6Zr0.4Ox
This place does not cover:
Mixed oxides of MgO and/or CaO with both alumina and silica, e.g. cordierite | |
Mixed oxides of MgO with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Mixed oxides of CaO with silica without alumina, e.g. wollastonite (CaSiO4) | |
Mixed oxides of MgO and/or CaO with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2683 |
Mixed oxides of MgO and/or CaO with chromium oxide, e.g. chromites | |
Mixed oxides of CaO with alumina, without silica, e.g. calcium aluminate | |
Mixed oxides of MgO with alumina, without silica, e.g. magnesium aluminate, spinel | |
Magnesium or calcium based phosphates | |
Mixed oxides of MgO and/or CaO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of MgO and/or CaO with zinc oxide and/or bismuth oxide, e.g. magnesium bismuthate | |
Mixed oxides of MgO and/or CaO with tin oxide, e.g. magnesium stannate | |
Mixed oxides of MgO and/or CaO with titanium oxides, such as magnesium titanate or calcium titanate | |
Mixed oxides of MgO and/or CaO with zirconium oxide, e.g. magnesium zirconate, containing more Zr than Mg and Ca | C04B 35/48 and subgroups |
Mixed oxides of MgO and/or CaO with zirconium oxide and titanium oxide, e.g. calcium titanate zirconate (CaTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of MgO and/or CaO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. magnesium tantalum niobate (MgNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Obtaining lime, magnesia or dolomite | C04B 2/00 and subgroups |
Alkaline earth oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. BeO | C04B 2235/3205 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of alkaline earth metals or magnesium | C09C 1/02 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic alkaline earth metal compounds |
This place covers:
Oxides based on single oxide phases of MgO, or mixed oxides of MgO with either alkali metal oxides and/or rare earth oxides, in which the MgO forms the largest fraction
This place does not cover:
Mixed oxides of MgO with both alumina and silica, e.g. cordierite | |
Mixed oxides of MgO with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Mixed oxides of MgO with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2683 |
Mixed oxides of MgO with chromium oxide, e.g. chromites | |
Mixed oxides of MgO with alumina, without silica, e.g. magnesium aluminate, spinel | |
Magnesium based phosphates | |
Mixed oxides of MgO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of MgO with zinc oxide and/or bismuth oxide, e.g. magnesium bismuthate | |
Mixed oxides of MgO with tin oxide, e.g. magnesium stannate | |
Mixed oxides of MgO with titanium oxides, such as magnesium titanate | |
Mixed oxides of MgO with zirconium oxide, e.g. magnesium zirconate | C04B 35/48 and subgroups |
Mixed oxides of MgO with zirconium oxide and titanium oxide, e.g. magnesium titanate zirconate (MgTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of MgO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. magnesium tantalum niobate (MgNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: magnesia | |
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone: magnesia; magnesium hydroxide | |
Magnesium oxide or magnesium carbonate cements | C04B 28/105, C04B 28/30 and subgroup |
Making fibres based on magnesium oxide | |
Coating or impregnating ceramic substrates with magnesium oxide | C04B 41/5029, C04B 41/5084 (cementitious) |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Materials for prostheses based on magnesia or magnesium oxide | |
Catalysts comprising the elements, oxides, or hydroxides of magnesium | |
Preparation of magnesium compound powders, e.g. magnesium oxide powder | C01F 5/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds containing only magnesium as metal |
This place covers:
MgO based refractories having large grains, the majority larger than 100 microns
This place does not cover:
MgO ceramics with the majority of the grain smaller than 100 microns |
Attention is drawn to the following places, which may be of interest for search:
Grain-sized alumina-based refractories | C04B 35/101 and subgroups |
Grain-sized titania-based refractories | C04B 35/46 and C04B 35/66 |
Grain-sized zirconia-based refractories | |
Grain-sized silicon carbide-based refractories | C04B 35/565 and subgroups, and C04B45/66 |
Monolithic refractories and refractory mortars | |
Using particles larger than 100 microns for making the ceramic | |
Bimodal, multi-modal or multi-fraction particle size distribution | |
Compositions of refractory mould or core materials; Grain structures thereof | B22C 1/00 and subgroups |
This place covers:
The majority of the refractory material is MgO, a minority a refractory metal oxide such alumina, zirconia, titania, or a refractory metal non-oxide such as a carbide or boride
Attention is drawn to the following places, which may be of interest for search:
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3804 and subgroups |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3817 and subgroups |
This place covers:
The refractory contains some type of chromium oxide
This place does not cover:
Fused magnesia refractories containing chromium oxide or chrome ore | |
Grain-sized alumina-based refractories containing chromium oxide or chromium ore |
Attention is drawn to the following places, which may be of interest for search:
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
This place covers:
Both refractories that are used directly after melting, either in particle or bulk form, as well as fused refractory that is sintered before use as refractory
This place does not cover:
Complete fusion of the magnesia refractory without subsequent heat treatment |
Attention is drawn to the following places, which may be of interest for search:
Fusing to make ceramic particles in general |
This place covers:
The refractory mixture has been sintered before use
This place covers:
The magnesia-based refractory has been melted
This place does not cover:
Magnesia-based refractory that has been melted and subsequently sintered |
Attention is drawn to the following places, which may be of interest for search:
Clay wares made by methods involving melting, fusion or softening | |
Alumina-based refractories made by fusion casting | C04B 35/107 and subgroup |
Zirconia-based refractories made by fusion casting | |
Fusing to make ceramic particles in general | |
Refractories in general made by fusion casting | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
This place covers:
Melted MgO based refractory containing also chromium oxide
This place does not cover:
Magnesia-based refractory containing chromium oxide or chrome ore that has been melted and subsequently sintered | |
Grain-sized alumina-based refractories containing chromium oxide or chromium ore |
Attention is drawn to the following places, which may be of interest for search:
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
This place covers:
MgO-based ceramics having a majority of grains with a size of below 100 microns. Oxides based on single oxide phases of MgO, or mixed oxides of MgO with either alkali metal oxides and/or rare earth oxides, in which the MgO forms the largest fraction.
This place does not cover:
Mixed oxides of MgO with both alumina and silica, e.g. cordierite | |
Mixed oxides of MgO with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Mixed oxides of MgO with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2683 |
Mixed oxides of MgO with chromium oxide, e.g. chromites | |
Mixed oxides of MgO with alumina, without silica, e.g. magnesium aluminate, spinel | |
Magnesium based phosphates | |
Mixed oxides of MgO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of MgO with zinc oxide and/or bismuth oxide, e.g. magnesium bismuthate | |
Mixed oxides of MgO with tin oxide, e.g. magnesium stannate | |
Mixed oxides of MgO with titanium oxides, such as magnesium titanate | |
Mixed oxides of MgO with zirconium oxide, e.g. magnesium zirconate | C04B 35/48 and subgroups |
Mixed oxides of MgO with zirconium oxide and titanium oxide, e.g. magnesium titanate zirconate (MgTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of MgO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. magnesium tantalum niobate (MgNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Using particles of size 1-100 microns for making a ceramic |
This place covers:
Oxides based on single oxide phases of CaO, or mixed oxides of MgO with either alkali metal oxides and/or rare earth oxides, in which the CaO forms the largest fraction. The ceramic can have all grain sizes.
This place does not cover:
Mixed oxides of CaO with both alumina and silica, e.g. cordierite | |
Mixed oxides of CaO with silica without alumina, e.g. wollastonite (CaSiO4) | |
Mixed oxides of CaO with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2683 |
Mixed oxides of CaO with chromium oxide, e.g. chromites | |
Mixed oxides of CaO with alumina, without silica, e.g. calcium aluminate | |
Calcium based phosphates | |
Mixed oxides of CaO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of CaO with zinc oxide and/or bismuth oxide, e.g. calcium bismuthate | |
Mixed oxides of CaO with tin oxide, e.g. calcium stannate | |
Mixed oxides of CaO with titanium oxides, such as calcium titanate | |
Mixed oxides of CaO with zirconium oxide, e.g. calcium zirconate | C04B 35/48 and subgroups |
Mixed oxides of CaO with zirconium oxide and titanium oxide, e.g. calcium titanate zirconate (CaTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of CaO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. calcium tantalum niobate (CaNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Hydraulic lime | |
Eliminating lime or iron from clay mixtures | |
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime | C04B 2235/3208 and subgroups |
Materials for prostheses based on calcia or calcium oxide CaO | |
The preparation of compounds of calcium, barium and strontium in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01F 11/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcium carbonates | C09C 1/021 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcium sulphates |
This place covers:
mixtures of CaO and MgO
Attention is drawn to the following places, which may be of interest for search:
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime | C04B 2235/3208 and subgroups |
Dolomite, i.e. mixed calcium magnesium carbonate, or oxides derived from dolomite as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Carbonates (CO32-) as starting material for making ceramics or present as secondary phase in the sintered ceramic |
In this place, the following terms or expressions are used with the meaning indicated:
Dolomite | (CaMg)(CO3)2 |
This place covers:
Oxide ceramics based on the single oxide phase of BeO.
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
Preparation of beryllium compound powders, e.g. beryllium oxide powder | C01F 3/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing beryllium compounds |
This place covers:
Ceramics or ceramic mixtures containing as the largest fraction the single oxide Al2O3
This place does not cover:
Ceramics containing as the largest fraction a mixed oxide of alumina with silica | C04B 33/00 (clay ceramics) or C04B 35/18 and subgroups (alumino-silicate ceramics) |
Ceramics containing as the largest fraction a mixed oxide of alumina with other metal oxides | C04B 35/44 (aluminates) |
Ceramics containing as the largest fraction a mixed oxide of alumina with magnesia | C04B 35/443 (magnesia-alumina spinel) |
Alumina containing a metallic binder, e.g. an alumina cermet with Al binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: alumina | |
Making fibres based on aluminium oxide | |
Coating or impregnating ceramic substrates with alumina | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Alumina or aluminate interlayer used for joining a ceramic with another substrate | |
Alumina or aluminate substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses or coatings of prostheses based on aluminium oxides | |
Materials for prostheses based on aluminium oxides | A61L 27/105 and subgroups |
Alumina-based membranes | |
Catalysts comprising alumina | |
Preparation of aluminium compound powders, e.g. aluminium oxide powder | C01F 7/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of aluminium | C09C 1/40 and subgroups |
Abrasives | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing aluminium | C09K 11/64 and subgroups |
Alumina single crystals | |
Aluminium oxide in machines or engines in general (F01) or machines for liquids ( F04) | F05C 2203/0869 and subgroup |
Materials for vessels of gas- or vapour discharge lamps | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate, the material containing aluminium, e.g. Al2O3 |
This place covers:
Alumina based refractories having large grains, the majority larger than 100 microns
Attention is drawn to the following places, which may be of interest for search:
Grain-sized magnesia-based refractories | C04B 35/043 and subgroups |
Grain-sized titania-based refractories | C04B 35/46 and C04B 35/66 |
Grain-sized zirconia-based refractories | |
Grain-sized silicon carbide-based refractories | C04B 35/565 and subgroups, and C04B45/66 |
Monolithic refractories and refractory mortars | |
Using particles larger than 100 microns for making the ceramic | |
Bimodal, multi-modal or multi-fraction particle size distribution | |
Compositions of refractory mould or core materials; Grain structures thereof | B22C 1/00 and subgroups |
Abrasive particles per se obtained by division of a mass agglomerated by sintering |
This place covers:
Refractories based on alumina containing other oxide refractories such as magnesia, titania
This place does not cover:
Grain-sized alumina-based refractories containing carbon | |
Grain-sized alumina-based refractories containing chromium oxide or chromium ore | |
Grain-sized refractory mixtures based on alumina containing zirconia |
Attention is drawn to the following places, which may be of interest for search:
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
This place covers:
Shaped alumina-based refractory ceramics or alumina-based refractory mixtures, containing non-oxides such as carbon, pitch, tar, carbides, nitrides, borides, silicides, fluorides, sulphides, any material that would be classified in C04B 35/515-C04B 35/597.
This place does not cover:
Grain-sized refractory mixtures based on alumina containing zirconia | |
Shaped alumina-based refractory ceramics or alumina-based refractory mixtures containing polymers such as polymeric binders | C04B 35/63404 and subgroups, C04B 35/63448 and subgroups and C04B 35/63492 and C04B 35/636 and subgroup |
Ceramic products containing reinforcing agents containing non-metallic materials (oxides and non-oxides only) such as fibres, filaments, whiskers, platelets or the like | |
Ceramic products containing reinforcing agents containing carbon nanotubes | |
Shaped alumina-based refractory ceramics or alumina-based refractory mixtures containing carbon as an impurity |
Attention is drawn to the following places, which may be of interest for search:
Oxide-based ceramics or ceramic mixtures in general containing carbon | |
Non-oxide based ceramics | C04B 35/515 and subgroups |
Ceramic powders coated with non-oxide ceramic materials | C04B 35/62828 and subgroups |
Ceramic fibers coated with non-oxide ceramic materials | C04B 35/62857 and subgroups |
Non-oxide additives for ceramics | C04B 2235/38 and subgroups |
Carbon additives for ceramics | C04B 2235/422 and subgroups |
Organic compounds becoming part of a ceramic after heat-treatment, e.g. phenol resins | C04B 2235/48 and subgroups |
Fibrous non-oxide additives for ceramics | |
Carbon nanotube additives for ceramics |
If the carbon additive is tar or pitch, C04B 35/63496 is given as well. The carbonaceous additives are further indicated with the codes C04B 2235/424 (carbon black), C04B 2235/425 (graphite) and C04B 2235/427 (diamond). Other non-oxide additives, such as silicon carbide or silicon nitride, are indicated with a symbol from C04B 2235/48. In the case polymeric additives from the classes C04B 35/63404 and subgroups, C04B 35/63448 and subgroups and C04B 35/63492 are added to an oxide ceramic mixture and are carbonised, C04B 2235/48 is given, but C04B 35/013 not.
This place covers:
Refractories based on alumina, containing also chromium oxide
Attention is drawn to the following places, which may be of interest for search:
Grain-sized refractory mixtures based on magnesia containing chromium oxide or chrome ore | C04B 35/047 and subgroups |
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
This place covers:
Refractories based on alumina, containing also zirconium oxide, possibly also containing silicon oxide
This place does not cover:
Alumina refractories containing zirconia, made by melt-casting |
Attention is drawn to the following places, which may be of interest for search:
Fine alumina ceramics containing zirconia | |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
Zirconates or hafnates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zircon (ZrSiO4) |
This place covers:
Both refractories that are used directly after melting, either in particle or bulk form, as well as fused refractory that is sintered before use as refractory
Attention is drawn to the following places, which may be of interest for search:
Clay wares made by methods involving melting, fusion or softening | |
Magnesia-based refractories made by fusion casting | C04B 35/05 and subgroup |
Zirconia-based refractories made by fusion casting | |
Fusing to make ceramic particles in general | |
Refractories in general made by fusion casting | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
Abrasive particles per se obtained by division of a mass agglomerated by melting, at least partially, e.g. with a binder |
This place covers:
Refractories based on alumina, made by melting, containing also zirconium oxide, possibly also containing silicon oxide
Attention is drawn to the following places, which may be of interest for search:
Grain-sized refractory mixtures based on alumina containing zirconia | |
Fine alumina ceramics containing zirconia | |
Zirconates or hafnates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zircon (ZrSiO4) |
This place covers:
Ceramics or ceramic mixtures having as the largest fraction alumina single phase material having an average grain size of below 100 microns
This place does not cover:
Ceramics containing as the largest fraction a mixed oxide of alumina with silica | C04B 33/00 (clay ceramics) or C04B 35/18 and subgroups (alumino-silicate ceramics) |
Ceramics containing as the largest fraction a mixed oxide of alumina with other metal oxides | C04B 35/44 (aluminates) |
Ceramics containing as the largest fraction a mixed oxide of alumina with magnesia | C04B 35/443 (magnesia-alumina spinel) |
Alumina containing a metallic binder, e.g. an alumina cermet with Al binder | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics or ceramic mixtures having as the largest fraction alumina single phase material having an average grain size of above 100 microns | C04B 35/101 and subgroups |
Using particles of size 1-100 microns for making the ceramic |
This place covers:
Mainly alumina particles that are bonded together into aggregates and used as abrasive
This place does not cover:
Bulk alumina objects |
Attention is drawn to the following places, which may be of interest for search:
Powdery starting material for making ceramics containing flakes, platelets or plates | |
Abrasive particles per se obtained by division of a mass agglomerated by sintering |
This place covers:
Ceramics or ceramic mixtures based on alumina(te) phases with the composition MAl11O18 or LnAl12O19
This place does not cover:
Other aluminates | C04B 35/44 or C04B 35/443 (spinel) |
Attention is drawn to the following places, which may be of interest for search:
Aluminates other than alumino-silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. spinel (MgAl2O4) | |
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Preparation of beta-alumina powders |
This place covers:
Sintered alumina ceramics that are translucent or transparent
Attention is drawn to the following places, which may be of interest for search:
Ceramic materials other than alumina that are transparent or translucent |
This place covers:
All sintered alumina ceramics that contain at least one secondary phase, where this secondary phase is neither a grain boundary phase nor a different type of alumina phase. The main phase can be for instance alpha-alumina, the secondary phase an aluminate.
This place does not cover:
Alumina refractories containing a secondary phase | |
Alumina ceramics containing a mixture of different alumina phases, e.g. alpha-alumina and beta-alumina |
Attention is drawn to the following places, which may be of interest for search:
Alumina ceramics containing shaped metallic materials, e.g. metallic fibers | C04B 35/74 and subgroup |
Alumina ceramics containing ceramic fibers, whiskers or platelets, e.g. an alumina particle matrix containing alumina fibers or alumina platelets | |
Ceramics containing one or more secondary phases | C04B 2235/80 and subgroups |
If the secondary phase is a ceramic fiber, whisker, platelet or similarly shaped ceramic particle, both C04B 35/80 and C04B 35/117 are given. The same logic applies to C04B 35/117 and C04B 35/74.
The secondary phases are indicated with codes from C04B 2235/32-C04B 2235/428. The code C04B 2235/80 does not need to be used, since the class itself already indicates that secondary phases are present.
This place covers:
All sintered alumina ceramics that contain at least one secondary zirconia phase, where this secondary zirconia phase is not a grain boundary phase
This place does not cover:
Alumina refractories containing a zirconia secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Zirconia-based ceramics containing an alumina secondary phase | |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
If the amount of alumina phase is larger than the amount of zirconia phase, C04B 35/119 is given, if the amounts are equal, e.g. C04B40/40, then both C04B 35/119 and C04B 35/4885 are given.
In patent documents, the following abbreviations are often used:
ZTA | Zirconia toughened alumina |
ATZ | Alumina toughened zirconia |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by a single oxide phase of chromium oxide
This place does not cover:
Grain-sized refractory mixtures based on magnesia containing chromium oxide or chrome ore | C04B 35/047 and subgroups |
Grain-sized alumina-based refractories containing chromium oxide or chromium ore | |
Mixed oxides of chromium with alkali metals, alkaline earth metals and rare earth metals | |
Mixed oxides of chromium with titanium oxide, containing more Cr, e.g. Cr0.6Ti0.4O2 | |
Mixed oxides of chromium with titanium oxide, containing more Ti, e.g. Cr0.4Ti0.6O2 | |
Chromium oxide based material with a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone chromium oxide | |
Magnesia-based refractories containing chromia | C04B 35/047 and subgroups, C04B 35/051 |
Alumina-based refractories containing chromia | |
Coating or impregnating ceramic substrates with chromium oxide | |
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
Refractory metal oxide interlayer used for joining a ceramic with another substrate | |
Refractory metal oxide substrate joined with another substrate or being part of a ceramic laminate | |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 37/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of chromium | C09C 1/34 and subgroups |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by a crystalline single oxide phase of SiO2, e.g. quartz or cristobalite
This place does not cover:
Ceramics or ceramics mixture of which the largest fraction is formed by a mixed oxide phase of SiO2, e.g. silicates such as cordierite, alumino-silicates in general, magnesium silicates such as forsterite, calcium silicates such as wollastonite | C04B 33/00 (clays), C04B 35/16 and subgroups (silicates) |
Materials having as largest fraction a form of crystalline SiO2 but also containing a glass matrix, e.g. 80% quartz and 20% glass matrix |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: granular materials: quartz; sand | C04B 14/06 and subgroups |
Making fibers based on silica | |
Coating or impregnating ceramic substrates with silica | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silica or silicate interlayer used for joining a ceramic with another substrate | |
Silica or silicate substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on silica or silicon oxide | |
Silica-based membranes | |
Catalysts comprising silica | |
Preparation of silica powders, sols, gels, dispersions and their after-treatments | C01B 33/113 and subgroups |
Processes specially adapted for the production of quartz or fused silica articles | |
Pure silica glass, e.g. pure fused quartz | C03B 2201/02 and subgroups, C03C 2201/02 |
Glass compositions with more than 90% silica by weight, e.g. quartz | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of silicon | C09C 1/28 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing silicon | C09K 11/59 and subgroups |
Quartz single crystals | |
Silica in machines or engines in general (F01) or machines for liquids ( F04) | |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate by gas or vapour deposition, the material containing silica | H10P 14/6328 and subgroup |
This place covers:
All silicates that are not clay (see C04B 33/00 for the definition of clays). A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate ([SiF6]2−) and other anions are also included. Silicate compounds, including the minerals, consist of silicate anions whose charge is balanced by various cations. Myriad silicate anions can exist, and each can form compounds with many different cations. Hence this class of compounds is very large. Both minerals and synthetic materials fit in this class. Silicates are mainly a mixed oxide phase of SiO2 with at least one other metal oxide, e.g. transition metal silicates such as iron silicate, or barium silicate, or rare earth silicates.
Materials having as largest fraction a crystalline silicate phase but also containing a glass matrix, e.g. 80% silicate and 20% glass matrix C03C
This place does not cover:
Clay wares | C04B 33/00 and subgroups |
Ceramics based on zirconium or hafnium silicates, e.g. zircon (ZrSiO4) |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone | C04B 14/04 and subgroups |
Coating or impregnating ceramic substrates with silicates | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. water glass (Na2SiO3) | C04B 2235/3427 and subgroups |
Silica or silicate interlayer used for joining a ceramic with another substrate | |
Silica or silicate substrate joined with another substrate or being part of a ceramic laminate | |
Preparation of silicate powders, sols, gels, dispersions and their after-treatments | C01B 33/20 and subgroups, C01B 37/005 |
Coating compositions, e.g. paints, varnishes or lacquers, based on alkali metal silicates | C09D 1/02 and subgroup |
Adhesives based on water-soluble alkali silicate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt as silicate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic halogen silicate compounds | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing refractory silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth silicates | C09K 11/77064, C09K 11/77214, C09K 11/77344, C09K 11/77494, C09K 11/7758, C09K 11/7764, C09K 11/77744, C09K 11/77924 |
Single crystals of silicates |
In this place, the following terms or expressions are used with the meaning indicated:
Silicate mineral | Mineralogically, silicate minerals are divided according to structure of their silicate anion into the following groups: Nesosilicates (lone tetrahedron) - [SiO4]4−, e.g. olivine. Sorosilicates (double tetrahedra) - [Si2O7]6−, e.g. epidote, melilite group. Cyclosilicates (rings) - [SinO3n]2n−, e.g. tourmaline group. Inosilicates (single chain) - [SinO3n]2n−, e.g. pyroxene group. Inosilicates(double chain) - [Si4nO11n]6n−, e.g. amphibole group. Phyllosilicates (sheets) - [Si2nO5n]2n−, e.g. micas and clays. Tectosilicates (3D framework) - [AlxSiyO2(x+y)]x−, e.g. quartz, feldspars, zeolites. Note that tectosilicates can only have additional cations if some of the silicon is replaced by a lower-charge cation such as aluminium . Al for Si substitution is common. |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by a mixed oxide phase of SiO2 with alumina, the alumino-silicates
This place does not cover:
Materials made of clay | C04B 33/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Making fibres based on silica, rich in aluminium oxide | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. mullite (3Al2O3-2SiO2) | C04B 2235/3463 and subgroups |
Catalysts comprising silica and alumina | |
Catalysts comprising Crystalline aluminosilicate zeolites; Isomorphous compounds thereof | B01J 29/06 and subgroups |
Preparation of aluminium containing silicate powders, sols, gels, dispersions and their after-treatments | C01B 33/26 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing aluminium silicates |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by a mullite phase
Materials having as largest fraction a mullite phase but also containing a glass matrix, e.g. 80% mullite and 20% glass matrix C03C
Attention is drawn to the following places, which may be of interest for search:
Mullite catalysts or catalysts supports |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by an alumino-silicate phase containing more alkali metal ions than ions of other type, such as alkaline earth metal ions
This place does not cover:
Materials having as largest fraction a spodumene phase but also containing a glass matrix, e.g. 80% spodumene and 20% glass matrix | C03C 10/0018 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Alkali oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Na2O, K2O | C04B 2235/3201 and subgroup |
Alkali metal alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. spodumene (LiAlSi2O6), alkali feldspars such as Albite (NaAlSi3O8) or Orthoclase (KAlSi3O8), micas such as Muscovite (KAl2(AlSi3)O10(OH)2), zeolites such as Natrolite (Na2Al2Si3O10·2H2O) |
This place covers:
Ceramics or ceramics mixture of which the largest fraction is formed by an alumino-silicate phase containing more alkaline earth metal ions than ions of other type, such as alkali metal ions
This place does not cover:
Materials having as largest fraction a cordierite phase but also containing a glass matrix, e.g. 80% cordierite and 20% glass matrix |
Attention is drawn to the following places, which may be of interest for search:
Cordierite honeycombs | C04B 38/0006 and subgroups |
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
Alkaline earth metal alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. cordierite ((Mg,Fe)2Al3(Si5AlO18)), beryl (Be3Al2(Si6O18)), micas such as margarite (CaAl2(Al2Si2)O10(OH)2), plagioclase feldspars such as anorthite (CaAl2Si2O8), zeolites such as chabazite (CaAl2Si4O12·6H2O) | |
Cordierite honeycombs containing a catalyst |
The cordierite honeycombs are normally classified in C04B 38/0006, but receive classification in C04B 35/195 as well, if specific details regarding the starting materials are given, or if the end-composition of the cordierite is specified, e.g. the presence of a certain secondary phase or the use of certain combinations of starting materials.
This place covers:
All silicate ceramics or ceramic mixtures containing a substantial amount of MgO, thus not containing MgO as an impurity
Materials having as largest fraction a forsterite phase but also containing a glass matrix, e.g. 80% forsterite and 20% glass matrix C03C
This place does not cover:
Magnesium alumino-silicates |
Attention is drawn to the following places, which may be of interest for search:
Magnesium silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. forsterite (Mg2SiO4) | |
Catalysts comprising silica and magnesia | |
Preparation of magnesium silicate powders, sols, gels, dispersions and their after-treatments |
This place covers:
All silicate ceramics or ceramic mixtures containing a substantial amount of CaO, thus not containing CaO as an impurity
Materials having as largest fraction a forsterite phase but also containing a glass matrix, e.g. 80% forsterite and 20% glass matrix C03C
This place does not cover:
Calcium alumino-silicates |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: alkaline-earth metal silicates, e.g. wollastonite | |
Calcium silicate based hydraulic cement | C04B 28/02 and subgroups |
Calcium silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. wollastonite (CaSiO3) | |
Preparation of alkaline earth metal silicate powders, sols, gels, dispersions and their after-treatments | |
Calcium silicates as compounding ingredient for polymers |
This place covers:
All oxidic ferrites, combinations between Fe2O3 and other oxides, such as FeO, ZnO, MnO, BaO, as well as Fe2O3 (hematite) itself
This place does not cover:
Metallic ferrite (Fe) | C22C 38/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: ferrites | |
Coating or impregnating ceramic substrates with ferrite | |
Ferrites as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. manganese ferrite (MnFe2O4) | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites | C01G 49/0018 and subgroups |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides iron, two or more other elements, with the exception of oxygen or hydrogen | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of iron | C09C 1/22 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt | C09K 11/60 and subgroups |
Recording by magnetisation or demagnetisation of a record carrier; Reproducing by magnetic means; Record carriers therefore: the pole pieces being ferrite | |
Recording by magnetisation or demagnetisation of a record carrier; Reproducing by magnetic means; Record carriers therefore: record carriers characterised by the selection of the material comprising one or more layers of magnetisable material homogeneously mixed with a bonding agent the magnetic material being a ferrite | G11B 5/70678 and subgroups |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of iron oxides or ferrites | |
Ferrite magnets | |
Hard magnetic material, e.g. ferrites | |
Soft magnetic material, e.g. ferrites | H01F 1/344 and subgroups |
Thin magnetic films, e.g. of one-domain structure made of ferrites | H01F 10/20 and subgroups |
Details of cathode ray tubes or electron beam tubes Electron beam control outside the vessel by magnetic fields Cores for field producing elements, e.g. ferrite | |
Loop aerials with a substantially uniform current distribution around the loop and having a directional radiation pattern in a plane perpendicular to the plane of the loop with ferrite rod or like elongated core | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
The effect of many metal oxide additives on the grain growth of ferrites is mentioned | document XP022314655, table 1 |
The sintered ferrite bodies are classified in C04B 35/00 as well as in H01F. Ferrite powders are classified in C01G 49/00, as well as H01F. The synthesis of ferrite powders is also classified in C04B 35/00 if the ferrite composition is a new composition or if the synthesis is preparatory to making a sintered body. More than one subgroup of C04B 35/26 can be attributed due to one ferrite composition.
In this place, the following terms or expressions are used with the meaning indicated:
Ferrite | Chemical compounds consisting of ceramic materials with iron (III) oxide (Fe2O3) as their principal component. Many of them are magnetic materials and they are used to make permanent magnets, ferrite cores for transformers, and in various other applications. Many ferrites are spinels with the formula AB2O4, where A and B represent various metal cations, usually including iron. Some ferrites have hexagonal crystal structure, e.g. barium ferrite BaO:6Fe2O3 or BaFe12O19. |
This place covers:
The metal ions can be part both of the main composition as additives to the main composition.
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead | |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing manganese or zinc, e.g. Mn-Zn ferrites | |
Other ferrites containing nickel, copper or cobalt | |
Other ferrites containing rare earth metals, e.g. rare earth ferrite garnets | |
Other ferrites containing alkaline earth metals or lead | |
Alkali oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Na2O, K2O | C04B 2235/3201 and subgroup |
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Manganese or rhenium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MnO | C04B 2235/3262 and subgroups |
Cobalt oxides, cobaltites or cobaltates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc cobaltite (ZnCo2O4) or bismuth cobaltate (BiCoO3) | C04B 2235/3275 and subgroup |
Nickel oxides, nickelates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. NiO | |
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroup |
Zinc oxides, zincates, cadmium oxides, cadmiates, mercury oxides, mercurates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. ZnO | |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one alkali metal | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing alkaline earth metal, magnesium or lead | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one rare earth metal, yttrium or scandium | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing zinc | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing manganese |
If the ferrite contains Mn/Zn/Ni/Cu/Co and rare earth and alkali/alkaline earth/lead both C04B 35/2608 and C04B 35/2641 are attributed. If it also contains both Mn/Zn and Ni/Cu/Co C04B 35/265 is attributed as well. Thus, C04B 35/2608, C04B 35/2641 and C04B 35/265 could be attributed to one and the same ferrite composition. If C04B 35/2608 is attributed for a certain ferrite, C04B 35/2658, C04B 35/2666, C04B 35/2675, C04B 35/2683 and C04B 35/2691 are not attributed for this ferrite composition. These classes could of course be attributed due to other ferrite compositions in the same document.
Since none of the individual metal ions of Mn/Zn/Ni/Cu/Co and rare earth and alkali/alkaline earth/lead necessarily needs to be present, when C04B 35/2608 is given, all metal ions present (except for Fe) need to be classified with symbols from C04B 2235/00.
This place covers:
The ferrite containing Mn, Zn, Ni, Cu or Co and also Li
Attention is drawn to the following places, which may be of interest for search:
Lithium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Li2O | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one alkali metal |
Since here the Li-ion necessarily needs to be present, the additional symbol (CCA) for Li (C04B 2235/3203) is not necessary
This place covers:
The ferrite containing Mn, Zn, Ni, Cu or Co and also Mg
Attention is drawn to the following places, which may be of interest for search:
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing alkaline earth metal, magnesium or lead |
This place covers:
Ferrites like barium hexaferrite, doped with Mn/Zn/Ni/Cu/Co.
Attention is drawn to the following places, which may be of interest for search:
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime | C04B 2235/3208 and subgroups |
Strontium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Barium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing alkaline earth metal, magnesium or lead | |
Soft magnetic material, e.g. Hexaferrites with decreased hardness or anisotropy, i.e. with increased permeability in the microwave (GHz) range | |
Thin magnetic films, e.g. of one-domain structure made of hexagonal ferrites |
This place covers:
The ferrite containing a rare earth like La, Nd, Ce and for instance Li, Na, K, Ba, Sr, Mg, Ca, W
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Other ferrites containing rare earth metals, e.g. rare earth ferrite garnets | |
Other ferrites containing alkaline earth metals or lead | |
Alkali oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Na2O, K2O | C04B 2235/3201 and subgroup |
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one alkali metal | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing alkaline earth metal, magnesium or lead | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one rare earth metal, yttrium or scandium |
If the ferrite contains Mn/Zn/Ni/Cu/Co and rare earth and alkali/alkaline earth/lead both C04B 35/2608 and C04B 35/2641 are attributed. If it also contains both Mn/Zn and Ni/Cu/Co C04B 35/265 is attributed as well. Thus, C04B 35/2608, C04B 35/2641 and C04B 35/265 could be attributed to one and the same ferrite composition. If C04B 35/2641 is attributed for a certain ferrite, C04B 35/2658, C04B 35/2666, C04B 35/2675, C04B 35/2683 and C04B 35/2691 are not attributed for this ferrite composition. These classes could of course be attributed due to other ferrite compositions in the same document.
This place covers:
The ferrite containing Mn or Zn and one of the group Ni, Cu, Co
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Other ferrites containing manganese or zinc, e.g. Mn-Zn ferrites | |
Other ferrites containing nickel, copper or cobalt | |
Manganese or rhenium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MnO | C04B 2235/3262 and subgroups |
Cobalt oxides, cobaltites or cobaltates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc cobaltite (ZnCo2O4) or bismuth cobaltate (BiCoO3) | C04B 2235/3275 and subgroup |
Nickel oxides, nickelates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. NiO | |
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroup |
Zinc oxides, zincates, cadmium oxides, cadmiates, mercury oxides, mercurates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. ZnO | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing zinc | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing manganese |
If the ferrite contains Mn/Zn/Ni/Cu/Co and rare earth and alkali/alkaline earth/lead both C04B 35/2608 and C04B 35/2641 are attributed. If it also contains both Mn/Zn and Ni/Cu/Co C04B 35/265 is attributed as well. Thus, C04B 35/2608, C04B 35/2641 and C04B 35/265 could be attributed to one and the same ferrite composition. If C04B 35/265 is attributed for a certain ferrite, C04B 35/2658, C04B 35/2666, C04B 35/2675, C04B 35/2683 and C04B 35/2691 are not attributed for this ferrite composition. These classes could of course be attributed due to other ferrite compositions in the same document.
This place covers:
The ferrite contains usually both Mn and Zn, the common Mn-Zn ferrite
This place does not cover:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt |
Attention is drawn to the following places, which may be of interest for search:
Manganese or rhenium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MnO | C04B 2235/3262 and subgroups |
Zinc oxides, zincates, cadmium oxides, cadmiates, mercury oxides, mercurates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. ZnO | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing zinc | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing manganese |
This place covers:
Ferrites containing Ni, Co, Cu, but not Zn or Mn
This place does not cover:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt |
Attention is drawn to the following places, which may be of interest for search:
Cobalt oxides, cobaltites or cobaltates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc cobaltite (ZnCo2O4) or bismuth cobaltate (BiCoO3) | C04B 2235/3275 and subgroup |
Nickel oxides, nickelates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. NiO | |
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroup |
This place covers:
Ferrites containing rare earth metal oxides such as La, Nd, Sm, but not alkaline earth metal oxides, Cu, Co, Zn, Ni, Mn
This place does not cover:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead |
Attention is drawn to the following places, which may be of interest for search:
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Garnet type symmetry | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one rare earth metal, yttrium or scandium |
This place covers:
Ferrites like barium hexaferrite.
This place does not cover:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead |
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. BeO | C04B 2235/3205 and subgroups |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing alkaline earth metal, magnesium or lead |
This place covers:
Ferrites containing alkali metal oxides but not rare earth metal oxides or oxides of Cu, Ni, Co, Mn, Zn
This place does not cover:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead |
Attention is drawn to the following places, which may be of interest for search:
Alkali oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Na2O, K2O | C04B 2235/3201 and subgroup |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites, containing one alkali metal |
This place covers:
Chromites and chromates. All ceramics containing as the largest phase mixed oxides of chromium with alkali metals, alkaline earth metals and rare earth metals, not containing other transition or post-transition metal oxides, or mixed oxides of chromium with other transition or post-transition metal oxides, in which the amount of chromium is larger than of any other transition or post-transition metal oxide, e.g. a mixture with titanium oxide, containing more Cr, e.g. Cr0.6Ti0.4O2.
This place does not cover:
Grain-sized refractory mixtures based on magnesia containing chromium oxide or chrome ore | C04B 35/047 and subgroups |
Grain-sized alumina-based refractories containing chromium oxide or chromium ore | |
Mixed oxides of chromium with other transition or post-transition metal oxides, in which there is at least one other transition or post-transition metal oxide in an amount larger than chromium, e.g. a mixture with titanium oxide, containing more Ti, e.g. Cr0.4Ti0.6O2. |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators | |
Chromium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Cr2O3 | C04B 2235/3241 and subgroup |
Chromates or chromites as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. aluminum chromate Al2(CrO4)3 or lanthanum strontium chromite (La1-xSrxCrO3) | |
Refractory metal oxide interlayer used for joining a ceramic with another substrate | |
Refractory metal oxide substrate joined with another substrate or being part of a ceramic laminate | |
Chromite containing catalysts | B01J 23/26, B01J 23/86 and subgroups |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides chromium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being chromates or bichromates | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: zinc chromate | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: lead chromate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten | C09K 11/68 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
Fuel cells containing Chromium complex oxides |
This place covers:
All mixed oxides in which alumina is mixed with alkali metal oxides, alkaline earth metal oxides or rare earth metal oxides.
This place does not cover:
Ceramics based on aluminate-silicate | C04B 33/00 and subgroups (clays) or C04B 35/18 and subgroups |
Ceramics based on beta-aluminas (MAl11O18 or LnAl12O19) |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: aluminates | |
Hydraulic aluminate cements | C04B 28/06 and subgroup, C04B 7/323 |
Ceramics based on alumina single oxide phase | C04B 35/10 and subgroups |
Coating or impregnating ceramic substrates with aluminate | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Aluminates other than alumino-silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. spinel (MgAl2O4) | |
Alumina or aluminate interlayer used for joining a ceramic with another substrate | |
Alumina or aluminate substrate joined with another substrate or being part of a ceramic laminate | |
Aluminate catalysts or catalysts carrier | |
Preparation of alkali metal aluminates powders | C01F 7/04 and subgroups |
Preparation of alkaline earth metal aluminates powders | C01F 7/16 and subgroups |
Purification of aluminates | C01F 7/47 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing refractory metal aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth aluminates | C09K 11/77062, C09K 11/77212, C09K 11/77342, C09K 11/77492, C09K 11/7758, C09K 11/7764, C09K 11/77742, C09K 11/77922 |
Devices characterised by the luminescent material |
This place covers:
Ceramics based on magnesium aluminate (MgOAl2O3 or MgAl2O4) having the spinel phase
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on magnesia single oxide phase | C04B 35/04 and subgroups |
Ceramics based on alumina single oxide phase | C04B 35/10 and subgroups |
Coating or impregnating ceramic substrates with spinels | |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Ceramics in general with the spinel symmetry | |
Catalysts comprising spinels |
If the class C04B 35/443 is given, C04B 2235/763 does not need to be given.
This place covers:
Ceramics based on inorganic phosphor-oxide compounds
This place does not cover:
Ceramics based on metal-phosphor compounds without oxygen, the phosphides | |
Ceramics having a phosphate binder | C04B 35/6306 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Phosphate cements | C04B 12/02 and subgroups |
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: phosphates, e.g. apatite | |
Making fibres based on phosphates | |
Coating or impregnating ceramic substrates with phosphates | C04B 41/5048, C04B 41/5092 (phosphate cements), C04B 41/67 |
Calcium phosphates, e.g. hydroxyapatite additives or secondary phases | |
Phosphates or phosphites (calcium phosphates C04B 2235/3212) as starting material for making ceramics, e.g. orthophosphate (PO43-), pyrophosphate (P2O74-), hypophosphite (H2PO2-), or present as secondary phase in the sintered ceramic | |
Materials for prostheses containing a phosphorus-containing compound, e.g. apatite | |
Phosphate catalysts | B01J 27/18 and subgroups, B01J 29/82 and subgroups |
Preparation of phosphates per se, e.g. phosphates powder, not preparative to making a phosphates ceramic | C01B 25/26 and subgroups, C01B 37/002 |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing phosphates | C09K 11/0855 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing phosphorus | C09K 11/70 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth phosphates | C09K 11/7464 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth phosphates | C09K 11/7709 and subgroups, C09K 11/7723 and subgroups, C09K 11/7737 and subgroups, C09K 11/7752 and subgroups, C09K 11/7777 and subgroups, C09K 11/7795 and subgroups |
Phosphate single crystals |
This place covers:
Precursor materials for ceramic superconductors and high critical-temperature superconductive materials characterised by the ceramic-forming technique or the ceramic composition based on cuprates.
Non superconductive ceramic copper oxides or solid solutions thereof with other oxides.
Single-crystals or homogeneous polycrystalline material with defined structure or crystallographic orientation characterised by the material or by the method: C30B
This place does not cover:
Mixed oxide of copper oxide and iron oxide: ferrite | C04B 35/2608 and subgroups, C04B 35/265, C04B 35/2666 |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: copper oxide or solid solutions thereof | |
Making fibres based on copper oxide | |
Coating or impregnating ceramic substrates with copper oxide ceramic material | C04B 41/5074 and subgroup |
Copper oxides, cuprates or oxide-forming salts thereof, e.g. CuO or Cu2O as additive for ceramics or as secondary phase | |
The preparation of copper compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides copper, two or more other elements, with the exception of oxygen or hydrogen | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing copper, silver or gold | C09K 11/58 and subgroups |
Ceramic superconductor Rope or cable materials | |
Superconductive conductors, cables, or transmission lines | H01B 12/00 and subgroups |
Superconducting magnets or coils | H01F 6/00 and subgroups |
Processes peculiar to the manufacture or treatment of composite superconductor filaments comprising copper oxide | H10N 60/0268 and subgroups |
Superconductors characterised by the material, containing copper oxide | H10N 60/857 and subgroup |
In patent documents, the following abbreviations are often used:
HTS or High-Tc | These abbreviations correspond to the term "high critical-temperature superconductor". |
Y-Ba-Cu-O | In patent literature this abbreviation is used for the general substance group, which includes e.g. the compounds Y1Ba2Cu3Ox or Y2Ba1Cu1O5 corresponding to the short cuts Y-123 or Y-211. |
Bi-Sr-Ca-Cu-O | In patent literature this abbreviation is used for the general substance group, which includes e.g. the compounds Bi2Sr2Ca2Cu3Ox or Bi2Sr2Ca1Cu2Ox corresponding to the short cuts Bi-2223 or Bi-2212. |
Hg-Ba-Ca-Cu-O | In patent literature this abbreviation is used for the general substance group, which includes e.g. the compound Hg1Ba2Ca2Cu3Ox corresponding to the short cut Hg-1223. |
Tl-Sr-Ca-Cu-O | In patent literature this abbreviation is used for the general substance group, which includes e.g. the compound Tl2Sr2Ca2Cu3Ox corresponding to the short cut Tl-2223. |
This place covers:
Ceramics based on yttrium, lanthanum or cerium oxide containing cuprates.
Attention is drawn to the following places, which may be of interest for search:
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Cuprates or oxide-forming salts thereof, as additive for ceramics or as secondary phase | |
Complex oxides based on rare earth copper oxide single crystals |
This place covers:
The compounds Y1Ba2Cu3Ox or Y2Ba1Cu1O5 corresponding to the short cuts Y-123 or Y-211
This place covers:
For instance ceramics based on the compound Tl2Sr2Ca2Cu3Ox corresponding to the short cut Tl-2223
Attention is drawn to the following places, which may be of interest for search:
Cuprates or oxide-forming salts thereof, as additive for ceramics or as secondary phase | |
Gallium oxides, gallates, indium oxides, indates, thallium oxides, thallates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc gallate (ZnGa2O4) |
This place covers:
The cuprate containing both thallium oxide and lead oxide
Attention is drawn to the following places, which may be of interest for search:
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) |
This place covers:
Ceramics based for instance on the compounds Bi2Sr2Ca2Cu3Ox or Bi2Sr2Ca1Cu2Ox corresponding to the short cuts Bi-2223 or Bi-2212.
Attention is drawn to the following places, which may be of interest for search:
Cuprates or oxide-forming salts thereof, as additive for ceramics or as secondary phase | |
Bismuth oxides, bismuthates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc bismuthate (Zn(BiO3)2) |
This place covers:
The cuprate containing both bismuth oxide and lead oxide
Attention is drawn to the following places, which may be of interest for search:
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) |
This place covers:
Ceramics based on the single metal oxide ZnO or Bi2O3. Mixed oxides of ZnO with gallium or indium oxide. Mixed oxides of ZnO with gallium or indium oxide and also tin oxide, containing more zinc oxide then tin oxide. Mixed oxides of alkali metal, alkaline metal oxide or rare earth metal oxide with bismuth oxide, the bismuthates.
This place does not cover:
Mixed oxide of zinc oxide and iron oxide: ferrite | C04B 35/2608 and subgroups, C04B 35/265, C04B 35/2658 |
Ceramics based on mixed oxides of bismuth with copper: cuprates | C04B 35/4521 and subgroup |
Ceramics based on mixed oxides of bismuth with titanium: bismuth titanate |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with zinc or bismuth oxides | |
Zinc oxides, zincates, cadmium oxides, cadmiates, mercury oxides, mercurates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. ZnO | |
Bismuth oxides, bismuthates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc bismuthate (Zn(BiO3)2) | |
The preparation of zinc compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 9/00 and subgroups |
The preparation of gallium, indium or thallium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 15/00 and subgroups |
The preparation of bismuth compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 29/00 and subgroups |
Transparent conductive oxide layers (TCO) being part of a multilayer coating on glass Layers comprising zinc oxide | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of bismuth and vanadium | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of zinc | C09C 1/04 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic Zn or Cd compounds | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth | C09K 11/74 and subgroups |
Obtaining zinc oxide | C22B 19/34 and subgroups |
Target materials for coating by Physical Vapour Deposition | |
Zinc oxide in machines or engines in general (F01) or machines for liquids ( F04) | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of zinc or cadmium oxide | |
Resistors, e.g. varistors based on ZnO | |
Fuel cells operating at high temperature, e.g. with stabilised ZrO2 electrolyte, the electrolyte consisting of oxides, the electrolyte containing bismuth oxide | |
Wideband gap semiconductor comprising zinc oxide, e.g. ZnO |
Bi13Mn13O40 is classified in C04B 35/016, Bi13Fe13O40 in C04B 35/26, Bi13Co13O40 in C04B 35/01, Bi13Mn6.5Fe6.5O40 in C04B 35/2658, Bi11Co7.5Cu7.5O40 is classified in C04B 35/01 and C04B 35/4521, Bi18.2Mn3.9Co3.9O40 is classified in C04B 35/016 and C04B 35/01. None of these compositions is classified in C04B 35/453
This place covers:
Ceramics based on the single oxide SnO2, or on mixed oxides of alkali metal, alkaline earth or rare earth metals with tin oxide. Ceramics based on mixed oxides of gallium or indium with tin, possibly also containing zinc, e.g. indium tin oxide (ITO) or indium tin zinc oxide (ITZO).
This place does not cover:
Ceramics based on mixed oxides of indium, tin and zinc containing more zinc than tin. |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with tin oxide | |
Tin oxides, stannates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g., indium tin oxide (ITO) | |
The preparation of tin compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 19/00 and subgroups |
Transparent conductive oxide layers (TCO) being part of a multilayer coating on glass Layers comprising indium tin oxide (ITO) | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead | C09K 11/66 and subgroups |
Thin film transistors having a semiconductor body comprising an oxide semiconductor material, e.g. zinc oxide, copper aluminium oxide, cadmium stannate | |
Transparent ITO electrodes |
This place covers:
Ceramics based on the single metal oxide phase TiO2 or on sub-oxides of titanium oxide, e.g. Ti2O3. Ceramics based on mixed metal oxides of titanium, the so-called titanates.
This place does not cover:
Ceramic compositions based on titanium oxides or titanates, containing also zirconium or hafnium oxides, zirconates or hafnates | C04B 35/49 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone Titanium oxide, e.g. titanates | |
Making fibres based on titanium oxide | |
Coating or impregnating ceramic substrates with titanium oxides or titanates | |
Titanium oxides or titanates, e.g. rutile or anatase as additive for making ceramics or as secondary phase in a ceramic | C04B 2235/3232 and subgroups |
Refractory metal oxide interlayer used for joining a ceramic with another substrate | |
Titania or titanate substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on titania or titanium oxide TiO | |
Catalysts or catalyst carriers comprising titanium; Oxides or hydroxides thereof | |
The preparation of titanium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 23/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of titanium | C09C 1/36 and subgroups |
Ceramic insulating or dielectric materials | |
Resistors, e.g. varistors, based on titanium oxide or titanates | |
Fixed capacitors containing a ceramic dielectric based on titanium oxides or titanates | |
Light-sensitive devices comprising an oxide semiconductor electrode comprising titanium oxide, e.g. TiO2 | |
Ceramic dielectric resonators | |
Wideband gap semiconductor comprising titanium oxide, e.g. TiO2 | |
Piezoelectric ceramics | |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate, the material containing titanium, e.g. TiO2 |
This place covers:
Ceramics based on mixed metal oxides of titanium, the so-called titanates.
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. zirconate-titanates such as PZT | C04B 35/49 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics or ceramic mixtures based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates containing also lead and also titanates | |
Titanates as additive for making ceramics or as secondary phase in a ceramic | C04B 2235/3234 and subgroup |
Zirconates or hafnates containing also titanium oxide or titanates as additive for making ceramics or as secondary phase in a ceramic | |
The preparation of titanium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides titanium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of titanate compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 23/003 and subgroups |
Single crystals of Titanates; Germanates; Molybdates; Tungstates | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient mainly consisting of perovskites, e.g. titanates | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of perovskites, e.g. titanates |
This place covers:
Ceramics based on mixed metal oxides of titanium with the alkaline earth metals Mg and/or Ca, e.g. magnesium titanate (MgTiO3) or calcium barium titanate with the formula Ca0.6Ba0.4TiO3
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth zirconate-titanates such as magnesium zirconate titanate |
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
Alkaline earth metal titanates as additive for making ceramics or as secondary phase in a ceramic | |
The preparation of alkaline earth metal titanate compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
Fixed capacitors containing a ceramic dielectric based on alkaline earth titanates | |
Insulating layers on semi-conductor bodies having a perovskite structure |
This place covers:
Ceramics based on mixed metal oxides of barium and titanium, containing more Ba than of any of the other alkaline earth metals, e.g. barium magnesium titanate containing more Ba than Mg.
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth zirconate-titanates such as barium zirconate titanate |
Attention is drawn to the following places, which may be of interest for search:
Barium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
Barium titanate normally has the perovskite structure. If the structure of the barium titanate material is not mentioned, it can normally be assumed it is a perovskite. This means that the head-class C04B 35/468 rarely needs to be used.
This place covers:
Ceramics based on mixed metal oxides of barium and titanium, containing more Ba than of any of the other alkaline earth metals, e.g. barium calcium titanate with the formula Ca0.4Ba0.6TiO3 Ceramics based on mixed metal oxides of titanium, the so-called titanates.
Attention is drawn to the following places, which may be of interest for search:
Ceramics having the perovskite structure, ABO3 | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
Barium titanate normally has the perovskite structure. If the structure of the barium titanate material is not mentioned, it can normally be assumed it is a perovskite. This means that the head-class C04B 35/468 rarely needs to be used.
This place covers:
Ceramics based on mixed metal oxides of barium and titanium, containing more Ba than of any of the other alkaline earth metals, and also containing some amount of Pb, e.g. as dopant
This place does not cover:
Lead titanate based ceramics | |
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth lead zirconate-titanates such as barium containing PZT |
Attention is drawn to the following places, which may be of interest for search:
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) |
This place covers:
Barium titanate normally has the perovskite structure. If the structure of the barium titanate material is not mentioned, it can normally be assumed it is a perovskite. Other barium titanate phases are BaTi4O9 and Ba2Ti9O20.
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth zirconate-titanates such as barium zirconate titanate |
This place covers:
Ceramics based on mixed metal oxides of barium and titanium, containing more Ba than of any of the other alkaline earth metals, and also containing some amount of Pb, e.g. as dopant
This place does not cover:
Lead titanate based ceramics | |
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth lead zirconate-titanates such as barium containing PZT |
Attention is drawn to the following places, which may be of interest for search:
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) |
Barium titanate normally has the perovskite structure. If the structure of the barium titanate material is not mentioned, it can normally be assumed it is a perovskite. Other barium titanate phases are not common.
This place covers:
Ceramics based on alkaline earth metal titanates, containing more Sr than of any other alkaline earth metal, e.g. Ba0.45Ca0.05Sr0.50TiO3
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. alkaline earth zirconate-titanates such as strontium zirconate-titanate | C04B 35/49 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Strontium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic |
This place covers:
Ceramics based on titanates, containing more Pb in the titanate phase than of any other metal ion, except for titanium, e.g. Al0.45Pb0.55TiO3
This place does not cover:
Barium titanate perovskite containing lead compounds based ceramic | |
Barium titanate containing lead compounds non-perovskite phase based ceramic | |
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. lead zirconate-titanates such PZT | C04B 35/491 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
Ceramic compositions for piezoelectric or electrostrictive devices |
This place covers:
Ceramics based on titanates, containing more Bi in the titanate phase than of any other metal ion, except for titanium, e.g. Pb0.3Al0.3Bi0.4TiO3
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. bismuth zirconate-titanate |
Attention is drawn to the following places, which may be of interest for search:
Bismuth oxides, bismuthates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zinc bismuthate (Zn(BiO3)2) |
This place covers:
Ceramics based on titanates, containing more Al in the titanate phase than of any other metal ion, except for titanium, e.g. Al0.3Pb0.2Bi0.2Ba0.2La0.1TiO3
This place does not cover:
Zirconium oxide based ceramics containing also titanium oxides or titanates, e.g. aluminium zirconate-titanate |
Attention is drawn to the following places, which may be of interest for search:
Ceramic honeycombs, e.g. aluminum titanate honeycombs | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Honeycomb filter for exhaust apparatus | |
Aluminium titanate in machines or engines in general (F01) or machines for liquids ( F04) |
This place covers:
All ceramic materials having a zirconia phase or zirconate phase as the largest fraction, e.g. yttria-stabilised-zirconia, monoclinic zirconia, lanthanum zirconate
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: zirconium oxide | |
Making fibres based on zirconium oxide | |
Coating or impregnating ceramic substrates with zirconium oxides or zirconates, hafnium oxides or hafnates | C04B 41/5042 and subgroup |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
Refractory oxide interlayer used for joining a ceramic with another substrate | |
Zirconia, hafnia, zirconate or hafnate substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on zirconia or zirconium oxide | |
Materials for prostheses based on hafnia or hafnium oxide | |
Catalysts comprising Zirconium or hafnium; Oxides or hydroxides thereof | |
The preparation of zirconium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 25/00 and subgroups |
Zirconium oxide in machines or engines in general (F01) or machines for liquids ( F04) | |
Fixed capacitors containing a ceramic dielectric based on zirconium oxides or zirconates | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate, the material containing hafnium, e.g. HfO2 | H10P 14/69392, H10P 14/6909, H10P 14/69473 (from the gas phase) |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate, the material containing zirconium, e.g. ZrO2 | H10P 14/69395, H10P 14/6912, H10P 14/69476 (from the gas phase) |
The head group C04B 35/48 only contains non-refractories of zirconia and/or zirconate with large grain sizes of at least 0,1 mm.
In patent documents, the following abbreviations are often used:
YSZ | Yttria-stabilised zirconia |
3Y-TZP | Zirconia partially stabilised in the tetragonal phase by 3 mol% yttria |
This place covers:
Zirconates containing silica, such as zircon (ZrSiO4), zirconia ceramics containing a silica or silicate binder, zirconia refractories containing quartz
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: zircon | |
Alumina based refractories containing zircon | |
Alumina based refractories containing zircon, made by melt-casting | |
Zirconates or hafnates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. zircon (ZrSiO4) | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic |
Documents that are classified in C04B 35/481 can also be classified in other sub-groups of C04B 35/48, e.g. a zirconia refractory containing quartz is classified in both C04B 35/481 and C04B 35/482. A fine ceramic containing as major phase zircon and having at least one secondary phase is also classified in C04B 35/488. Classification in C04B 35/486 is not necessary, if the silica-containing zirconia ceramic is a fine ceramic with grain sizes below 100 microns. In practice C04B 35/482, C04B 35/484, C04B 35/488 and C04B 35/4885 are used in combination with C04B35/48A.
Zircon is in principle the only silicate that is not classified as a silicate, but is classified according to the other metal cation(s) present in the silicate.
This place covers:
Zirconia based refractories having large grains, the majority larger than 100 microns
Attention is drawn to the following places, which may be of interest for search:
Grain-sized magnesia-based refractories | C04B 35/043 and subgroups |
Grain-sized alumina-based refractories | C04B 35/101 and subgroups |
Grain-sized alumina-based refractories, containing zirconia or zircon | |
Grain-sized titania-based refractories | C04B 35/46 and C04B 35/66 |
Grain-sized silicon carbide-based refractories | C04B 35/565 and subgroups, and C04B45/66 |
Monolithic refractories and refractory mortars | |
Using particles larger than 100 microns for making the ceramic | |
Bimodal, multi-modal or multi-fraction particle size distribution | |
Compositions of refractory mould or core materials; Grain structures thereof | B22C 1/00 and subgroups |
Abrasive particles per se obtained by division of a mass agglomerated by sintering |
This place covers:
Refractories that are used directly after melting, either in particle or bulk form, as well as fused refractory that is sintered before use as refractory
Attention is drawn to the following places, which may be of interest for search:
Clay wares made by methods involving melting, fusion or softening | |
Magnesia-based refractories made by fusion casting | C04B 35/05 and subgroup |
Alumina-based refractories made by fusion casting | C04B 35/107 and subgroup |
Alumina-based refractories made by fusion casting, containing zirconia or zircon | |
Fusing to make ceramic particles in general | |
Refractories in general made by fusion casting | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
This place covers:
All ceramic materials having a zirconia phase or zirconate phase as the largest fraction, e.g. yttria-stabilised-zirconia, monoclinic zirconia, lanthanum zirconate, where the major phase has an average grain size of below 100 micron
Attention is drawn to the following places, which may be of interest for search:
Using particles of size 1-100 microns for making the ceramic | |
Protective coatings for engine blades | |
Fuel cells operating at high temperature, e.g. with stabilised ZrO2 electrolyte, the electrolyte consisting of oxides, the electrolyte containing zirconium oxide |
This place covers:
All ceramic materials having a zirconia phase or zirconate phase as the largest fraction, e.g. yttria-stabilised-zirconia, monoclinic zirconia, lanthanum zirconate, but containing also at least one secondary phase, which is not a grain boundary phase. This secondary phase normally is another ceramic phase, but could also be a metallic non-continuous phase. The composite can also be a mixture of a zirconia and a zirconate phase or of two different zirconate phases.
This place does not cover:
Zirconia refractories containing a secondary phase | |
Mixtures of different zirconia phases, e.g. a mixture of cubic and tetragonal zirconia or a mixture of tetragonal and monoclinic zirconia |
Attention is drawn to the following places, which may be of interest for search:
Zirconia ceramics containing shaped metallic materials, e.g. metallic fibers | C04B 35/74 and subgroup |
Zirconia ceramics containing ceramic fibers, whiskers or platelets, e.g. an zirconia particle matrix containing alumina fibers or alumina platelets | |
Ceramics containing one or more secondary phases | C04B 2235/80 and subgroups |
If the secondary phase is a ceramic fiber, whisker, platelet or similarly shaped ceramic particle, both C04B 35/80 and C04B 35/488 are given. The same logic applies to C04B 35/488 and C04B 35/74.
The secondary phases are indicated with symbols from C04B 2235/32-C04B 2235/428. The symbol C04B 2235/80 does not need to be used, since the class itself already indicates that secondary phases are present.
This place covers:
All sintered zirconia ceramics that contain at least one secondary alumina phase, where this secondary alumina phase is not a grain boundary phase
This place does not cover:
Zirconia refractories containing an alumina secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Alumina-based ceramics containing a zirconia secondary phase | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
If the amount of zirconia phase is larger than the amount of alumina phase, C04B 35/4885 is given, if the amounts are equal, e.g. C04B40/40, then both C04B 35/119 and C04B 35/4885 are given.
In patent documents, the following abbreviations are often used:
ZTA | Zirconia toughened alumina |
ATZ | Alumina toughened zirconia |
This place covers:
Titanium-zirconates, zirconium-titanates, titanate-zirconates, for instance barium zirconate-titanate, mixed oxides containing at least zirconia and titania.
Attention is drawn to the following places, which may be of interest for search:
Titanates | C04B 35/462 and subgroups |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
Titanate as starting material for making ceramics or as secondary phase of a sintered ceramic, not containing zirconium, e.g. aluminium titanate (Al2TiO5) or mixed niobate-titanates | C04B 2235/3234 and subgroup. |
Zirconates or hafnates containing also titanium oxide or titanates as starting material for making ceramics or as secondary phase in a ceramic, e.g. lead zirconate titanate (PZT, PbTi1-xZrxO3). | |
Ceramic insulating or dielectric materials | |
Resistors, e.g. varistors, based on metal oxides | |
Fixed capacitors containing a ceramic dielectric based on zirconium oxides containing also titanates | |
Ceramic dielectric resonators | |
Piezoelectric ceramics |
If the amount of ZrO2 is quite small, e.g. BaTiO3 with only 1 wt% of zirconia dopant, then both C04B 35/49 and C04B 35/4682 are given.
This place covers:
Lead zirconate titanate, doped possibly with other elements such as La.
Attention is drawn to the following places, which may be of interest for search:
Lead titanate based ceramics | |
Lead zirconate based ceramics | |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) | |
Ceramic probes, e.g. lead zirconate titanate (PZT) probes | |
Piezoelectric devices; Electrostrictive devices; Magnetostrictive devices; Processes or apparatus peculiar to the manufacture or treatment thereof or of parts thereof; Details thereof of devices of ceramic compositions | |
Insulating layers on semi-conductor bodies having a perovskite structure |
These materials normally have a perovskite structure. C04B 2235/768 can be added to indicate the presence of a perovskite structure.
In patent documents, the following abbreviations are often used:
PLZT | Lead zirconate titanate doped with lanthanum |
This place covers:
PZT doped for instance with Mg, Nb, Ni or other elements that take the B position in the ABO3 perovskite structure of PZT, while the A-position is taken by Pb
Attention is drawn to the following places, which may be of interest for search:
Lead titanate | |
Lead niobate titanate (zirconate) | |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Niobates or tantalates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver niobate (AgNbO3) |
These materials normally have a perovskite structure, ABO3. C04B 2235/768 can be added to indicate the presence of a perovskite structure. If there is any element at the B-position that is present in an amount larger than Zr and Ti together, then the material is classified in the class of this element, e.g. PbNb0.4Ti0.3Zr0.3 is classified in C04B 35/493, but PbNb0.55Ti0.3Zr0.15 is classified in C04B 35/499. PbAl0.4Ti0.1Zr0.5 is classified in C04B 35/493, but PbAl0.55Ti0.1Zr0.35 is classified in C04B 35/44. PbNb0.4Mg0.05Ti0.1Zr0.45 is also classified in C04B 35/493, since Ti and Zr together form the largest fraction of B-atoms. PbNb0.3W0.25Zr0.4Ti0.05 is classified in C04B 35/499 though, since Nb and W together form a larger fraction than Zr and Ti together.
This place covers:
Ceramics based on the single metal oxide phase Nb2O5, Ta2O5, MoOx, WOx, V2O5 or on sub-oxides such as niobium suboxide. Ceramics based on mixed metal oxides of V, Nb, Ta, Mo or W.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on titanium oxide or titanates | C04B 35/46 and subgroups |
Ceramics based on titanium oxide or titanates containing also zirconium or hafnium oxides, zirconates or hafnates | C04B 35/49 and subgroups |
Coating or impregnating ceramic substrates with niobium oxides or niobates | |
Vanadium oxides, vanadates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. magnesium vanadate (Mg2V2O7). | |
Niobium or tantalum oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Nb2O5 or Ta2O5 | C04B 2235/3251 and subgroups |
Molybdenum oxides, molybdates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. cadmium molybdate (CdMoO4) | |
Tungsten oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. scheelite (CaWO4) | C04B 2235/3258 and subgroup |
Refractory metal oxide interlayer used for joining a ceramic with another substrate | |
Refractory metal oxide substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on tantalum oxide | |
Catalysts comprising metals or metal oxides or hydroxides of vanadium | |
Catalysts comprising metals or metal oxides or hydroxides of molybdenum | |
Catalysts comprising metals or metal oxides or hydroxides of tungsten | |
The preparation of vanadium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 31/00 and subgroups |
The preparation of niobium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 33/00 and subgroups |
The preparation of tantalum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 35/00 and subgroups/ |
The preparation of molybdenum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 39/00 and subgroups |
The preparation of tungsten compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 41/00 and subgroups/ |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of molybdenum | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of bismuth and vanadium | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten | C09K 11/68 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium | C09K 11/69 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
Single crystals of Niobates; Vanadates; Tantalates | |
Single crystals of Titanates; Germanates; Molybdates; Tungstates | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having positive temperature coefficient mainly consisting of Vanadium oxides or oxidic compounds, e.g. VOx | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of Vanadium oxides or oxidic compounds, e.g. VOx | |
Fixed capacitors containing a ceramic dielectric based on niobium or tungsten, tantalum oxides or niobates, tantalates | H01G 4/1254 and subgroup |
Details of surface acoustic wave devices of lithium niobate or lithium-tantalate substrates | |
Ceramic compositions for piezoelectric or electrostrictive devices | |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate, the material containing tantalum, e.g. Ta2O5 | H10P 14/69393, H10P 14/691, H10P 14/69474 (from the gas phase) |
Materials for prostheses based on niobium oxide | K61F2/00A6B2N |
This place covers:
Lead niobate (PbNbO3), tantalate, etc., possibly doped with other elements such as Mg, Ni, Zr, Fe
Attention is drawn to the following places, which may be of interest for search:
Lead titanate | |
Lead zirconate | C04B 35/486 and subgroups |
Lead titanate zirconate | C04B 35/491 and subgroup |
Lead oxides, plumbates or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver plumbate (Ag5Pb2O6) |
This place covers:
PZT-like material for instance with large amount of Nb, more than the amount of Ti and Zr together.
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with niobium oxides or niobates | |
Titanate as starting material for making ceramics or as secondary phase of a sintered ceramic, not containing zirconium, e.g. aluminium titanate (Al2TiO5) or mixed niobate-titanates | C04B 2235/3234 and subgroup |
The preparation of vanadium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides vanadium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of niobium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides niobium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of tantalum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides tantalum, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of molybdenum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides molybdenum, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of tungsten compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides tungsten, two or more other elements, with the exception of oxygen or hydrogen |
These materials normally have a perovskite structure, ABO3. C04B 2235/768 can be added to indicate the presence of a perovskite structure. If the combined amount of Nb/Ta/W/Mo/V is lower than the combined amount of Zr and Ti, then the material is classified in C04B 35/00-C04B 35/493, e.g. PbNb0.4Ti0.3Zr0.3 is classified in C04B 35/493, but PbNb0.55Ti0.3Zr0.15 is classified in C04B 35/499. PbAl0.4Ti0.1Zr0.5 is classified in C04B 35/493, but PbAl0.55Ti0.1Zr0.35 is classified in C04B 35/44. PbNb0.4Mg0.05Ti0.1Zr0.45 is also classified in C04B 35/493, since Ti and Zr together form the largest fraction of B-atoms. PbNb0.3W0.25Zr0.4Ti0.05 is classified in C04B 35/499 though, since Nb and W together form a larger fraction than Zr and Ti together.
This place covers:
Ceramic materials containing as the largest fraction a phase consisting out of rare earth oxides or out of mixtures of rare earth oxides with alkali metals or alkaline earth metals, e.g. gadolinium cerate, GdxCe1-xO3, barium cerate, BaCeO3, magnesium lanthanate, MgLaO3, yttrium scandium oxide, YScOx (also classified C04B 35/505). Ceramic materials containing a mixture of rare earth metals and zirconia and/or hafnia, where the amount of rare earth metals is higher than the amount of zirconia, e.g. Ce0.3La0.3Zr0.4Ox.
This place does not cover:
Mixed oxides of rare earth metals with silica without alumina | |
Mixed oxides of rare earth metals with both alumina and silica | |
Mixed oxides of rare earth metals with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2641, C04B 35/2675 |
Mixed oxides of rare earth metals with chromium oxide, e.g. lanthanum chromites | |
Mixed oxides of rare earth metals with alumina, without silica, e.g. scandium aluminate | |
Rare earth phosphates | |
Mixed oxides of rare earth metals with copper oxide, e.g. superconducting LaBa-cuprate | C04B 35/4504 and subgroup |
Mixed oxides of rare earth metals with zinc oxide and/or bismuth oxide, e.g. dysprosium bismuthate | |
Mixed oxides of rare earth metals with tin oxide, e.g. neodymium stannate | |
Mixed oxides of rare earth metals with titanium oxides, such as lanthanum titanate or cerium titanate | |
Mixed oxides of rare earth metals with zirconium oxide, e.g. cerium zirconate, containing more Zr than rare earth metals | C04B 35/48 and subgroups |
Mixed oxides of rare earth metals with zirconium oxide and titanium oxide, e.g. ytterbium titanate zirconate (YbTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of rare earth metals with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. erbium tantalum niobate (ErNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Ceramics based on non-oxide rare earth compounds | |
Rare earth oxide interlayer used for joining a ceramic with another substrate |
Attention is drawn to the following places, which may be of interest for search:
Rare earth non-oxide ceramics | |
Coating or impregnating ceramic substrates with rare earth oxides | |
Rare earth oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Sc2O3, Lu2O3, Nd2O3 | |
Catalysts comprising metals or metal oxides or hydroxides of rare earths | |
The preparation of rare earth compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01F 17/00 and subgroups |
Luminescent materials containing rare earth metals | C09K 11/77 and subgroups, C09K 11/0822 |
Shades containing photoluminescent material | |
Refractors containing photoluminescent material | |
Reflectors containing photoluminescent material | |
Elements containing photoluminescent material distinct from or spaced from the light source and subgroups | |
Elements with provision for controlling the spectral properties or intensity containing photoluminescent material | |
Scintillation detectors | |
Luminescent screens | |
Fuel cells operating at high temperature, e.g. with stabilised ZrO2 electrolyte, the electrolyte consisting of oxides, the electrolyte containing cerium oxide | |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate, the material containing at least one rare earth metal element, e.g. oxides of lanthanides, scandium or yttrium |
If any of cerium or lanthanum oxide is present, C04B 2235/3229 (Ce) or C04B 2235/3227 (La) is used. C04B 2235/3224 does not need to be given, if C04B 35/50 is given for a certain composition.
In this place, the following terms or expressions are used with the meaning indicated:
Rare earth oxides | The oxides of scandium (Sc), yttrium (Y), lutetium (Lu), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) |
This place covers:
Ceramic materials containing as the largest fraction a phase consisting out of yttria or out of mixtures of yttria with other rare earth oxides, where yttria forms the largest fraction, e.g. yttrium scandium oxide, YScOx (also classified in C04B 35/50). Ceramic materials containing as the largest fraction a phase that is a mixture of yttria with alkali metals or alkaline earth metals. Ceramic materials containing a mixture of yttria and zirconia and/or hafnia, where the amount of yttria is higher than the amount of zirconia, e.g. Y0.6Zr0.4Ox.
This place does not cover:
Mixed oxides of yttrium with silica without alumina | |
Mixed oxides of yttrium with both alumina and silica | |
Mixed oxides of yttrium with iron oxides and possible other metal oxides, e.g. ferrites | C04B 35/2608 and subgroups, C04B 35/2641, C04B 35/2675 |
Mixed oxides of yttrium with chromium oxide, e.g. chromites | |
Mixed oxides of yttrium with alumina, without silica, e.g. yttrium aluminium garnet (YAG, Y3Al5O12) | |
Yttrium phosphates | |
Mixed oxides of yttrium with copper oxide, e.g. superconducting LaBa-cuprate | C04B 35/4504 and subgroup |
Mixed oxides of yttrium with zinc oxide and/or bismuth oxide | |
Mixed oxides of yttrium with tin oxide | |
Mixed oxides of yttrium with titanium oxides | |
Mixed oxides of yttrium with zirconium oxide, containing more Zr than yttrium, e.g. YSZ, yttria-stabilised-zirconia | C04B 35/48 and subgroups |
Mixed oxides of yttrium with zirconium oxide and titanium oxide | C04B 35/49 and subgroups |
Mixed oxides of yttrium with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide | C04B 35/495 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Rare earth non-oxide ceramics | |
Yttrium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic |
This place covers:
Ceramics having as the largest fraction an oxide based on actinides, e.g. uranium oxide
This place does not cover:
Non-oxide actinide ceramics, e.g. uranium carbide |
Attention is drawn to the following places, which may be of interest for search:
Actinide oxides, mixed metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Catalysts comprising metals or metal oxides or hydroxides of actinides | |
Compounds of thorium | |
The preparation of uranium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 43/00 and subgroups |
The preparation of transuranic element compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 56/00 and subgroups |
Ceramic nuclear fuel materials | G21C 3/62 and subgroups |
This place covers:
Ceramics having as the largest fraction a non-oxide material, e.g. a carbide, nitride, boride, silicide, fluoride, sulphide, selenide.
This place does not cover:
Non-oxide ceramics having a metallic binder | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Making fibres based on non-oxide ceramic material | |
Ceramic products containing macroscopic reinforcing agents containing non-metallic materials (oxides and non-oxides only) such as fibres, filaments, whiskers, platelets, or the like | |
Coating or impregnating ceramic substrates with non-oxide ceramics | |
Non-oxides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/38 and subgroups |
Non-oxide interlayer used for joining a ceramic with another substrate | C04B 2237/08 and subgroup |
Non-oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/36 and subgroups |
Non-oxide glass compositions, e.g. binary or ternary halides, sulphides or nitrides of germanium, selenium or tellurium | C03C 3/32, C03B 2201/80 and subgroups |
Non-oxide coatings on glass | C03C 17/3429 and subgroups |
Non-oxide ceramics in MACHINES OR ENGINES IN GENERAL (F01) OR machines for liquids ( F04) | F05C 2203/0804 and subgroups |
This place covers:
Sintered ceramics having as the largest fraction a chloride, bromide or iodide phase, or a mixture of these phases
This place does not cover:
Starting powder mixtures based on halogenides used to make ceramics |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with salts or salty compositions: containing halogen in the anion | |
Halogens per se | C01B 7/00, C01B 9/00, C01B 11/00 and subgroups |
Halides of sodium, potassium or alkali metals in general | C01D 3/00 and subgroups |
Halide glasses other than fluoride glasses, i.e. Cl, Br or I glasses, e.g. AgCl-AgBr "glass" |
This place covers:
Sintered ceramics having as the largest fraction a phosphide phase. Phosphides are metal-phosphor compounds that do not contain oxygen.
This place does not cover:
Phosphate ceramics, metal-phosphor-oxygen compounds |
Attention is drawn to the following places, which may be of interest for search:
Metal salts chosen for the nature of the anions as starting material for making ceramics, e.g. phosphides, hydrides, acetylacetonate, hydroxides, or present as secondary phase in the sintered ceramic | C04B 2235/44 and subgroups |
Preparation of phosphides per se, e.g. phosphide powder, not preparative to making a phosphide ceramic | C01B 25/08 and subgroups |
Boride or phosphide coating on glass | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing phosphides | |
Single crystals based on gallium phosphide | |
Phosphides used as active substance in electrodes for fuel cells and batteries | |
Forming inorganic semiconducting materials on a substrate, the substrate being a phosphide |
This place covers:
Sintered ceramics having as the largest fraction a rare earth non-oxide phase, e.g., a lanthanum carbide, yttrium nitride, cerium boride, scandium silicide, dysprosium fluoride
Attention is drawn to the following places, which may be of interest for search:
Rare earth oxide based ceramics | |
Yttrium oxide based ceramics | |
Ceramics or ceramic mixtures based on carbides, e.g. rare earth carbides | |
Ceramics or ceramic mixtures based on nitrides, e.g. rare earth nitrides | |
Ceramics or ceramic mixtures based on borides, e.g. rare earth borides | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rare earth boride | |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rare earth carbide | |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2), rare earth nitride | |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with one or more rare earth metals |
The documents classified in this class are also classified in the class of the specific type of non oxide, e.g. lanthanum carbide is classified in C04B 35/5156 and C04B 35/56, cerium nitride is classified in C04B 35/5156 and C04B 35/58.
This place covers:
The synthesis of actinide carbides, nitrides, borides, silicides, fluorides, sulphides, selenides
Attention is drawn to the following places, which may be of interest for search:
Actinide oxide based ceramics | |
Ceramics or ceramic mixtures based on carbides, e.g. actinide carbides | |
Ceramics or ceramic mixtures based on nitrides, e.g. actinide nitrides | |
Ceramics or ceramic mixtures based on borides, e.g. actinide borides | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. actinide boride | |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. actinide carbide | |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2), actinide nitride | |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi, U, Pt | C04B 2235/40 and subgroups |
The preparation of nitride powders, with one or more actinides, e.g. UN, PuN | |
The preparation of actinide carbide powders | |
Ceramic nuclear fuel materials | G21C 3/62 and subgroups |
The documents classified in this class are also classified in the class of the specific type of non oxide, e.g. uranium carbide is classified in C04B 35/5158 and C04B 35/56, plutonium nitride is classified in C04B 35/5158 and C04B 35/58.
This place covers:
All inorganic objects containing as largest fraction an inorganic carbon phase or carbon-based mixtures used for making carbon-based bodies or other ceramic objects, e.g. sintered carbon electrodes, characterised by their composition or their synthesis
This place does not cover:
Ceramics or ceramic mixtures based on carbides | C04B 35/56 and subgroups |
A carbon-based matrix containing carbon fibers | |
Diamond bodies containing a metallic binder | |
Carbon electrodes used in electrolytic capacitors, rectifiers, detectors, switching devices, light-sensitive or temperature-sensitive devices | H01G 9/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Oxide-based ceramics or ceramic mixtures in general containing carbon | |
Alumina-based refractories containing carbon | |
Ceramic powders coated with carbon | |
Ceramic fibers coated with carbon | |
Ceramics or ceramic mixtures containing carbon fibers or carbon whiskers | C04B 35/80 and subgroups and C04B 2235/5248 |
Ceramics or ceramic mixtures containing carbon nanotubes | C04B 35/80 and subgroups and C04B 2235/5288 |
Coating or impregnating a ceramic substrates with carbon | C04B 41/5001 and subgroups |
Carbon additives for ceramics | C04B 2235/422 and subgroups |
Organics compounds becoming part of a ceramic after heat-treatment, e.g. phenol resins | C04B 2235/48 and subgroups |
Fibrous carbon additives for ceramics | |
Carbon nanotube additives for ceramics | |
Ceramics or ceramic mixtures containing carbon as an impurity | |
Carbon interlayer used for joining a ceramic with another substrate | |
Carbon substrate joined with another substrate or being part of a ceramic laminate | |
Carbon materials for grafts or prostheses or for coating grafts or prostheses | |
Carbon-based inorganic membranes | |
Presses for the formation of diamonds or boronitride | B01J 3/065 and subgroup |
Catalysts comprising carbon | B01J 21/18, and subgroup, C07C 2521/18 |
High pressure synthesis of diamond | |
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: non-consumable electrodes; C-electrode | |
Carbon electrodes for use in soldering, welding, or cutting | |
The preparation of carbon powders per se, not preparative to the making of carbon ceramics | C01B 32/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: carbon | C09C 1/44 and subgroups |
Electrodes for electrolytic processes based on carbon | |
Carbon ceramics in machines or engines in general (F01) or machines for liquids ( F04) | |
Constructions of heat-exchange apparatus characterised by the selection of particular materials, of carbon, e.g. graphite | |
Carbonic moderators in nuclear reactors | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of Carbon or carbides | |
Field emission cathodes common to discharge tubes: carbon type | H01J 2201/30453 and subgroups |
Field emission cathodes common to electron emission display panels: carbon type | H01J 2329/0444 and subgroups |
Electrodes based on carbonaceous material, e.g. graphite-intercalation compounds or CFx for fuel cells and batteries | H01M 4/133 , H01M 4/1393, H01M 4/583 and subgroup, H01M 4/663 |
Carbon-based electrodes for fuel cells and batteries | |
Ohmic-resistance heating, heater elements characterised by the composition or nature of the conductive material being carbon only, e.g. carbon black, graphite | H05B 3/145, C03B 2205/63 (for fiber drawing) |
Electrodes mainly consisting of carbon for heating by electric discharge | H05B 7/085 and subgroup |
Carbon-based electrodes for electric arc lamps | H05B 31/08 and subgroups |
Forming inorganic semiconducting materials on a substrate, the substrate being carbon, e.g. diamond-like carbon |
The carbonaceous additives used for making the carbon-based body are further indicated with the codes C04B 2235/424 (carbon black), C04B 2235/425 (graphite) and C04B 2235/427 (diamond).
Diamond based composites are classified normally in C04B 35/52 rather than in C04B 35/528.
In patent documents, the following abbreviations are often used:
C/C | Carbon fibres in a carbon matrix |
This place covers:
The carbonisable material normally is carbonised through a heat treatment. The end-product contains mainly carbon phase.
This place does not cover:
Porous carbon is infiltrated with Si-containing polymer that is carbonised to form a product containing mainly SiC | |
Impregnation of a carbon product with Si in order to form SiC | |
Porous carbon is infiltrated with Si-and N-containing polymer that is carbonised to form a product containing mainly Si3N4 | |
Impregnation of a fibrous carbon product with a carbonisable material |
Attention is drawn to the following places, which may be of interest for search:
Impregnation of carbon products with materials that lead to the formation of other phases than carbon, where one of these other phases forms the largest fraction of the end-product (thus the end product does not have carbon as largest fraction anymore) | Classification is in the class of this largest fraction, e.g. impregnation with alumina to such an extent that the end-product contains more alumina than carbon leads to the class C04B 35/117 (alumina composites) and the code C04B 2235/422 (carbon additive or secondary phase in the end-product) |
The synthesis and properties of porous carbon bodies | C04B 38/00 and subgroups |
Porous mortars, concrete, artificial stone or ceramic ware obtained by a chemical conversion or reaction other than those relating to the setting or hardening of cement-like material or to the formation of a sol or a gel, e.g. by carbonising or pyrolysing preformed cellular materials based on polymers, organo-metallic or organo-silicon precursors | C04B 38/0022 and subgroups |
Impregnation of carbon products with materials that lead to the formation of other phases than carbon, where none of these other phases form the largest fraction of the end-product | C04B 41/00 and subgroups |
Materials with friction-reduced moving parts, e.g. ceramics lubricated by impregnation with carbon | |
Gas infiltration of green bodies or pre-forms | |
Liquid infiltration of green bodies or pre-forms | |
Impregnated carbon catalyst carriers | B01J 21/18, and subgroup, C07C 2521/18 |
Impregnation of carbon electrodes |
The symbols C04B 2235/614 and C04B 2235/616 are used in combination with C04B 35/521 to indicate whether the infiltration is through gas or liquid, respectively.
This place covers:
All shaped products or mixtures for making a shaped product that have graphite as the largest fraction
This place does not cover:
All shaped products or mixtures for making a shaped product that have expanded graphite as the largest fraction |
Attention is drawn to the following places, which may be of interest for search:
Use of graphite as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of graphite specially adapted to enhance their filling properties in mortars, concrete or artificial stone | |
Creating porosity in ceramic products by burning out graphite | |
Graphite as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Graphite materials for grafts or prostheses | |
Graphite reactor vessels | |
The preparation and after-treatment of graphite powders | C01B 32/20 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: graphite | |
Sliding surface consisting mainly of graphite |
This place covers:
Carbon containing polymers are carbonised, leading to a product that has as largest fraction a carbon phase, e.g. carbonising a shaped phenol resin
This place does not cover:
Mixtures of polymer precursors and carbon particles, where the amount of carbon particles is larger than the amount of polymer precursors | |
Carbon and silicon containing polymers are carbonised, leading to a product that has as largest fraction a silicon carbide phase, e.g. carbonising a shaped polysilane resin | |
Carbon, silicon and nitrogen containing polymers are carbonised, leading to a product that has as largest fraction a silicon nitride phase, e.g. carbonising a shaped polysilazane resin |
Attention is drawn to the following places, which may be of interest for search:
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
This place covers:
The shaping of carbon particles into a compact and possible further densification through heat treatment
This place does not cover:
The shaping of graphite particles into a compact | C04B 35/522, C04B 35/536 (expanded graphite) |
This place covers:
The shaping of carbon particles into a compact and possible further densification through heat treatment, whereby binders such as pitch, tar, phenolic resin, etc., all possible binders from the range C04B 35/632-C04B 35/6365 can be used, as long as the further (heat) treatment is in non-oxidising atmosphere.
This place does not cover:
Carbon shaped bodies where the binder is not added to starting powder mixture, but is impregnated or infiltrated into an already shaped carbonaceous body | |
Mixtures of carbon particles with carbonisable binder, where the amount of carbonisable binder is larger than the amount of carbon particles | C04B 35/524 and C04B 2235/422 (carbon particles) |
Attention is drawn to the following places, which may be of interest for search:
Organics compounds becoming part of a ceramic after heat-treatment | C04B 2235/48 and subgroups |
Preparation of active carbon using carbonaceous precursors per se and binders, e.g. pitch, and producing the granules |
If a carbonisable binder is used, classification in C04B 35/528 is not necessary, unless also the possibility of not using the carbonisable binder is disclosed. C04B 35/532 is also given to graphite powders containing a carbonisable binder, together with C04B 35/522 (or C04B 35/536).
Since the class C04B 35/532 already indicates that organics are carbonised and become part of the ceramic, the code C04B 2235/48 is superfluous.
This place covers:
Shaped objects of expanded graphite, possibly further heat-treated, or preparing of expanded graphite preparatory to the shaping of the mixture
This place does not cover:
Creating porosity in the ceramic product by expanding the graphite |
Attention is drawn to the following places, which may be of interest for search:
Graphite as starting material for making ceramics or as secondary phase of a sintered ceramic | |
The preparation and after-treatment of intercalated graphite powders | |
The preparation and after-treatment of expanded or exfoliated graphite powders | |
Intercalated carbon- or graphite fibres | |
Electrodes based on carbonaceous material, e.g. graphite-intercalation compounds or CFx for fuel cells and batteries | H01M 4/133 , H01M 4/1393, H01M 4/583 and subgroup, H01M 4/663 |
This place covers:
Ceramics having as the largest fraction a sulphide, selenide or telluride phase, or a mixture of these phases
This place does not cover:
Starting powder mixtures based on sulphides, selenides or tellurides used to make ceramics |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with salts or salty compositions: containing sulphur in the anion, e.g. sulphides | |
Coating or impregnating ceramic substrates with sulphides or selenides | |
Catalysts comprising sulphides | B01J 27/04 and subgroups |
Sulphide compounds per se | C01B 17/20 and subgroups |
Selenides and tellurides per se | |
Preparation of sulphides metal compounds in general | |
Chalcogenide glasses, e.g. containing S, Se, Te | C03C 3/321 and subgroup, C03B 2201/86, C03B 2201/88 |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing sulphides | C09K 11/56 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing two or more rare earth metals: oxysulfides | |
Single crystals based on Sulphur-, selenium- or tellurium-containing compounds | C30B 29/46 and subgroups |
Sulphide ceramics in machines or engines in general (F01) or machines for liquids ( F04) | F05C 2203/0856 and subgroup |
Electrodes for accumulators with non-aqueous electrolyte based on inorganic compounds other than oxides or hydroxides, e.g. sulphides, selenides, tellurides, halogenides or LiCoFy | |
Forming inorganic semiconducting materials on a substrate, the substrate being a sulphide | |
Forming inorganic semiconducting materials on a substrate, the substrate being a selenide | |
Forming inorganic semiconducting materials on a substrate, the substrate being a telluride |
This place covers:
Ceramics having as the largest fraction a fluoride phase
This place does not cover:
Starting powder mixtures based on fluorides used to make ceramics |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with fluorine compounds | |
Coating or impregnating ceramic substrates with fluorides | |
Catalysts containing fluoride | |
Fluorides per se | C01B 7/19 and subgroups, C01B 9/08, C01B 11/24 |
Fluorides of sodium, potassium or alkali metals in general | |
Fluoride glasses | C03C 3/325, C03B 2201/82 and subgroup |
This place covers:
Ceramics having as the largest fraction a carbide phase, a compound between carbon and a metal or semi-metal, e.g. e.g. potassium carbide, magnesium carbide, Cerium carbide (CeC2), Manganese carbide (Mn3C), Iron carbide (Fe3C), Cobalt carbide (CoC), Nickel carbide (Ni3C), Copper carbide (Cu2C), Zinc carbide (ZnC), Germanium carbide (GeC), Gold carbide (Au2C2), Silver carbide (Ag2C2), Antimony carbide (SbC).
This place does not cover:
Carbo-nitride ceramics | C04B 35/58 and subgroups |
Carbides containing a metallic binder | C22C 29/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: carbides | C04B 14/322 and subgroups |
Making fibres based on carbides | |
Coating or impregnating ceramic substrates with carbides | |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rare earth carbide | C04B 2235/3817 and subgroups |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
Carbide interlayer used for joining a ceramic with another substrate | |
Materials for prostheses based on metal carbides | A61F 2310/00269 and subgroups |
Coating materials for prostheses based on metal carbides | A61F 2310/0073 and subgroups |
Carbide catalysts | B01J 27/22, C07C 2527/22 and subgroups |
Casting non-ferrous metals with a high melting point, e.g. metallic carbides | |
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting comprising refractory compounds, e.g. carbides | |
Making carbide powders | C01B 32/90 and subgroups |
Materials for coating a single layer on glass: carbides, silicides | |
Making hard metals based on borides, carbides, nitrides, oxides or silicides; Preparation of the powder mixture used as the starting material | |
Making hard metals based on borides, carbides, nitrides, oxides, silicides starting from a melt | |
Metallic alloys based carbides, but not containing other metal compounds | C22C 29/06 and subgroups |
Coating by vacuum evaporation, by sputtering or by ion implantation of the coating forming material: carbides | |
Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition (CVD) processes: carbides | C23C 16/32 and subgroup |
Single crystals of carbides | |
Carbide ceramics in machines or engines in general (F01) or machines for liquids ( F04) | F05C 2203/0813 and subgroups |
Friction linings | |
Non-adjustable resistors formed as one or more layers or coatings; Non-adjustable resistors made from powdered conducting material or powdered semi-conducting material with or without insulating material having negative temperature coefficient mainly consisting of carbon or carbides | |
Varistor cores, Carbide, e.g. SiC type | |
Electrical contacts having a noble metal as the basic material and containing carbides | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: conductive ceramics, e.g. metal oxides, metal carbides, barium titanate, ferrites, zirconia, vitreous compounds | H05B 3/141 and subgroup |
Encapsulations with oxides or nitrides or carbides, e.g. ceramics, glass, e.g. encapsulating layers, coatings of semi-conductors |
Carbonitrides are seen as nitrides. If a ceramic is however a mixture of separate carbide and nitride phases, then classification occurs in the class that corresponds to the phase that is present as the largest fraction, which could be a carbide class.
This place covers:
All oxy-carbides and all carbides that contain oxygen in the principal carbide phase
This place does not cover:
Carbide ceramics containing oxide secondary phases, e.g. a carbide containing a silica sintering aid | C04B 35/56 or subgroups, except C04B 35/5603 and symbols from C04B 2235/32- C04B 2235/365 to indicate the oxide phase |
Attention is drawn to the following places, which may be of interest for search:
Non-oxides with a defined oxygen content as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiOC, SiON, TiON | |
Making powders of oxycarbides, sulfocarbides or mixtures of carbides with other bodies, e.g. graphite; Carbides of other non-metals, e.g. silicocarbides, borocarbides | |
Silicon oxycarbide, oxynitride or oxycarbonitride glasses | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate: the material containing Si, O, and at least one of H, N, C, F, or other non-metal elements, e.g. SiOC, SiOC:H or SiONC | |
Manufacture or treatment of semiconductor devices or of parts thereof, forming insulating materials on a substrate by gas or vapour deposition, the material containing carbon doped silicon oxide, e.g. SiOC |
The oxy-carbides are also classified in the other sub-groups of C04B 35/56, e.g. titanium oxy-carbide is classified in C04B 35/5603 and C04B 35/5611. Silicon oxy-carbide is classified in C04B 35/5603 and in C04B 35/565 or one of the subgroups of C04B 35/565. Carbides that are normally classified in the head group C04B 35/56 are only classified in C04B 35/5603, in the case it is an oxy-carbide and not in C04B 35/56.
This place covers:
Ceramics based on refractory metal carbides or refractory metal oxy-carbides
This place does not cover:
Refractory carbides other than refractory metal carbides, e.g. a SiC refractory or boron carbide refractory | C04B 35/565 respectively C04B 35/563 |
Cemented refractory carbides | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Oxide ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Refractory metal nitride ceramics | C04B 35/58007 and subgroups |
Refractory metal boride ceramics | C04B 35/58064 and subgroups/ |
Refractory metal silicide ceramics | C04B 35/58092 and subgroups/ |
Refractory mortars or monolithic refractories | |
Refractory metal carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. VC, Cr3C2, ZrC, HfC, NbC, TaC, MoC or Mo2C | C04B 2235/3839 and subgroups |
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium (Ti), chromium (Cr), tantalum (Ta) | |
Coating for prosthesis made of tantalum carbide | |
Coating for prosthesis made of chromium carbide | |
Coating for prosthesis made of niobium carbide | |
The preparation of tungsten or molybdenum carbide powders |
In this place, the following terms or expressions are used with the meaning indicated:
Refractory carbides | titanium carbide, vanadium carbide, chromium carbide, zirconium carbide, niobium carbide, molybdenum carbide, hafnium carbide, tantalum carbide, tungsten carbide |
This place covers:
Ceramics based on titanium carbides or titanium oxy-carbides
This place does not cover:
Titanium (oxy)carbonitride ceramics | |
Titanium (oxy)carbide with a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Titanium oxide based ceramics | C04B 35/46 and subgroups |
Titanium (oxy)nitride ceramics | C04B 35/58014 and subgroup |
Titanium (oxy)boride ceramics | |
After-treatment of mortars, concrete, artificial stone or ceramics; Treatment of natural stone: with titanium carbide | |
Titanium carbide as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiC | |
Materials for prostheses, containing titanium carbide | |
Making titanium (oxy)carbide powders | |
Titanium carbide ceramics in machines or engines in general (F01) or machines for liquids ( F04) | |
Materials for prostheses, coatings containing titanium carbide | K6F2/00B22B4T |
In the case of mixed refractory carbides, e.g. TiCrC, both C04B 35/5607 (for the Cr) and C04B 35/5611 are added, since the amount of Ti and Cr is the same. Ti0.9Cr1.1C is only classified in C04B 35/5607 (possibly using an additional symbol (CCA) to indicate the presence of Ti, e.g. C04B 2235/3843 or C04B 2235/404)
This place covers:
Ceramics based on titanium carbides or titanium oxy-carbides that also contain silicon, or silicon carbides or silicon oxy-carbides that also contain titanium
This place does not cover:
Silicon carbide based ceramics | C04B 35/565 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with titanium carbide | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic |
The relation between Ti and Si can vary to almost any extent, thus both Ti0.9Si0.1C and Ti0.1Si0.9C are classified in this group and neither in C04B 35/5611 nor in C04B 35/565 and subgroups. Only when the amount of Ti or Si is very low, classification in C04B 35/5611 or in C04B 35/565 and subgroups might be considered.
This place covers:
Ceramics based on titanium carbides or titanium oxy-carbides that also contain aluminium, or aluminium carbides or aluminium oxy-carbides that also contain titanium
Attention is drawn to the following places, which may be of interest for search:
Aluminium carbide based ceramics | |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. aluminum carbide | |
Aluminium as starting material for making ceramics or as secondary phase of a sintered ceramic |
The relation between Ti and Al can vary to almost any extent, thus both Ti0.9Al0.1C and Ti0.1Al0.9C are classified in this group and neither in C04B 35/5611 nor in C04B 35/56. Only when the amount of Ti or Al is very low, classification in C04B 35/5611 or in C04B 35/56 might be considered.
This place covers:
Ceramics based on zirconium or hafnium carbides or zirconium or hafnium oxy-carbides
This place does not cover:
Zirconium or hafnium (oxy)carbonitride ceramics |
Attention is drawn to the following places, which may be of interest for search:
Zirconium oxide based ceramics | C04B 35/48 and subgroups |
Zirconium or hafnium (oxy)nitride ceramics | |
Zirconium or hafnium (oxy)boride ceramics | |
Materials for prostheses, coatings made of zirconium carbide | |
Materials for prostheses, coatings made of hafnium carbide |
In the case of mixed refractory carbides, e.g. ZrTiC, both C04B 35/5611 (for the Ti) and C04B 35/5622 are added, since the amount of Ti and Zr is the same. Ti0.9Zr1.1C is only classified in C04B 35/5622 (possibly using an additional symbol(CCA) to indicate the presence of Ti, e.g. C04B 2235/3843 or C04B 2235/404)
This place covers:
Ceramics based on tungsten carbides or tungsten oxy-carbides
This place does not cover:
Tungsten (oxy)carbonitride ceramics | |
Tungsten carbide with a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Tungsten oxide based ceramics | C04B 35/495 and subgroups |
Tungsten (oxy)nitride ceramics | |
Tungsten (oxy)boride ceramics | |
Tungsten carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. WC | |
Coating for prosthesis made of tungsten carbide | |
The preparation of tungsten or molybdenum carbide powders | |
Metallic alloys based on tungsten carbide | |
Tungsten carbide ceramics in machines or engines in general (F01) or machines for liquids ( F04) |
In the case of mixed carbides, e.g. WSiC, both C04B 35/565 (for the Si) and C04B 35/5626 are added, since the amount of W and Si is the same. Si0.9W1.1C is only classified in C04B 35/5626 (possibly using an additional symbol (CCA) to indicate the presence of Si, e.g. C04B 2235/3826 or C04B 2235/428)
This place covers:
Ceramics based on boron carbides or boron oxy-carbides
This place does not cover:
Ceramics based on boron carbo-nitride | C04B 35/583 and subgroup |
Boron carbide with a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: boron carbide | |
Ceramics based on boron oxide | C04B 35/01 together with C04B 2235/3409 |
Ceramics based on boron oxycarbide | |
Coating or impregnating ceramic substrates with boron carbide | |
Boron oxide or borate as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron carbide as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. B4C | |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Materials for prostheses based on boron carbide | |
Coatings for prostheses based on boron carbide | |
Preparation of boron carbide powders | |
Metallic alloys based on B4C |
In the case of mixed carbides, e.g. SiBC, both C04B 35/565 (for the Si) and C04B 35/563 are added, since the amount of B and Si is the same. Si0.9B1.1C is only classified in C04B 35/563 (possibly using an additional symbol (CCA) to indicate the presence of Si, e.g. C04B 2235/3826 or C04B 2235/428). B0.9Si1.1C is only classified in C04B 35/565 or subgroup of C04B 35/565 (possibly using an additional symbol (CCA) to indicate the presence of B, e.g. C04B 2235/3821 or C04B 2235/421).
This place covers:
Ceramics based on silicon carbides or silicon oxy-carbides
This place does not cover:
Silicon carbide based ceramics containing also Ti, e.g. Ti0.1Si0.9C | |
Ceramics based on silicon carbo-nitride | C04B 35/584 and subgroups |
Silicon carbide with a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: silicon carbide | |
Ceramics based on silicon oxide | |
Ceramics based on silicon oxycarbide | C04B 35/5603 and C04B 35/565 and subgroups |
Ceramics based on silicon nitride | C04B 35/584 and subgroups |
Making fibres based on silicon carbide | |
Coating or impregnating ceramic substrates with silicon carbide | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes | |
Cubic symmetry, e.g. beta SiC | |
Hexagonal symmetry, alpha SiC | |
Silicon carbide substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on silicon carbide SiC | |
Coating materials for prostheses, the coating material based on silicon carbide SiC | |
Silicon carbide catalyst | B01J 27/224 and subgroup, C07C 2527/224 |
Preparation of silicon carbide powders | |
Metallic alloys based on SiC | |
Chemical coating by decomposition of gaseous compounds, without leaving reaction products of surface material in the coating, i.e. chemical vapour deposition (CVD) processes: silicon carbide | |
Rope or cable materials: silicon carbides | |
Silicon carbide ceramics in machines or engines in general (F01) or machines for liquids ( F04) | |
Varistor cores, Carbide, e.g. SiC type | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: silicon, e.g. silicon carbide, magnesium silicide, heating transistors or diodes | |
Thin film transistors having a semiconductor body comprising semiconductor materials of the fourth group not being silicon, or alloys including an element of the group IV, e.g. Ge, SiN alloys, SiC alloys | |
Semiconductor devices sensitive to infrared radiation, light, electromagnetic radiation of shorter wavelength or corpuscular radiation and adapted either for the conversion of the energy of such radiation into electrical energy or for the control of electrical energy by such radiation; Processes or apparatus peculiar to the manufacture or treatment thereof or of parts thereof; details thereof: characterised by their semiconductor bodies: including, apart from doping materials or other impurities, only AIVBIV compounds, e.g. SiC | H10F 71/1218, H10F 71/1035, H10F 77/1226, H10F 77/1648.H10F 77/1665 |
Forming inorganic semiconducting materials on a substrate, the substrate being silicon carbide | H10P 14/2904, H10D 62/8325 and subgroups, H10D 62/8325, H10D 84/035 |
Forming inorganic semiconducting materials on a substrate, the coating being silicon carbide |
Both Ti0.9Si0.1C and Ti0.1Si0.9C are classified in C04B 35/5615 and not in C04B 35/5611 nor in C04B 35/565 and subgroups. Only when the amount of Ti or Si is very low, classification in C04B 35/5611 or in C04B 35/565 and subgroups might be considered.
In the case of mixed carbides, e.g. SiBC, both C04B 35/565 (for the Si) and C04B 35/563 are added, since the amount of B and Si is the same. Si0.9B1.1C is only classified in C04B 35/563 (possibly using an additional symbol (CCA) to indicate the presence of Si, e.g. C04B 2235/3826 or C04B 2235/428). B0.9Si1.1C is only classified in C04B 35/565 or subgroup of C04B 35/565 (possibly using an additional symbol (CCA) to indicate the presence of B, e.g. C04B 2235/3821 or C04B 2235/421).
For all other mixed carbides containing Si the reasoning is as with SiBC, classification is in the carbide group of the metal element that is most abundant, with the exception of TiSi-carbides.
If the main phase is alpha SiC, C04B 2235/767 (hexagonal phase) is attributed, if the main phase is beta SiC, C04B 2235/762 (cubic phase) is attributed.
SiC/SiC | Silicon carbide reinforced with silicon carbide fibers |
This place covers:
Silicon carbide ceramics made by pyrolysing silicone resins, (poly)silanes, (poly)siloxanes, (poly)silazanes etc., or porous ceramics that are infiltrated with a silicon-containing resin and pyrolysed to a product that contains mainly silicon carbide
This place does not cover:
Porous carbon is infiltrated with Si-containing polymer that is carbonised to form a product containing mainly carbon | |
Carbon and silicon containing polymers are carbonised, leading to a product that has as largest fraction a carbon phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on carbon obtained from polymer or organic precursors | |
Ceramics based on silicon nitride obtained from polymer or organic precursors | |
Si-containing organic compounds, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes used for becoming part of a ceramic after heat-treatment, e.g. phenol resins |
The end-product of the pyrolysis needs to have as largest fraction a silicon carbide phase. If after pyrolysis the product is hot-pressed, C04B 35/575 is added as well.
This place covers:
A carbon containing material and a silicon containing material are reacted to form in-situ a SiC containing ceramics, e.g. a porous carbon body is infiltrated with molten Si and reacted to SiC or a porous carbon body is infiltrated with gaseous SiOx and reacted to SiC, or carbon powder and SiO2 and/or Si powder are mixed, shaped and heated to a temperature and in an atmosphere where they react to SiC
This place does not cover:
Infiltration of porous carbon product with molten Si, with the end-product containing more carbon phase than SiC | |
Infiltration of porous carbon product with molten Si, with the end-product containing more unreacted silicon phase than SiC |
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering to make silicon nitride based ceramics | |
Reaction sintering to make ceramics in general | C04B 35/65 and subgroups |
A paper sheet which after carbonisation will react with silicon to form a porous silicon carbide porous body] | |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic |
The end-product of the reaction sintering needs to have as largest fraction a silicon carbide phase. If after reaction sintering the product is hot-pressed, C04B 35/575 is added as well. Silicon is not regarded as a metallic phase, thus silicon carbide materials that contain a large amount of silicon phase are not regarded as ceramics having a metallic binder, which are classified in C22C 29/00 and subgroups, but as a ceramic with a secondary phase.
If a SiC ceramic is made by mixing 55 wt% SiC with 45 wt% of Si/C mixture, and this mixture is reaction sintered, C04B 35/573 should not be given, but C04B 35/565, since the majority of the material does not result from reaction sintering.
When classifying in C04B 35/573, classification in C04B 35/65 is superfluous.
This place covers:
A SiC-containing or forming material is densified under mechanical pressure, leading to a product having SiC as the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Pressure sintering to make silicon nitride based ceramics | |
Pressure sintering to make ceramics in general |
When classifying in C04B 35/575, classification in C04B 35/645 is superfluous. C04B 35/575 can be used in combination with C04B 35/571 or C04B 35/573, when pressure sintering a silicon containing polymer respectively when pressure sintering a reaction sintered SiC based ceramic.
This place covers:
A SiC-containing or forming material is densified under gas pressure, leading to a product having SiC as the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Gas pressure sintering to make silicon nitride based ceramics | |
Hot isostatic pressing to make ceramics in general |
When classifying in C04B 35/5755, classification in C04B 35/6455 is superfluous. C04B 35/5755 can be used in combination with C04B 35/571 or C04B 35/573, when gas pressure sintering a silicon containing polymer respectively when gas pressure sintering a reaction sintered SiC based ceramic.
This place covers:
Making nitride ceramics, compounds between nitrogen and a metal or semi-metal, e.g. aluminum nitride, alkali nitrides, alkaline earth metal nitrides, rare earth nitrides, gallium nitride, indium nitride, carbonitrides, oxynitrides
This place does not cover:
The preparation of nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/06 and subgroups, C01B 21/082 and subgroups |
Carbonitrides containing a metallic binder | |
Nitrides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: granular materials, e.g. microballoons: carbides; nitrides; borides; silicides | C04B 14/32 and subgroups |
Making fibres based on nitrides | |
Coating or impregnating ceramic substrates with borides, nitrides or silicides | |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2), rare earth nitride | C04B 2235/3852 and subgroups |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Gases other than oxygen used as reactant for making a ceramic phase, e.g. nitrogen used to make a nitride phase | C04B 2235/46 and subgroup |
Materials for prostheses based on metal nitrides | A61F 2310/00299 and subgroups |
Coating or prosthesis-covering structure made of compounds based on metal nitrides | A61F 2310/00856 and subgroups |
High pressure synthesis of gallium nitrides | |
The preparation of oxynitride powders per se, not preparative to the making of nitride ceramics | |
The preparation of aluminium oxynitride powders per se, not preparative to the making of nitride ceramics | |
The preparation of carbonitrides or oxycarbonitrides of metals, boron or silicon per se, not preparative to the making of nitride ceramics | |
Silicon oxycarbide, oxynitride or oxycarbonitride glasses | |
Nitride glasses | |
Nitride coating on glass | |
Nitride coatings on glass | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing nitrides | |
Making hard metals based on borides, carbides, nitrides, oxides or silicides; Preparation of the powder mixture used as the starting material | |
Making hard metals based on borides, carbides, nitrides, oxides, silicides starting from a melt | |
Alloys based on carbonitrides | |
Single crystals of nitrides | C30B 29/38, C30B 29/403 and subgroup |
Nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) | F05C 2203/083 and subgroups |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: silicon, e.g. silicon carbide, magnesium silicide, heating transistors or diodes | |
Making conductor-insulator-semiconductor electrode the insulator being formed after the semiconductor body, the semiconductor being silicon: making the insulator on single crystalline silicon, e.g. using a liquid, i.e. chemical oxidation in a nitrogen-containing ambient, e.g. nitride deposition, growth, oxynitridation, NH3 nitridation, N2O oxidation, thermal nitridation, RTN, plasma nitridation, RPN | |
Semiconductor devices with at least one potential-jump barrier or surface barrier specially adapted for light emission; Processes or apparatus specially adapted for the manufacture or treatment thereof or of parts thereof; Details thereof, Processes for devices with an active region comprising only III-V compounds comprising nitride compounds | |
Semiconductor devices with at least one potential-jump barrier or surface barrier specially adapted for light emission; Processes or apparatus specially adapted for the manufacture or treatment thereof or of parts thereof; Details thereof, Processes for devices with an active region comprising only III-V compounds with a substrate not being a III-V compound comprising nitride compounds | |
Processes or apparatus peculiar to the manufacture or treatment of superconducting devices comprising nitrides or carbonitrides | |
Forming inorganic semiconducting materials on a substrate, the substrate being a nitride | |
Forming inorganic semiconducting materials on a substrate, the substrate containing a nitride coating layer | |
Treatment of semiconductor bodies to form insulating layers thereon,e.g. for masking or by using photolithographic techniques composed of nitrides | |
Treatment of semiconductor bodies to form insulating layers thereon, e.g. for masking or by using photolithographic techniques composed of alternated layers or of mixtures of nitrides and oxides or of oxynitrides, e.g. formation of oxynitride by oxidation of nitride layers | |
Encapsulations with oxides or nitrides or carbides, e.g. ceramics, glass, e.g. encapsulating layers, coatings of semi-conductors |
Carbonitrides are seen as nitrides. If a ceramic is however a mixture of separate carbide and nitride phases, then classification occurs in the class that corresponds to the phase that is present as the largest fraction, which could be a carbide class.
This place covers:
Ceramics based on refractory metal nitrides or refractory metal oxy-nitrides
This place does not cover:
Refractory nitrides other than refractory metal nitrides, e.g. a silicon nitride refractory or boron nitride refractory | C04B 35/584 respectively C04B 35/583 |
Cemented refractory nitrides |
Attention is drawn to the following places, which may be of interest for search:
Oxide ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Refractory metal carbide ceramics | C04B 35/5607 and sub/classes |
Refractory metal boride ceramics | C04B 35/58064 and sub/classes |
Refractory metal silicide ceramics | C04B 35/58092 and sub/classes |
Refractory mortars or monolithic refractories | |
Refractory metal nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. vanadium nitride (VN), tungsten nitride (WN2) | |
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium (Ti), chromium (Cr), tantalum (Ta) | |
Coating or prosthesis-covering structure made of chromium nitride | |
Coating or prosthesis-covering structure made of niobium nitride | |
Coating or prosthesis-covering structure made of tantalum nitride | |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with vanadium, niobium or tantalum | |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with chromium, molybdenum or tungsten |
In this place, the following terms or expressions are used with the meaning indicated:
Refractory nitrides | titanium nitride, vanadium nitride, chromium nitride, zirconium nitride, niobium nitride, molybdenum nitride, hafnium nitride, tantalum nitride, tungsten nitride |
This place covers:
Ceramics based on titanium nitrides or titanium oxy-nitrides
Attention is drawn to the following places, which may be of interest for search:
Titanium oxide based ceramics | C04B 35/46 and subgroups |
Titanium (oxy)carbide based ceramics | C04B 35/5611 and subgroups |
Titanium (oxy)boride based ceramics | |
Coating or impregnating ceramic substrates with titanium nitride | |
Materials for prostheses based on titanium nitrides | |
Coating or prosthesis-covering structure made of titanium nitride | |
The preparation of titanium, zirconium or hafnium nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/076 and subgroups, C01B 21/076 |
Titanium nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) | |
Making conductor-insulator-semiconductor electrodes the insulator being formed after the semiconductor body, the semiconductor being silicon the final conductor layer next to the insulator being a composite, e.g. TiN |
In the case of mixed refractory nitrides, e.g. TiCrN, both C04B 35/58007 (for the Cr) and C04B 35/58014 are added, since the amount of Ti and Cr is the same. Ti0.9Cr1.1N is only classified in C04B 35/5607.
This place covers:
Ceramics based on titanium carbonitrides or titanium oxycarbonitrides
Attention is drawn to the following places, which may be of interest for search:
Carbonitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium carbonitride, zirconium carbonitride |
This place covers:
Ceramics based on zirconium or hafnium nitrides or zirconium or hafnium oxy-nitrides
Attention is drawn to the following places, which may be of interest for search:
Zirconium oxide based ceramics | C04B 35/48 and subgroups |
Zirconium or hafnium (oxy)carbide ceramics | |
Zirconium or hafnium (oxy)boride ceramics | |
Coating or prosthesis-covering structure made of hafnium nitride | |
Coating or prosthesis-covering structure made of zirconium nitride | |
The preparation of titanium, zirconium or hafnium nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/076 and subgroups, C01B 21/076 |
Zirconium nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) |
In the case of mixed refractory nitrides, e.g. ZrTiN, both C04B 35/58014 (for the Ti) and C04B 35/58028 are added, since the amount of Ti and Zr is the same. Ti0.9Zr1.1N is only classified in C04B 35/58028.
This place covers:
Ceramics based on zirconium or hafnium carbonitrides or zirconium or hafnium oxycarbonitrides
Attention is drawn to the following places, which may be of interest for search:
Carbonitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium carbonitride, zirconium carbonitride |
This place covers:
Nitride ceramics based on iron nitride, nickel nitride or cobalt nitride
Attention is drawn to the following places, which may be of interest for search:
Cobalt oxide based ceramics | C04B 35/01 together with C04B 2235/3275 or C04B 2235/3277 |
Nickel oxide based ceramics | C04B 35/01 together with C04B 2235/3279 |
Iron oxide based ceramics | C04B 35/26 and subgroups |
Iron group carbide based ceramics | |
Iron group boride based ceramics | |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2), rare earth nitride, iron group metal nitrides | |
Iron group metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. nickel (Ni) or cobalt (Co) | |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with iron, cobalt or nickel |
This place covers:
Ceramic materials based one or more boride phases, a compound between boron and a metal or semi-metal, e.g. e.g. aluminium boride, Rare earth boride, e.g. dysprosium boride (DyB2), Lanthanum boride (LaB6), Manganese boride (Mn2B, MnB or MnB2), Iron boride (Fe2B, FeB), Cobalt boride (CoB), Nickel boride (NiB), Copper boride (Cu3B2), Gallium boride (GaB12), Scandium Iridium Boride (Sc3Ir5B2), Silver boride (AgB2), Nickel bismuth boride (Ni23-xBixB6), Silicon boride (SiBn)
This place does not cover:
Ceramics based on boron carbide | |
Ceramics based on boron nitride | C04B 35/583 and subgroup |
Borides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with borides | |
Boron oxide or borate as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rare earth boride | C04B 2235/3804 and subgroups |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Materials for prostheses based on metal borides | |
Coating or prosthesis-covering structure made of compounds based on metal borides | A61F 2310/0067 and subgroups |
Preparation of metal boride powders | |
Boride or phosphide coating on glass | |
Boride or phosphide coating on glass | |
Making hard metals based on borides, carbides, nitrides, oxides or silicides; Preparation of the powder mixture used as the starting material | |
Making hard metals based on borides, carbides, nitrides, oxides, silicides starting from a melt | |
Alloys based on borides | |
Details of electrodes, of magnetic control means, of screens, or of the mounting or spacing thereof, common to two or more basic types of discharge tubes or lamps: main electrodes: solid thermionic cathodes with compounds having metallic conductive properties, e.g. lanthanum boride, as an emissive material |
This place covers:
Ceramics based on a magnesium boride phase, whether superconducting or not
Attention is drawn to the following places, which may be of interest for search:
Magnesium oxide based ceramics | C04B 35/04 and subgroups |
Magnesium carbide based ceramics | |
Magnesium nitride based ceramics | |
Alkaline earth metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Mg | |
Processes or apparatus peculiar to the manufacture or treatment of superconducting devices comprising metal borides, e.g. MgB2 | |
Superconducting devices comprising a junction of dissimilar materials, namely Josephson-effect devices comprising metal borides, e.g. MgB2 | |
Permanent superconductor devices comprising metal borides, e.g. MgB2 |
This place covers:
Ceramics based on refractory metal borides or refractory metal oxy-borides, Vanadium diboride (VB2), Chromium boride (CrB or CrB2), Niobium or tantalum diboride (NbB2 or TaB2), Molybdenum boride (Mo2B or Mo2B5), Tungsten boride (W2B, WB or W2B5)
This place does not cover:
Refractory borides other than refractory metal borides, e.g. a silicon boride refractory | |
Cemented refractory borides |
Attention is drawn to the following places, which may be of interest for search:
Oxide ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Refractory metal carbide ceramics | C04B 35/5607 and subgroups |
Refractory metal nitride ceramics | C04B 35/58007 and subgroups |
Refractory metal silicide ceramics | C04B 35/58092 and subgroups |
Refractory mortars or monolithic refractories | |
Refractory metal borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiB2, HfB2 | |
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium (Ti), chromium (Cr), tantalum (Ta) | |
Coating or prosthesis-covering structure made of compounds based on chromium boride | |
Coating or prosthesis-covering structure made of compounds based on molybdenum boride | |
Coating or prosthesis-covering structure made of compounds based on vanadium boride | |
Coating or prosthesis-covering structure made of compounds based on tungsten boride |
This place covers:
Ceramics based on titanium borides or titanium oxy-borides, Titanium diboride (TiB2)
This place does not cover:
Titanium oxide based ceramics | C04B 35/46 and subgroups |
Titanium (oxy)carbide based ceramics | C04B 35/5611 and subgroups |
Titanium (oxy)nitride based ceramics | C04B 35/58014 and subgroup |
Titanium (oxy)silicide based ceramics |
Attention is drawn to the following places, which may be of interest for search:
Coating or prosthesis-covering structure made of compounds based on titanium borides | K61F2/00L22B8T |
In the case of mixed refractory borides, e.g. TiCrB, both C04B 35/58064 (for the Cr) and C04B 35/58071 are added, since the amount of Ti and Cr is the same. Ti0.9Cr1.1B is only classified in C04B 35/58064.
This place covers:
Ceramics based on zirconium borides or zirconium oxy-borides, Zirconium of hafnium diboride (ZrB2 or HfB2).
Attention is drawn to the following places, which may be of interest for search:
Zirconium oxide based ceramics | C04B 35/48 and subgroups |
Zirconium or hafnium (oxy)carbide ceramics | |
Zirconium or hafnium (oxy)nitride ceramics | C04B 35/58028 and subgroup |
Coating or prosthesis-covering structure made of compounds based on zirconium borides |
In the case of mixed refractory borides, e.g. ZrTiB, both C04B 35/58071 (for the Ti) and C04B 35/58078 are added, since the amount of Ti and Zr is the same. Ti0.9Zr1.1B is only classified in C04B 35/58078.
This place covers:
Ceramics based on silicides or oxy-silicides
This place does not cover:
Silicides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with silicides | |
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic, i.e. chemical compounds between silicon and a one or more metals, e.g. chromium silicide (CrSi2), molybdenum disilicide (MoSi2), iron silicide (FeSi, FeSi2), cobalt silicide (Co2Si, CoSi, CoSi2) | |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
The preparation of metal silicide powders | |
Materials for coating a single layer on glass: carbides, silicides | |
Making hard metals based on borides, carbides, nitrides, oxides or silicides; preparation of the powder mixture used as the starting material | |
Making hard metals based on borides, carbides, nitrides, oxides, silicides starting from a melt | |
Alloys based on silicides | |
Heater elements characterised by the composition or nature of the materials or by the arrangement of the conductor: silicon, e.g. silicon carbide, magnesium silicide, heating transistors or diodes | |
Manufacture of electrodes on semiconductor bodies from a gas or vapour, e.g. condensation of conductive layers on semiconductor bodies comprising elements of the fourth group of the Periodic Table the conductive layers comprising silicides | |
Field-effect transistors with an insulated gate using self aligned silicidation, i.e. silicide | H10D 64/0112 and subgroup |
Making conductor-insulator-semiconductor electrodes the final conductor layer being next to the insulator being silicon, e.g. polysilicon, with or without impurities, the conductor comprising a silicide layer formed by the silicidation reaction of silicon with a metal layer | |
Making conductor-insulator-semiconductor electrodes the final conductor layer being next to the insulator being silicon, e.g. polysilicon, with or without impurities, the conductor comprising a metal or metallic silicIde formed by deposition, e.g. sputter deposition, i.e. without a silicidation reaction | |
Making conductor-insulator-semiconductor electrodes the insulator being formed after the semiconductor body, the semiconductor being silicon the final conductor layer next to the insulator being a metallic silicide | |
Forming inorganic semiconducting materials on a substrate, the substrate being conductive materials, e.g. metallic silicides | |
Manufacture or treatment of semiconductor devices or of parts thereof, the devices having at least one potential-jump barrier or surface barrier, e.g. PN junction, depletion layer, carrier concentration layer, the devices having semiconductor bodies comprising elements of the fourth group of the Periodic Table or AIIIBV compounds with or without impurities, e.g. doping material; Deposition of semiconductor materials on a substrate, e.g. epitaxial growth the substrate being of crystalline conducting material, e.g. metallic silicides | |
Treatment of semiconductor bodies to form insulating layers thereon; deposition of non-insulating-, e.g. conductive- or resistive-, layers on insulating layers; after-treatment of these layer; deposition of metallic of metal-silicide layers | |
Applying interconnections to be used for carrying current between separate components within a device characterised by the formation and the after-treatment of the conductors; modifying permanently or temporarily the pattern or the conductivity of conductive members, e.g. formation of alloys, reduction of contact resistances by forming silicides of refractory metals |
This place covers:
Ceramics based on refractory metal silicides or refractory metal oxy-silicides
Attention is drawn to the following places, which may be of interest for search:
Oxide ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Refractory metal carbide ceramics | C04B 35/5607 and subgroups |
Refractory metal nitride ceramics | C04B 35/58007 and subgroups |
Refractory metal boride ceramics | C04B 35/58064 and subgroups |
Refractory mortars or monolithic refractories |
In the case of mixed silicides, e.g. MoFeSix both C04B 35/58092 (for the Mo) and C04B 35/58085 (for the Fe) are added, since the amount of Mo and Fe is the same. Fe0.9Mo1.1Six is only classified in C04B 35/58092.
This place covers:
Ceramics based on aluminium nitrides or aluminium oxynitrides or aluminium carbonitrides
This place does not cover:
Ceramics based on aluminium silicon oxynitride (Sialon) | |
Nitrides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: granular materials, Aluminium nitride | |
Ceramics based on aluminium carbide | |
Ceramics based on aluminium boride | |
Coating or impregnating ceramic substrates with aluminium nitride | |
Aluminum nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3865 and subgroup |
Aluminium as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Aluminum nitride substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on aluminium nitride | |
Coatings for prostheses based on aluminium nitride | |
High pressure synthesis of aluminium nitrides | |
The preparation of aluminium nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/072 and subgroups, C01B 21/0825 (oxy-nitrides) |
Aluminium nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) |
Silicon-aluminium-oxynitrides are all in C04B 35/597. Silicon-aluminium-nitrides are in C04B 35/581 if the amount of aluminium is larger and in C04B 35/584 and subgroups if the amount of silicon is larger.
This place covers:
Ceramics based on boron nitrides or boron oxynitrides or boron carbonitrides, having for instant the hexagonal phase
This place does not cover:
Nitrides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on boron carbide | |
Making fibres based on boron nitride | |
Coating or impregnating ceramic substrates with boron nitride | |
Boron oxide or borate as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron nitride starting material for making ceramics or secondary phase of a sintered ceramic | |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Boron nitride substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on boron nitride | |
Coatings for prostheses based on boron nitride | |
Presses for the formation of diamonds or boronitride | B01J 3/065 and subgroup |
High pressure synthesis of boronitrides | |
The preparation of boron nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/064 and subgroups |
Alloys containing diamond or cubic or wurtzitic boron nitride, fullerenes or carbon nanotubes | |
Alloys based on nitrides | |
Boron nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) |
Materials of silicon boron nitride are classified in C04B 35/583 if the amount of boron is larger than the amount of silicon and in C04B 35/584 and subgroups if the amount of silicon is larger. The same accounts for aluminium boron nitrides and other mixed boron nitrides.
In this place, the following terms or expressions are used with the meaning indicated:
hBN | Hexagonal boron nitride |
This place covers:
Ceramics based on boron nitrides, boron oxynitrides or boron carbonitrides, having the cubic structure
This place does not cover:
Nitrides containing a metallic binder |
In this place, the following terms or expressions are used with the meaning indicated:
cBN | Cubic boron nitride |
This place covers:
Ceramics based on silicon nitrides or silicon carbonitrides
This place does not cover:
Silicon oxynitride | |
Nitrides containing a metallic binder |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on silicon oxide | |
Ceramics based on silicon carbide | C04B 35/565 and subgroups |
Ceramics based on silicon boride | |
Making fibres based on silicon nitrides | |
Coating or impregnating ceramic substrates with silicon nitride | |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes | |
Silicon nitride substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses based on silicon nitride | |
Coatings based on silicon nitride on prostheses | |
The preparation of silicon nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/068 and subgroups, C01B 21/0823 (oxy-nitrides) |
Silicon nitride ceramics in machines or engines in general (F01) or machines for liquids ( F04) | |
Treatment of semiconductor bodies to form insulating layers thereon, e.g. for masking or by using photolithographic techniques composed of silicon nitrides | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate the material being a silicon nitride not containing oxygen, e.g. SixNy or SixByNz |
In the case of mixed nitrides, e.g. SiBN, both C04B 35/584 (for the Si) and C04B 35/583 are added, since the amount of B and Si is the same. Si0.9B1.1N is only classified in C04B 35/583 (possibly using an additional symbol (CCA) to indicate the presence of Si, e.g. C04B 2235/3873 or C04B 2235/428). B0.9Si1.1N is only classified in C04B 35/584 or subgroup of C04B 35/584 (possibly using an additional symbol (CCA) to indicate the presence of B, e.g. C04B 2235/386 or C04B 2235/421).
For all other mixed nitrides containing Si the reasoning is as with SiBN, classification is in the nitride group of the metal element that is most abundant.
If the main phase is alpha Si3N4, C04B 2235/766 (trigonal symmetry) is attributed, if the main phase is beta Si3N4, C04B 2235/767 (hexagonal symmetry) is attributed.
This place covers:
Silicon nitride ceramics having grains smaller than 100 microns.
Attention is drawn to the following places, which may be of interest for search:
Using particles of size 1-100 microns for making the ceramic |
This class is not used in practice. All silicon nitride ceramics are either in C04B 35/584 or in C04B 35/589-C04B 35/5935.
This place covers:
Silicon nitride or carbonitride ceramics made by pyrolysing silicone resins, (poly)silanes, (poly)siloxanes, (poly)silazanes etc., or porous ceramics that are infiltrated with a silicon-containing resin and pyrolysed to a product that contains mainly silicon nitride or carbonitride
This place does not cover:
Porous carbon is infiltrated with Si-containing polymer that is carbonised to form a product containing mainly carbon | |
Carbon and silicon containing polymers are carbonised, leading to a product that has as largest fraction a carbon phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on carbon obtained from polymer or organic precursors | |
Ceramics based on silicon carbide obtained from polymer or organic precursors | |
Si-containing organic compounds, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes used for becoming part of a ceramic after heat-treatment, e.g. phenol resins |
The end-product of the pyrolysis needs to have as largest fraction a silicon nitride phase. If after pyrolysis the product is hot-pressed, C04B 35/593 is added as well.
This place covers:
A nitrogen containing material and a silicon containing material are reacted to form in-situ a Si3N4 containing ceramics, e.g. a silicon body is infiltrated with gaseous N2 and reacted to Si3N4
This place does not cover:
Infiltration of porous silicon product with nitrogen, with the end-product containing more unreacted silicon phase than Si3N4 | C04B 41/45 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering to make silicon carbide based ceramics | |
Reaction sintering to make ceramics in general | C04B 35/65 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Gases other than oxygen used as reactant for making a ceramic phase, e.g. nitrogen used to make a nitride phase | C04B 2235/46 and subgroup |
Treatment of semiconductor bodies to form insulating layers thereon, e.g. for masking or by using photolithographic techniques: deposition of non-insulating-, e.g. conductive- or resistive-, layers on insulating layers; after treatment: nitridation of silicon-containing layers |
The end-product of the reaction sintering needs to have as largest fraction a silicon nitride phase. If after reaction sintering the product is hot-pressed, C04B 35/593 is added as well. Silicon is not regarded as a metallic phase, thus silicon nitride materials that contain a large amount of silicon phase are not regarded as ceramics having a metallic binder, which are classified in C22C 29/00 and subgroups, but as a ceramic with a secondary phase.
When classifying in C04B 35/591, classification in C04B 35/65 is superfluous.
This place covers:
A Si3N4-containing or forming material is densified under mechanical pressure, leading to a product having Si3N4 as the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Pressure sintering to make silicon carbide based ceramics | |
Pressure sintering to make ceramics in general |
When classifying in C04B 35/593, classification in C04B 35/645 is superfluous. C04B 35/593 can be used in combination with C04B 35/589 or C04B 35/591, when pressure sintering a silicon containing polymer respectively when pressure sintering a reaction sintered Si3N4 based ceramic.
This place covers:
A Si3N4-containing or forming material is densified under gas pressure, leading to a product having Si3N4 as the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Gas pressure sintering to make silicon carbide based ceramics | |
Hot isostatic pressing to make ceramics in general |
When classifying in C04B 35/5935, classification in C04B 35/6455 is superfluous. C04B 35/5935 can be used in combination with C04B 35/589 or C04B 35/591, when gas pressure sintering a silicon containing polymer respectively when gas pressure sintering a reaction sintered Si3N4 based ceramic.
This place covers:
Ceramics based on oxynitrides containing both aluminium and silicon, possibly further containing rare earths
This place does not cover:
Aluminium oxynitride based ceramics |
Attention is drawn to the following places, which may be of interest for search:
Aluminium nitride based ceramics | |
Silicon nitride based ceramics | C04B 35/584 and subgroups |
Coating or impregnating ceramic substrates with silicon oxynitrides, e.g. SIALON | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Aluminum nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3865 and subgroup |
Aluminum oxynitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. AlON or sialon | |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Aluminium as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
The preparation of sion powders per se, not preparative to the making of nitride ceramics | |
The preparation of sialon powders per se, not preparative to the making of nitride ceramics | |
Silicon oxy-nitride glasses | |
Silicon oxycarbide, oxynitride or oxycarbonitride glasses | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate the material being a nitride into which oxygen is introduced, e.g. changing SiN to SiON | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate the material being an oxide into which nitrogen is introduced, e.g. changing SiO to SiON | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate: the material being a silicon oxynitride, e.g. SiON or SiON:H |
This place covers:
All processes for producing and treating ceramic powders or powders that are used for making ceramics, where these powders subsequently are used to make shaped ceramics. Making and treating ceramic fibers. Additives used for shaping ceramics. The shaping of (pre)ceramic powders or slurries. Heat treatments of (pre)ceramic powders and shaped ceramic materials.
working by grinding or polishing B24
Mechanical features relating to the shaping of clay or other ceramic compositions, B28B
Preparing clay or like ceramic compositions; Producing mixtures containing clay or like ceramic compositions B28C
Working stone or stone-like materials B28D
Layered products B32B
Chemical preparations of powders of inorganic compounds C01
This place does not cover:
After- treatment of ceramics, e.g. coating or impregnation | |
Articles characterised by particular shape, e.g. linings for casting ladles, tundishes, cups or the like | |
Injection moulding of clay or other ceramic compositions | |
Slip-casting clay or other ceramic compositions | |
Applying clay or other ceramic compositions on to a core to form a layer thereon |
Attention is drawn to the following places, which may be of interest for search:
Aspects relating to the preparation, properties or mechanical treatment of green bodies or pre-forms | C04B 2235/60 and subgroups |
Patterns for foundry moulding; Manufacture thereof so far as not provided for in other classes | |
Manufacture of workpieces or articles from metallic powder characterised by the manner of sintering by using electric current, laser radiation or plasma | |
Working by laser beam | |
Layered products essentially comprising ceramics, e.g. refractory products | |
Photomechanical, e.g. photolithographic, production of textured or patterned surfaces | |
Exposure, e.g. with laser beam |
In this place, the following terms or expressions are used with the meaning indicated:
Rapid Prototyping (RP) | RP is a forming method in which resin or powder material is used. RP devices build up a prototype body layer by layer, rapidly generating a three-dimensional free form. In the ceramic art, two kinds of RP are mainly applied. One is "3D Printing", the other is "Selective Laser Sintering" (see glossary of C04B 35/64). |
3D Printing (3DP) | 3DP is a general forming technique which is also used in the ceramic art, developed from stereolithography. Light-sensitive monomers are polymerised by a laser beam and solidified by gelation in this way. Through the gelation and solidification of aqueous ceramic slurry, which contains the light-sensitive monomer, a component is built up in layers. |
In patent documents, the following words/expressions are often used as synonyms:
- "Rapid Prototyping technologies", "Solid Freeform Fabrication", "Layer Manufacturing technologies " and "Desktop Manufacturing"
This place covers:
Waste material is mixed with ceramic or refractory material to be sintered into a ceramic or refractory product
Disposal of solid waste B09B
This place does not cover:
Waste material is mixed with clay to make a fired clay product | C04B 33/132 and subgroups |
Alumino-silicate products made by sintering waste materials, without adding any clay or ceramic material. | |
Waste materials that are added to the ceramic material to create porosity after a heat treatment | |
Removing ash, clinker, or slag from combustion chamber | F23J 1/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Cements containing slag | C04B 7/14 and subgroups |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone | C04B 18/04 and subgroups |
Use of agglomerated or waste materials or refuse as fillers for mortars, concrete or artificial stone, or treatment of agglomerated or waste materials or refuse, specially adapted to enhance their filling properties in mortars, concrete or artificial stone: waste material from metallurgical processes being silica fume | C04B 18/146 and subgroups |
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: waste inorganic materials | |
Coating or impregnating of mortars, concrete, artificial stone or ceramics with waste materials | |
Manufacture of articles from scrap or waste metal particles | |
Active carbon from waste materials, e.g. tyres, spent sulphite pulp liquor | |
Preparation of alkali metal aluminates; Aluminium oxide or hydroxide there from by treating aluminous minerals or waste-like raw materials with alkali hydroxide, | |
Melting in furnaces of glass-forming waste materials | |
Use of waste materials, e.g. slags as ingredients generally applicable to manufacture of glasses, glazes, or vitreous enamels | |
Devitrified glass ceramics containing waste materials, e.g. slags | |
Foundations for pavings characterised by material or composition used, e.g. waste or recycled material |
This place covers:
Wood materials are carbonised to make a carbon product, which could be further reacted with silicon to make silicon carbide, e.g. cellulose is carbonised and becomes part of a ceramic object.
This place does not cover:
Burning out woody material, e.g. cellulose, to obtain a porous ceramic |
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues from burning wood | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: wood, e.g. sawdust, wood shaving | C04B 18/26 and subgroup |
Using cellulose as additive for making ceramics | |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
Incinerators or other apparatus for consuming industrial waste, of wood waste |
This place covers:
Rice bran or rice hulls are pyrolysed into silicon oxide material or are treated in reducing atmosphere to make a silicon (oxy)carbide material.
Attention is drawn to the following places, which may be of interest for search:
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: combustion residues: burned rice husks or other burned vegetable material | |
Use of waste materials or refuse as fillers for mortars, concrete or artificial stone: vegetable refuse, e.g. rice husks, maize-ear refuse; Cellulosic materials, e.g. paper, cork | C04B 18/24 and subgroups |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
This place covers:
Obtaining free-standing ceramic films or sheets, e.g. making ceramic paper, ceramic layers, where the emphasis lies on how to obtain the free-standing film, e.g. through tape casting, or using a method that is normally used for making coatings, to make a free-standing film, e.g., CVD. Not meant for standard tape casting or standard sheet making.
Obtaining ceramic films that remain on a metallic substrate C23C
This place does not cover:
Obtaining ceramic films that remain on a substrate of mortars, concrete, artificial or natural stone or ceramics | C04B 41/45 and subgroups |
Making ceramic tapes by tape casting | |
Obtaining ceramic films that remain on a glass substrate | C03C 17/00, C03C 2217/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Laminating ceramic pre-shaped layers |
If the symbol C04B 2235/6025 is given, C04B 35/62218 could be given as well, since tape casting always leads to a freestanding film. This is normally not done, however. Only if there are new or special aspects in the tape casting process also C04B 35/62218 is given.
This place covers:
Ceramic coatings on bulk objects, where the substrate is not defined, making it impossible to classify in C03C 17/00, C04B 41/00, C23C, H01 or any other field where ceramic coatings could be classified. This is for instance the case when making thick coatings from suspensions, such as by screen printing, on an undefined substrate.
Obtaining ceramic films that remain on a metallic substrate C23C
Obtaining ceramic coatings on silicon for semi-conductor purposes H10W
This place does not cover:
Obtaining ceramic coatings on inorganic particles that are subsequently used in a ceramic material | |
Obtaining ceramic coatings on inorganic fibers that are subsequently used in a ceramic material | |
Obtaining ceramic coatings that remain on a substrate of mortars, concrete, artificial or natural stone or ceramics | C04B 41/45 and subgroups |
Linings or coatings, e.g. removable, absorbent linings, permanent anti-stick coatings; Linings becoming a non-permanent layer of the moulded article of Moulds; Cores; Mandrel | B28B 7/36 and subgroups |
Mechanical aspects of coating ceramic objects | B28B 11/04, B28B 19/00 and subgroups |
Obtaining ceramic coatings that remain on a glass substrate | C03C 17/00, C03C 2217/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Mechanical aspects of coating ceramic tubes | |
Laminating ceramic pre-shaped layers |
In practice this class is hardly used. Examples of documents where this class has been used are EP1186579 and WO9818741.
This place covers:
All documents describing the synthesis of ceramic fibers, both oxide and non-oxide fibers, except for carbon fibers, and all documents that describe ceramic fibers having a new or uncommon composition. The fibers can be obtained either in individual form or in certain shaped form, such as woven fibers. Also for the synthesis of (nano) wires, whiskers, needles, pins.
Obtaining fibers or threads in general D01
This place does not cover:
Coating of ceramic fibers | C04B 35/62844 and subgroups |
Making metallic fibers per se | |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Glass-ceramic fiber compositions | |
The synthesis of carbon fibers |
Attention is drawn to the following places, which may be of interest for search:
Clay wares reinforced with fibers | |
Coating ceramic and carbon fibers | C04B 35/62844 and subgroups |
Ceramic material reinforced with fibers | C04B 35/71 and subgroups, e.g. C04B 35/83, C/C composites |
Fibers used in ceramic compositions | C04B 2235/5208 and subgroups |
Mechanical aspects of shaping ceramic objects containing fibers | |
Glass fibre or filament compositions | C03C 13/00 and subgroups |
Use of inorganic fibers as ingredient for polymers | C08K 7/02 and subgroups |
Fibers of inorganic material, not being glass or ceramic |
The method of making the fibers is usually classified in D01, e.g. spinning or electro-spinning ceramic fibers.
If the making of ceramic fibers is not described but just the use of them in a ceramic composite is mentioned, C04B 35/62227 is not used, but C04B 2235/5208 and its subgroups together with C04B 35/80 and it's subgroups.
The making of ceramic fibers is normally not classified in the general oxide classes C04B 35/01-C04B 35/51 or general non-oxide classes C04B 35/515-C04B 35/597, unless the fiber composition is a new composition for that material in general or in the case the synthesis contains a new aspect that would be applicable also for making a bulk ceramic, e.g. using a new combination of starting materials that also could be used to make a bulk ceramic.
This place covers:
The obtaining of ceramic fibers based on oxide ceramics, e.g. ferrite, manganite, chromite, fibers
This place does not cover:
Coating fibers with oxide ceramic | C04B 35/62847 and subgroups |
The obtaining of glass-ceramic fibers | C03C 13/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Oxidic fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/46 and subgroups |
Obtaining oxide ceramics in general | C04B 35/01 and subgroups |
Metal oxides, mixed metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/32 and subgroups |
Non-metal oxides, mixed non-metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/34 and subgroups |
Use of fibers based on oxides in making ceramics | |
Use of inorganic oxygen-containing fibers as ingredient for polymers | |
Inorganic fibres based on oxides or oxide ceramics, e.g. silicates, Ceramic |
This place covers:
The obtaining of ceramic fibers based on aluminium oxide ceramics, e.g. spinel, alumina, YAG (yttrium aluminate garnet) fibers
This place does not cover:
The obtaining of fibers based on alumino-silicates | |
Coating fibers with alumina or aluminates |
Attention is drawn to the following places, which may be of interest for search:
Alumina fibers as filler for concrete, cement, mortar or artificial stone | |
Obtaining alumina-based ceramics in general | C04B 35/10 and subgroups |
Obtaining aluminate-based ceramics in general | C04B 35/44 and subgroup |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Use of alumina or aluminate fibers in making ceramics |
This place covers:
The obtaining of ceramic fibers based on silicon oxide ceramics, e.g. silica, forsterite, wollastonite fibers
This place does not cover:
Coating fibers with silica or silicates | |
The synthesis of silica based glass or glass-ceramic fibers | C03B 37/01 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Silica fibers as filler for concrete, cement, mortar or artificial stone | |
Silicate fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/4656 and subgroups |
Obtaining silica-based ceramics in general | |
Obtaining silicate-based ceramics in general | |
Obtaining magnesium silicate-based ceramics in general | |
Obtaining calcium silicate-based ceramics in general | |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. water glass (Na2SiO3) | C04B 2235/3427 and subgroups |
Use of Silica or silicates other than aluminosilicates fibers in making ceramics | |
Use of inorganic silicon-containing fibers as ingredient for polymers | C08K 7/08 and subgroup |
This place covers:
The obtaining of ceramic fibers based on alumino-silicate ceramics, e.g. mullite, cordierite, kyanite, zeolite, spodumene, vermiculite, albite, anorthite fibers
Attention is drawn to the following places, which may be of interest for search:
Alumino-silicate fibers as filler for concrete, cement, mortar or artificial stone | |
Obtaining alumino-silicate-based ceramics in general | C04B 35/18 and subgroups |
Alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. mullite (3Al2O3-2SiO2) | C04B 2235/3463 and subgroups |
Use of fibers based on Silica and alumina, including aluminosilicates in making ceramics |
This place covers:
The obtaining of ceramic fibers based on zirconium oxide ceramics, e.g. zirconia, YSZ (yttria-stabilised-zirconia), zircon, zirconate, zirconate-titanates such as PZT (lead zirconate titanate) fibers
This place does not cover:
Coating fibers with refractory metal oxides |
Attention is drawn to the following places, which may be of interest for search:
Zirconia or zircon fibers as filler for concrete, cement, mortar or artificial stone | |
Obtaining zirconia or zirconate-based ceramics in general | C04B 35/48 and subgroups |
Obtaining titanate-zirconate-based ceramics in general | C04B 35/49 and subgroups |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
Use of fibers based on zirconia in making ceramics |
This place covers:
The obtaining of ceramic fibers based on copper oxide ceramics, e.g. cuprate fibers such as superconducting YBaCuO fibers
This place does not cover:
Obtaining copper oxide containing ferrite based fibers |
Attention is drawn to the following places, which may be of interest for search:
Obtaining copper oxide or cuprate-based ceramics in general | C04B 35/45 and subgroups |
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroup |
Processes peculiar to the manufacture or treatment of superconducting filaments or superconducting composite wires |
This place covers:
The obtaining of ceramic fibers based on titanium oxide ceramics, e.g. titania such as rutile and anatase, titanates such as alkaline earth titanates, e.g. barium or strontium titanates, rare earth titanates, alkali titanates, lead titanates, bismuth titanates, aluminium titanates such as tialite
This place does not cover:
Zirconate-titanate fibers such as PZT fibers | |
Coating fibers with refractory metal oxides |
Attention is drawn to the following places, which may be of interest for search:
Titanate fibers as filler for concrete, cement, mortar or artificial stone | |
Obtaining titania-based ceramics in general | |
Obtaining titanate-based ceramics in general | C04B 35/462 and subgroups |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
This place covers:
The obtaining of ceramic fibers based on oxides based on single oxide phases of MgO or mixed oxides of MgO and CaO such as dolomite, or mixed oxides of MgO with either alkali metal oxides and/or rare earth oxides, in which the MgO forms the largest fraction. Mixed oxides of magnesia with zirconium oxide, in which the amount of magnesia is larger than the amount of zirconia, e.g. Mg0.6Zr0.4Ox
This place does not cover:
Obtaining magnesium ferrite based fibers | |
Obtaining magnesium chromite based fibers | |
Obtaining magnesium niobate based fibers | |
Obtaining magnesium aluminate based fibers, such as spinel fibers | |
Obtaining magnesium silicate based fibers, such as forsterite fibers | |
Obtaining magnesium alumino silicate based fibers, such as cordierite fibers | |
Obtaining magnesium zirconate based fibers | |
Obtaining magnesium titanate zirconate based fibers | |
Obtaining magnesium cuprate based fibers | |
Obtaining magnesium titanate based fibers | |
Obtaining magnesium phosphate based fibers |
Attention is drawn to the following places, which may be of interest for search:
Obtaining magnesia based ceramics in general | C04B 35/04 and subgroups |
Obtaining dolomite based ceramics in general | |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic |
This place covers:
The obtaining of ceramic fibers based on inorganic phosphor-oxide compounds
This place does not cover:
Obtaining phosphide based fibers | |
Coating fibers with metal salts, e.g. phosphates |
Attention is drawn to the following places, which may be of interest for search:
Obtaining phosphate based ceramics in general | |
Calcium phosphate as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. hydroxyapatite | |
Phosphates or phosphites (calcium phosphates C04B 2235/3212) as starting material for making ceramics, e.g. orthophosphate (PO43-), pyrophosphate (P2O74-), hypophosphite (H2PO2-), or present as secondary phase in the sintered ceramic | |
Preparation of phosphates per se, e.g. phosphates powder, not preparative to making a phosphates ceramic | C01B 25/26 and subgroups |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a non-oxide material, e.g. a carbide, nitride, boride, silicide, fluoride, sulphide, selenide.
This place does not cover:
Coating fibers with non-oxide ceramics | C04B 35/62828 and subgroups |
The synthesis of carbon nanotubes | |
The synthesis of carbon fibers |
Attention is drawn to the following places, which may be of interest for search:
Non-oxide fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/4687 and subgroup |
Obtaining non-oxide based ceramics in general | C04B 35/515 and subgroups |
Non-oxides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/38 and subgroups |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
Use of fibers based on non-oxides in making ceramics | |
Non-oxide glass compositions for glass fibers | C03C 13/041 and subgroups |
Inorganic fibres based on Carbides; Nitrides; Silicides; Borides | D10B 2101/14 and subgroup |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a carbide, e.g. boron carbide, titanium carbide, tungsten carbide, an oxy-carbide
This place does not cover:
The obtaining of carbo-nitride based fibers or whiskers | |
Coating fibers with carbides | C04B 35/6286 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Obtaining carbide based ceramics in general | C04B 35/56 and subgroups |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rare earth carbide | C04B 2235/3817 and subgroups |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a silicon carbide phase, e.g. alpha- or beta-silicon carbide or silicon oxy-carbide, silicon carbide whiskers
This place does not cover:
Carbon and silicon containing polymers are carbonised, leading to a product that has as largest fraction a silicon carbide phase, e.g. carbonising a shaped polysilane resin | |
Carbon, silicon and nitrogen containing polymers are carbonised, leading to a product that has as largest fraction a silicon nitride phase, e.g. carbonising a shaped polysilazane resin | |
The obtaining of silicon carbo-nitride based fibers or whiskers | |
Coating fibers with silicon carbide |
Attention is drawn to the following places, which may be of interest for search:
Silicon carbide fibers as filler for concrete, cement, mortar or artificial stone | |
Obtaining silicon carbide based ceramics in general | C04B 35/565 and subgroups |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes | |
Use of fibers based on silicon carbide in making ceramics | |
Inorganic fibres based on Silicon carbide |
In patent documents, the following abbreviations are often used:
SiC/SiC | Silicon carbide reinforced with silicon carbide fibers |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a nitride, e.g. aluminium nitride, titanium nitride, tungsten nitride, a carbonitride phase, an oxy-nitride such as AlON
This place does not cover:
Coating fibers with nitrides | C04B 35/62865 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Obtaining nitride based ceramics in general | C04B 35/58 and subgroups |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2), rare earth nitride, iron group metal nitrides | C04B 2235/3852 and subgroups |
Gases other than oxygen used as reactant for making a ceramic phase, e.g. nitrogen used to make a nitride phase | C04B 2235/46 and subgroup |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a boron nitride phase, e.g. hexagonal boron nitride, cubic boron nitride, boron carbonitride
This place does not cover:
Coating fibers with boron nitride |
Attention is drawn to the following places, which may be of interest for search:
Obtaining boron nitride based ceramics in general | C04B 35/583 and subgroup |
Boron oxide or borate as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron nitride starting material for making ceramics or secondary phase of a sintered ceramic | |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl |
This place covers:
The obtaining of ceramic fibers based on ceramics having as the largest fraction a silicon nitride phase, e.g. alpha- or beta-silicon nitride or silicon oxy- nitride such as SiAlON
This place does not cover:
Coating fibers with silicon nitride |
Attention is drawn to the following places, which may be of interest for search:
Obtaining silicon nitride based ceramics in general | C04B 35/584 and subgroups |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes |
This place covers:
Processes in which a liquid containing inorganic sol particles is gelified.
This place does not cover:
Sol-gel processes for making a porous ceramic | C04B 38/0045 and subgroup |
Gel casting of a ceramic slurry | |
Depositing a ceramic layer on a metallic substrate by sol-gel processing |
Attention is drawn to the following places, which may be of interest for search:
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder: sol-gel binders | |
Porous mortars, concrete, artificial stone or ceramic ware; preparation thereof by a process involving the formation of a sol or a gel, e.g. sol-gel or precipitation processes | C04B 38/0045 and subgroup |
Coating or impregnating a ceramic substrate by the sol-gel process | |
Inorganic membrane formation by sol-gel transition | |
Preparation of sols of inorganic materials in water in general | B01J 13/0008 and subgroups |
Preparation of gels containing inorganic material and water | B01J 13/0056 and subgroup |
Precipitating catalyst gels | |
Compositions of refractory mould or core materials; Grain structures thereof characterised by the use of binding agents; Mixtures of binding agents of inorganic agents N: sols, colloids or hydroxide gels] | |
Methods for preparing oxides or hydroxides in general by oxidation or hydrolysis of elements or compounds in the liquid or solid state or in non-aqueous solution, e.g. sol-gel process | |
Preparation of colloidal silica , e.g. sols, gels, dispersions and their after-treatments | C01B 33/14 and subgroups |
Making glass by wet processes, e.g. sol-gel processes | C03C 2203/20 and subgroups, C03C 2214/32 |
Making coatings on glass by sol-gel processes | |
Manufacture or treatment of semiconductor devices or of parts thereof: forming insulating materials on a substrate: liquid deposition, e.g. spin-coating, sol-gel techniques, spray coating] | H10P 14/6342 and subgroups |
This place covers:
All processes for producing and treating ceramic powders or fibers, or powders or fibers that are used for making ceramics, where these powders or fibers subsequently are used to make shaped ceramics, e.g. coating ceramic particles, heat treating ceramic (precursor) particles. Coating ceramic fibers. Additives used for shaping ceramics, such as inorganic and organic binders.
This place does not cover:
Apparatus or methods for producing or processing clay suspensions, e.g. slip | B28C 1/02 and subgroups |
Apparatus or methods for processing clay-containing substances in non-fluid condition | B28C 1/10 and subgroups |
Apparatus or methods for mixing clay or ceramic with other substances | |
Controlling the operation of apparatus for producing mixtures of clay, ceramic or cement with other substances; Supplying or proportioning the ingredients for mixing clay or cement with other substances; Discharging the mixture | B28C 7/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Aspects relating to the preparation, properties or mechanical treatment of green bodies or pre-forms | C04B 2235/60 and subgroups |
Pigments for ceramics |
C04B 35/626 and subgroups apply to powder and powder like materials, e.g. fibers whiskers, platelets, slurries, dispersions.
This place covers:
Processes for producing and treating ceramic powders or powders that are used for making ceramics, where these powders subsequently are used to make shaped ceramics, e.g. heat treating ceramic (precursor) particles, sieving ceramic powders
This place does not cover:
Supplying or proportioning the ingredients | B28C 7/04 and subgroups |
This place covers:
Milling treatments of powder particles such as ball milling or grinding, usually in order to reduce the particle size, normally in wet condition, but dry conditions can be used as well. Milling is the process by which materials are reduced from a large size to a smaller size. Milling may involve breaking up cemented material (in which case individual particles retain their shape) or pulverization (which involves grinding the particles themselves to a smaller size). Milling is generally done by mechanical means, including attrition (which is particle-to-particle collision that results in agglomerate break up or particle shearing), compression (which applies a forces that results in fracturing), and impact (which employs a milling medium or the particles themselves to cause fracturing). Attrition milling equipment includes the wet scrubber (also called the planetary mill or wet attrition mill), which has paddles in water creating vortexes in which the material collides and break up. Compression mills include the jaw crusher, roller crusher and cone crusher. Impact mills include the ball mill, which has media that tumble and fracture the material. Shaft impactors cause particle-to particle attrition and compression.
Crushing, pulverising or disintegrating in general B02C
This place does not cover:
Mechanical aspects of methods specially adapted for comminuting clay or ceramic in non-fluid condition | B28C 1/18 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: comminuting, e.g. by grinding or breaking; defibrillating fibres other than asbestos | |
Grinding catalysts | |
Milling balls | |
Crushing, grinding or milling of metallic powders | |
Producing suspensions, e.g. by blunging or mixing; with means for removing stones | |
Grinding, deagglomeration, disintegration of aluminium oxide; Aluminium hydroxide; Aluminates | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: grinding silicic acid | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: grinding titania | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: grinding |
This place covers:
Milling with high energy in order to activate powders or to cause a chemical reaction leading to a different phase composition
This place covers:
A powder is calcined and then milled before it is either calcined again and/or used to make a ceramic. A green or sintered ceramic is destroyed by milling after which the resulting powder is calcined and/or used to make a ceramic,
This place covers:
The making and treating of mixtures of a liquid and solids where the wet mixture is used to make a ceramic material, e.g. obtaining a slurry with a certain viscosity
This place does not cover:
Making a clay slurry | |
Apparatus or methods for producing or processing clay or ceramic suspensions, e.g. slip | B28C 1/02 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating a ceramic substrate with a emulsion, dispersion or suspension | |
Feeding a slurry or a ceramic slip to moulds or apparatus for producing shaped articles | |
Abrasive powders, suspensions and pastes for polishing | C09K 3/1454 and subgroups |
Stabilised aqueous aluminosilicate suspensions for detergents |
This place covers:
Obtaining a slurry with a certain defined relation between the amount of solids and liquid is obtained, e.g. with 40-50 wt% solids or 20-30 vol% solids
Attention is drawn to the following places, which may be of interest for search:
Using specific drying method to reduce the solids loading |
This place covers:
Specific methods for mixing the solid and liquid components are used, e.g. a specific mixer is used, or a mixer is used with a specific rotation speed, ultra-sonification is used.
This place does not cover:
Mixing solids and liquids by a milling technique, e.g. wet ball milling | C04B 35/6261 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
At least two separate mixing steps are used to add different components to the ceramic mixture | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: aspects relating to the mixing step of the mortar preparation | C04B 40/0028 with subgroups |
Producing suspensions, e.g. by blunging or mixing; with means for removing stones | |
Apparatus or methods for mixing clay or ceramics with other substances | B28C 3/00 and subgroups |
Apparatus or methods for producing mixtures of cement with other substances, e.g. slurries, mortars, porous or fibrous compositions | B28C 5/00 and subgroups |
Controlling the operation of apparatus for producing mixtures of clay or cement with other substances; Supplying or proportioning the ingredients for mixing clay or cement with other substances; Discharging the mixture | B28C 7/00 and subgroups |
This place covers:
Using a specific liquid for a ceramic containing slurry or a slurry that is used for making a ceramic, e.g. water with a specific pH, a specific mixture of water with organic solvents, a specific mixture of organic solvents, using an unusual organic solvent
This place does not cover:
The use of organic solvents in coatings of ceramic substrates |
This place covers:
Thermal treatments of non-shaped ceramic or pre-ceramic powders or mixtures, such as calcining a ceramic powder mixture, pyrolysing an inorganic-organic pre-ceramic mixture, carbonising organic material into carbon or other non-oxide material, e.g. silicon carbide
This place does not cover:
Preheating, burning calcining or cooling of lime, magnesia or dolomite | C04B 2/10 and subgroups |
Burning methods for clay wares | C04B 33/32 and subgroup |
Curing of mixtures |
Attention is drawn to the following places, which may be of interest for search:
Heat treatment, e.g. precalcining, burning, melting; Cooling of hydraulic cements | C04B 7/43 and subgroups |
Methods and apparatus for] dehydrating gypsum | C04B 11/028 and subgroups |
Fillers added to cement, concrete, mortar or artificial stone: thermal treatment | C04B 20/04 and subgroups |
Burning or sintering processes for ceramics | |
Aspects relating to heat treatment of green bodies, e.g. burning, sintering or melting processes [N0808] | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
Processing clay- or ceramic containing substances in non-fluid condition by heating, drying | |
Producing mixtures of clay or cement with other substances; Heating, e.g. using steam | |
Making magnesia by calcining magnesium hydroxide | |
Dehydration of aluminium oxide or hydroxide, by calcination | C01F 7/441 and subgroups |
Drying; Calcining; After treatment of titanium oxide | |
Drying, calcination of silicic acid to enhance its pigmenting or filling properties | |
Drying, calcination of titania to enhance its pigmenting or filling properties | |
Drying, calcination of inorganic materials in general, other than fibrous fillers, to enhance their pigmenting or filling properties | |
Methods of preparing the interference pigments by wet methods, e.g. co-precipitation comprising a drying or calcination step after applying each layer | |
Methods of preparing the interference pigments by wet methods, e.g. co-precipitation comprising only a drying or calcination step of the finally coated pigment | |
Shaft or like vertical or substantially vertical furnaces wherein no smelting of the charge occurs, e.g. calcining or sintering furnaces | |
Rotary-drum furnaces, i.e. horizontal or slightly inclined Arrangements of preheating devices for the charge | |
Type of treatment of the charge: Calcining |
The class C04B 35/62645 is for instance given if the time of the heat treatment is of specific importance. If the specific temperature used is of significant importance, C04B 35/62675 is given.
This place covers:
Heating methods that result in the oxidation or reduction of powders, preparatory to the making of a ceramic material, e.g. reducing an oxide powder to a carbide powder in order to make a carbide ceramic, or oxidising a metallic powder to make an oxide ceramic
This place does not cover:
Directional oxidation or solidification, e.g. Lanxide process | |
Reduction treatment in general of a shaped ceramic |
Attention is drawn to the following places, which may be of interest for search:
Oxidative annealing of shaped ceramics | |
Reductive annealing of shaped ceramics |
This place covers:
The drying of unshaped ceramic mixtures or mixtures that can be used to make a ceramic, e.g. ceramic slurries, dispersions, hydrated powder, where the liquid can be water or any organic solvent.
Spraying or atomising in general B05
Drying solid materials or objects by removing liquid therefrom F26B
This place does not cover:
Drying clay or porcelain powder mixtures | |
Curing of starting mixtures for making ceramics or of green bodies | |
Drying of green ceramic or refractory bodies |
Attention is drawn to the following places, which may be of interest for search:
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: making use of electric or wave energy or particle radiation | C04B 40/0003 with subgroups |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: freeze-drying | |
Removal of physically bonded water from cement or ceramics, e.g. drying of hardened concrete | |
Drying a catalyst slurry, e.g. spray drying | |
Freeze drying, i.e. lyophilisation of catalysts | |
Spray drying of solutions or suspensions of metallic powders | B22F 9/026 or B22F 9/08 and subgroups |
Mechanical aspects of drying clay objects | |
Processing clay- or ceramic containing substances in non-fluid condition by heating, drying | |
Surface treatment of glass not in the form of fibres or filaments: drying; dehydroxylation] | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: drying silicic acid | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: drying titania | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: drying |
This place covers:
All drying methods where the humidity of the atmosphere is quantified, e.g. drying in a chamber with 50% humidity
Attention is drawn to the following places, which may be of interest for search:
Hardening mortars, concrete or artificial stone in an atmosphere of increased relative humidity |
This place covers:
The making of ceramic powders that are used to make ceramic objects by a method in which the raw material is molten or by which the raw material is passed through a flame, e.g. oxygen flame methods
This place does not cover:
Melting, fusion or softening of clay materials | |
Magnesium oxide refractories from grain sized mixtures containing chromium oxide or chrome ore obtained from fused grains | |
Melting of ceramic or refractory material to make a bulk ceramic | C04B 35/653 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Burning; Melting of hydraulic cements | C04B 7/44 and subgroups |
Burning; Melting of hydraulic cements, using plasmas or radiations | |
Melted agglomerated or melted waste materials or melted refuse as fillers for mortars, concrete or artificial stone | |
Ceramic products containing macroscopic reinforcing agents, e.g. fused silica | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: involving melting of at least part of the composition | |
Thermally activated mortars, e.g. by melting ingredients | |
Plasma sintering | |
Heat treatments such as; Fusing in general | B01J 6/005 and subgroup |
Preparation of catalyst particles by melting | |
Preparation of oxide powder in general by plasma method | |
Preparation of AlN powder by plasma method | |
Preparation of alumina powder by fusion or vaporisation | |
Treatment involving fusion or vaporisation of Aluminium oxide; Aluminium hydroxide; Aluminates | |
Preparation of alumina powder by plasma method | |
Melting temperature of inorganic powders | |
Hot gas, e.g. plasma, flame, burner for drawing optical glass fibers | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: plasma treatment |
This place covers:
Making ceramic powder by pyrolysing or carbonising raw material, or by using an auto-combustion reaction, e.g. using a mixture of metal nitrates with urea and heating this mixture until the auto-combustion starts
This place does not cover:
Carbonisation of organic material into carbon powders |
Attention is drawn to the following places, which may be of interest for search:
Porous mortars, concrete, artificial stone or ceramic ware obtained by a chemical conversion or reaction other than those relating to the setting or hardening of cement-like material or to the formation of a sol or a gel, e.g. by carbonising or pyrolysing preformed cellular materials based on polymers, organo-metallic or organo-silicon precursors | C04B 38/0022 and subgroups |
Inorganic membrane formation by carbonisation or pyrolysis | |
Heat treatments such as Pyrolysis in general | |
Decomposition and pyrolysis for making catalysts | B01J 37/082 and subgroups |
Making metal compounds by pyrolysis | B22F 9/30 and subgroup |
Making BN by pyrolysis | |
Making TiN, ZrN or HfN by pyrolysis | |
Multi-step carbonising or coking processes | |
Electrodes made by methods involving thermal decomposition pyrolysis |
This place covers:
The calcination of ceramic powders or ceramic fibers, where the used calcination temperature is of importance.
This place does not cover:
The sintering into a shaped ceramic at a certain specific temperature |
Attention is drawn to the following places, which may be of interest for search:
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcining silicic acid | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcining titania | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcination |
This place covers:
If the atmosphere during the heat treatment is not 1 atmosphere, but higher or lower, or if a specific gas is used, such as hydrogen, water, carbon monoxide, carbon dioxide, or if an unexpected atmosphere is used, e.g. heat treating oxide material in nitrogen or argon or heat treating non-oxide material in air
This place does not cover:
Used atmosphere during sintering of a shaped ceramic or bulk melting treatment | C04B 2235/658 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Selection of the kiln atmosphere during Heat treatment, e.g. precalcining, burning, melting; Cooling of hydraulic cements | |
Processes of utilising sub-atmospheric or super-atmospheric pressure to effect chemical or physical change of matter; Apparatus therefore |
This place covers:
Not all components are mixed together at the same moment, first a first mixture is made, which then receives a treatment such as calcination and/or milling, and then other components are added, thus there are at least two separate mixing steps in which components are added
Attention is drawn to the following places, which may be of interest for search:
Mixing solids and liquids by a milling technique, e.g. wet ball milling | C04B 35/6261 and subgroups |
Mixing components for ceramic mixtures in wet conditions general, e.g. with specific mixers | |
Apparatus or methods for mixing clay or ceramics with other substances | B28C 3/00 and subgroups |
Controlling the operation of apparatus for producing mixtures of clay or cement with other substances; Supplying or proportioning the ingredients for mixing clay or cement with other substances; Discharging the mixture | B28C 7/00 and subgroups |
This place covers:
Powder mixtures or pressed powder mixtures containing polymers or pre-polymers are heat treated in order to cure/set/harden the polymers or pre-polymer.
This place does not cover:
Drying clay or porcelain powder mixtures or clay green bodies | |
Drying, e.g. freeze-drying, spray-drying, microwave or supercritical drying of powder mixtures, slurries | |
Drying of green ceramic or refractory bodies | |
Heat treatments on green ceramic bodies | C04B 2235/65 and subgroups |
Mechanical aspects of curing ceramics | B28B 11/24 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: selection of the hardening environment | C04B 40/02 and subgroups |
Crosslinking, e.g. vulcanising, of macromolecules in general | C08J 3/24 and subgroups |
This place covers:
The ceramic material is granulated for instance by spray-drying, or is pelletised by using for instance a drum pelletiser, or by pressing
This place does not cover:
Devices for shaping artificial aggregates from ceramic mixtures |
Attention is drawn to the following places, which may be of interest for search:
Dehydrating; Forming, e.g. granulating of hydraulic cements in general | |
Pelletizing of Calcium sulphate cements before starting the manufacture | |
Granular material as fillers, e.g. pigments, for mortars, concrete or artificial stone | C04B 14/02 and subgroups |
Pelletizing flue dust | |
Processes or devices for granulating materials, e.g. fertilisers in general | B01J 2/00 and subgroups |
Granulating catalysts | |
Granulating metals | B22F 9/00 and subgroups |
Mechanical aspects of working of plastics or substances in a plastic state to make granules | B29B 9/00 and subgroups |
Granulation of active carbon | |
Granulation, agglomeration of Aluminium oxide; Aluminium hydroxide; Aluminates | |
Attrition-index or crushing strength of granulates | |
Pelletisation or prereacting of powdered raw materials for making glass | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: agglomeration, granulation, pelleting silicic acid | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: agglomeration, granulation, pelleting titania | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: agglomeration, granulation, pelleting |
This place covers:
Inorganic additives that are used for making ceramics are coated, for instance with organic surfactant
Spraying or atomising in general; applying liquids or other fluent materials to surfaces, in general B05F
This place does not cover:
Coating ceramic fibers | |
Coating bulk ceramic objects | C04B 41/45 and subgroups |
Coating or impregnating of particulate or fibrous ceramic material, that is not subsequently used in a ceramic material |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone | C04B 20/10 and subgroups |
Constituents for ceramics and secondary phases of ceramics not being of a fibrous nature | C04B 2235/30 and subgroups |
Coating metallic powders with an organic coating |
Organic coatings on inorganic powders are classified in C04B 35/628.
The particles that are coated are indicated with symbols from C04B 2235/30-C04B 2235/448.
This place covers:
Non-fibrous inorganic additives that are used for making ceramics are coated, for instance with silicon or boron, e.g. diamond particles are coated with Si that is used to make SiC bonded diamond
This place does not cover:
Coating non-fibrous inorganic additives that are used for making ceramics with an organic layer |
Attention is drawn to the following places, which may be of interest for search:
Coating of granules in general | |
Coating of catalyst particles | B01J 37/0221 and subgroup |
Coating metallic powders per se | B22F 1/16 and subgroup |
Coating; Grafting; Microencapsulation of active carbon | |
Coated silica sol particles | |
Coating or hydrophobisation of silica gel | |
Inorganic particles per se consisting of a mixture of two or more inorganic phases, one phase coated with the other | C01P 2004/84 and subgroups |
Pigments exhibiting interference colours comprising a core coated with only one layer having a high or low refractive index | |
Pigments exhibiting interference colours comprising a stack of coating layers with alternating high and low refractive indices, wherein the first coating layer on the core surface has the high refractive index | C09C 1/0024 and subgroups |
Pigments exhibiting interference colours comprising a stack of coating layers with alternating low and high refractive indices, wherein the first coating layer on the core surface has the low refractive index | C09C 1/0051 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: coating silicic acid | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: coating titania | |
Treatment in general of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: coating | |
Interference pigments characterized by the core material | C09C 2200/10 and subgroups |
Interference pigments comprising a layer with a concentration gradient or a gradient of the refractive index | C09C 2200/20 and subgroups |
Interference pigments comprising an outermost surface coating | C09C 2200/40 and subgroups |
Interference pigments comprising a layer or a core consisting of or comprising discrete particles, e.g. nanometric or submicrometer-sized particles; Inorganic particles, e.g. oxides, nitrides or carbides | |
Abrasive composite particles per se, e.g. coated particles | C09K 3/1436 and subgroup |
This class is used for inorganic coatings on inorganic particles, where this coating cannot be classified in any of the subgroups for oxide, non-oxide or metal coating.
This place covers:
Inorganic particles are coated with an oxide layer or with material that converts to an oxide layer upon heating, such as with a metal nitrate salt, a metal carbonate salt, a metal halide salt, a metal phosphate, or with organo-metallics such as metal acetate. The coating contains for the majority oxide material, but can also contain a minority of non-oxide material.
This place does not cover:
Coating inorganic fibers with an oxide layer | C04B 35/62847 and subgroups |
Coating of bulk ceramic objects with an oxide coating | C04B 41/5025, C04B 41/5072 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Light-sensitive devices comprising an oxide semiconductor electrode comprising mixed oxides, e.g. ZnO covered TiO2 particles |
This place covers:
Inorganic particles are coated with a layer that contains for the majority a silica or silicate phase or with material that converts for the majority to a silica or silicate phase upon heating, for instance alumino-silicates such as cordierite, mullite, spodumene, alkaline earth silicates such as forsterite, wollastonite
This place does not cover:
Coating inorganic fibers with a silica or silicate layer | |
Coating of bulk ceramic objects with a silicate or silica coating | |
Coating of bulk ceramic objects with a clay or kaolin coating |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone with silicate |
This place covers:
Inorganic particles are coated with a layer that contains for the majority an alkaline earth oxide phase or with material that converts for the majority to an alkaline earth oxide phase upon heating, such as with an alkaline earth nitrate salt, an alkaline earth carbonate salt, an alkaline earth halide salt, or with organo-metallics such as an alkaline earth acetate.
This place does not cover:
Mixed oxide coatings on inorganic particles of alkaline earth oxides with copper oxide, e.g. cuprates | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with zinc oxide and/or bismuth oxide, e.g. magnesium bismuthate | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with tin oxide, e.g. magnesium stannate | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with both alumina and silica, e.g. cordierite | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with silica without alumina, e.g. wollastonite (CaSiO4) | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with alumina, without silica, e.g. calcium aluminate | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with alumina, without silica, e.g. magnesium aluminate, spinel | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with chromium oxide, e.g. chromites | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. magnesium tantalum niobate (MgNb0.5Ta0.5O3) | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with titanium oxides, such as magnesium titanate or calcium titanate | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with zirconium oxide, e.g. magnesium zirconate, containing more Zr than Mg and Ca | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with zirconium oxide and titanium oxide, e.g. calcium titanate zirconate (CaTi0.5Zr0.5O3) | |
Mixed oxide coatings on inorganic particles of alkaline earth oxides with iron oxides and possible other metal oxides, e.g. ferrites | |
Coating inorganic fibers with an alkaline earth oxide layer | |
Coating of bulk ceramic objects with a magnesia coating |
The alkaline earth oxide is not mixed with other metal oxides, except for alkali metal oxides or rare earth metal oxides. If the alkaline earth metal is mixed with other metal oxides, normally other materials such as alkaline earth metal titanates, aluminates, silicates, zirconates, niobates, chromates, ferrites, manganates, etc. are formed. These coatings are classified in the classes for these materials, e.g. alkaline earth aluminates in C04B 35/62813, alkaline earth titanate in C04B 35/62821, alkaline earth chromates and niobates in C04B 35/62818, alkaline earth zirconates in C04B 35/62823, alkaline earth ferrites in C04B 35/62826, alkaline earth silicates in C04B 35/62807, alkaline earth manganates, stannates C04B 35/62805. Mixtures of alkaline earth, alkali and rare earth oxide are classified according to which of these 3 groups is present in the largest amount, e.g. a coating containing 40 wt% alkaline earth oxide, 30 wt% alkali oxide and 30 wt% rare earth oxide is classified in C04B 35/6281.
This place covers:
Inorganic particles are coated with a layer that contains for the majority an alumina or aluminate phase or with material that converts for the majority to an alumina or aluminate phase upon heating, such as with an aluminum nitrate salt, an aluminum carbonate salt, an aluminum halide salt, or with organo-metallics such as an aluminum acetate. The aluminate can for instance be a spinel, calcium aluminate, lanthanum aluminate, etc.
This place does not cover:
Alumino-silicate coating on inorganic particles | |
Coating inorganic fibers with an alumina or aluminate layer | |
Coating of bulk ceramic objects with an alumina or aluminate coating | C04B 41/5031 and subgroup |
Coating of bulk ceramic objects with a spinel coating |
This place covers:
Inorganic particles are coated with a layer that contains for the majority a rare earth oxide phase or with material that converts for the majority to a rare earth oxide phase upon heating, such as with a rare earth nitrate salt, a rare earth carbonate salt, a rare earth halide salt, or with organo-metallics such as a rare earth acetate.
This place does not cover:
Mixed oxide coatings on inorganic particles of rare earth oxide with copper oxide, e.g. superconducting LaBa-cuprate | |
Mixed oxide coatings on inorganic particles of rare earth oxide with zinc oxide and/or bismuth oxide, e.g. dysprosium bismuthate | |
Mixed oxide coatings on inorganic particles of rare earth oxide with tin oxide, e.g. neodymium stannate | |
Mixed oxide coatings on inorganic particles of rare earth oxide with silica without alumina | |
Mixed oxide coatings on inorganic particles of rare earth oxide with both alumina and silica | |
Mixed oxide coatings on inorganic particles of rare earth oxide with alumina, without silica, e.g. scandium aluminate | |
Mixed oxide coatings on inorganic particles of rare earth oxide with chromium oxide, e.g. lanthanum chromites | |
Mixed oxide coatings on inorganic particles of rare earth oxide with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. erbium tantalum niobate (ErNb0.5Ta0.5O3) | |
Mixed oxide coatings on inorganic particles of rare earth oxide with titanium oxides, such as lanthanum titanate or cerium titanate | |
Mixed oxide coatings on inorganic particles of rare earth oxide with zirconium oxide, e.g. cerium zirconate, containing more Zr than rare earth metals | |
Mixed oxide coatings on inorganic particles of rare earth oxide with zirconium oxide and titanium oxide, e.g. ytterbium titanate zirconate (YbTi0.5Zr0.5O3) | |
Mixed oxide coatings on inorganic particles of rare earth oxide with iron oxides and possible other metal oxides, e.g. ferrites | |
Coating inorganic fibers with a rare earth oxide layer | |
Coating of bulk ceramic objects with a rare earth oxide coating |
The rare earth oxide is not mixed with other metal oxides, except for alkali metal oxides or rare earth metal oxides. If the rare earth metal is mixed with other metal oxides, normally other materials such as rare earth metal titanates, aluminates, silicates, zirconates, niobates, chromates, ferrites, manganates, etc. are formed. These coatings are classified in the classes for these materials, e.g. rare earth aluminates in C04B 35/62813, rare earth titanate in C04B 35/62821, rare earth chromates and niobates in C04B 35/62818, rare earth zirconates in C04B 35/62823, rare earth ferrites in C04B 35/62826, rare earth silicates in C04B 35/62807, rare earth manganates, stannates C04B 35/62805. Mixtures of alkaline earth, alkali and rare earth oxide are classified according to which of these 3 groups is present in the largest amount, e.g. a coating containing 30 wt% alkaline earth oxide, 30 wt% alkali oxide and 40 wt% rare earth oxide is classified in C04B 35/62815.
This place covers:
Inorganic particles are coated with a layer that contains for the majority a refractory metal oxide phase or with material that converts for the majority to a refractory metal oxide phase upon heating, such as with refractory metal nitrate salt, refractory metal carbonate salt, refractory metal halide salt, or with organo-metallics such as refractory metal acetate. The refractory metal can for instance be chromium, vanadium, niobium, tungsten etc., the oxide phase can also be a mixed oxide such as a chromate, niobate, tungstate, vanadate.
This place does not cover:
Coating inorganic fibers with a refractory metal oxide layer | |
Coating of bulk ceramic objects with a chromium oxide coating | |
Coating of bulk ceramic objects with a niobium oxide or niobate coating |
This place covers:
Inorganic particles are coated with a layer that contains for the majority a titania or titanate phase or with material that converts for the majority to a titania or titanate phase upon heating, such as with titanium nitrate salt, titanium carbonate salt, titanium halide salt, or with organo-metallics such as titanium acetate. The titanates can be for instance calcium titanate, barium titanate, aluminium titanate, bismuth titanate, lead titanate, strontium titanate, sodium titanate, etc.
This place does not cover:
Titanate-zirconate coating on an inorganic particle | |
Coating inorganic fibers with a titanium oxide or titanate layer | |
Coating of bulk ceramic objects with a titanium oxide or titanate coating |
This place covers:
Inorganic particles are coated with a layer that contains for the majority a zirconia or zirconate phase or with material that converts for the majority to a zirconia or zirconate phase upon heating, such as with zirconium nitrate salt, zirconium carbonate salt, zirconium halide salt, or with organo-metallics such as zirconium acetate. The zirconates can be for instance calcium zirconate, barium zirconate, bismuth zirconate, lead zirconate, strontium zirconate, sodium zirconate, or titanate-zirconates such as lead titanate zirconate (PZT), etc.
This place does not cover:
Coating inorganic fibers with a zirconium oxide or zirconate layer | |
Coating of bulk ceramic objects with a zirconium oxide, hafnium oxide, zirconate or hafnate coating | C04B 41/5042 and subgroup |
This place covers:
Inorganic particles are coated with a layer that contains for the majority an iron oxide or ferrite phase or with material that converts for the majority to an iron oxide or ferrite phase upon heating, such as with ferric or ferrous nitrate salt, ferric or ferrous carbonate salt, ferric or ferrous halide salt, or with organo-metallics such as ferric or ferrous acetate. The ferrites can be for instance calcium ferrite, barium ferrite, bismuth ferrite, lead ferrite, strontium ferrite, sodium ferrite, Mn-Zn ferrite, etc.
This place does not cover:
Coating inorganic fibers with an iron group metal oxide layer | |
Coating of bulk ceramic objects with a ferrite coating |
This place covers:
Inorganic particles are coated with a non-oxide layer, e.g. with a boride or silicide coating. The coating contains for the majority non-oxide material, but can also contain a minority of oxide material.
This place does not cover:
Coating inorganic fibers with a non-oxide ceramic layer | |
Coating of bulk ceramic objects with a non-oxide coating | C04B 41/5072 and subgroups |
This place covers:
Inorganic particles are coated with a carbide layer, e.g. with titanium carbide, boron carbide, tungsten carbide, zirconium carbide, oxy carbides. The coating contains for the majority carbide material, but can also contain a minority other material such as oxides, nitrides, borides.
This place does not cover:
Coating of particles with carbo-nitrides | |
Coating inorganic fibers with a carbide layer | |
Coating of bulk ceramic objects with a carbide coating | C04B 41/5057 and subgroups |
This place covers:
Inorganic particles are coated with a silicon carbide layer, e.g. with silicon oxy-carbide, with alpha silicon carbide, beta silicon carbide. The coating contains for the majority silicon carbide material, but can also contain a minority other material such as oxides, nitrides, borides.
This place does not cover:
Coating of particles with silicon carbo-nitrides | |
Coating inorganic fibers with a silicon carbide layer | |
Coating of bulk ceramic objects with a silicon carbide coating |
This place covers:
Inorganic particles are coated with a nitride layer, e.g. with titanium nitride, boron nitride, tungsten nitride, zirconium nitride, aluminium nitride, silicon nitride, sialon, oxy nitrides in general, carbonitrides. The coating contains for the majority nitride material, but can also contain a minority other material such as oxides, carbides, borides.
This place does not cover:
Coating inorganic fibers with a nitride layer | C04B 35/62865 and subgroups |
Coating of bulk ceramic objects with a nitride coating | C04B 41/5062, C04B 41/5063, C04B 41/5064, C04B 41/5066, C04B 41/5067, C04B 41/5068 |
This place covers:
Inorganic particles are coated with a carbon layer or carbon-like layer, e.g. with diamond, graphite, carbon black, pitch, tar, anthracene. The coating contains for the majority carbon material, but can also contain a minority other material such as oxides, carbides, borides, nitrides.
This place does not cover:
Coating of particles with carbides | C04B 35/62831 and subgroup |
Coating inorganic fibers with a carbon layer | |
Coating of bulk ceramic objects with carbon or carbonisable material coating | C04B 41/5001 and subgroups |
This place covers:
Inorganic particles to be used in making a ceramic are coated with a metal layer, e.g. with cobalt, nickel, iron, aluminium, titanium, silver, gold, platinum, palladium, chromium, copper. The coating contains for the majority metallic material, but can also contain a minority other material such as oxides, carbides, borides, nitrides, carbon.
This place does not cover:
Coating inorganic fibers with a metallic layer, to be used as reinforcement in ceramics | |
Metalising bulk ceramic substrates, or metalising ceramic powders that are not used for making ceramics | C04B 41/51 and subgroups |
Coating ceramic particles with a metallic layer, where the coated particles are subsequently used to make a cermet | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone with metal | |
Coating metallic powder with a metallic coating |
In most cases coating ceramic particles with a metallic coating will not be usable to make a ceramic, but only for making a cermet, since the metal coating will easily form a metallic binder phase. In some cases, however, a metallic binder phase will not be formed, for instance if a ceramic coating layer is coated on top of the metallic coating layer and the ceramic coating encloses the metallic coating layer completely. The metallic coating layer might also react to a ceramic phase, e.g. to an oxide through oxidation, or to a carbide or nitride. In such a case the particle is usable for making a ceramic.
This place covers:
All ceramic fibers that are coated, whether they are used in a ceramic composite or for any other purpose; other fibers, e.g. glass or metallic fibers, that are used as additive for a ceramic composite that are coated; the coating can for instance be an organic surfactant
- Spraying or atomising in general; applying liquids or other fluent materials to surfaces, in general B05F
- Treating of textile materials by liquids, gases or vapours D06B
This place does not cover:
Making ceramic fibers | C04B 35/62227 and subgroups |
Coating or impregnating of particulate or fibrous ceramic material, that is not subsequently used in a ceramic material | |
Coating glass fibers that are not used to make ceramics | C03C 25/10 and subgroups |
The coating of fibers that are used as additive to metallic alloys |
Attention is drawn to the following places, which may be of interest for search:
Fibrous materials and whiskers added to cement, concrete, mortar or artificial stone: composite fibres, e.g. fibres with a core and sheath of different material | |
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone: fillers characterised by the shape, e.g. fibrous materials | |
Coating inorganic particles that are used for making ceramics | C04B 35/62802 and subgroups |
Fibers used in ceramic composition | C04B 2235/5208 and subgroups |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture | D01F 11/10 and subgroups |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with inorganic substances or complexes thereof | D06M 11/00 and subgroups |
Organic coatings, e.g. a surfactant coating, on the fibers are classified in C04B 35/62844. The fiber that is coated is in principle indicated by symbols from the range C04B 2235/5208-C04B 2235/5248. If the synthesis or the composition of the fiber that is coated is of particular importance, a class from C04B 35/62227 and subgroups is given. In that case it is not necessary anymore to give a symbol from the range C04B 2235/5208-C04B 2235/5248 to indicate the fiber substrate that is coated.
This place covers:
Fibers are coated with an oxide layer. The coating contains for the majority oxide material, but can also contain a minority of non-oxide material.
This place does not cover:
Making oxide ceramic fibers | C04B 35/62231 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating inorganic particles used for making ceramics with an oxide layer | C04B 35/62805 and subgroups |
Coating of bulk ceramic objects with an oxide coating | C04B 41/5025, C04B 41/5072 and subgroups |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with oxides | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with oxides, hydroxides or mixed oxides; with salts derived from anions with an amphoteric element-oxygen bond | D06M 11/36 and subgroups |
This place covers:
Fibers are coated with a layer that contains for the majority a silica or silicate phase, for instance alumino-silicates such as cordierite, mullite, spodumene, alkaline earth silicates such as forsterite, wollastonite
This place does not cover:
Making fibers based on silica | C04B 35/6224 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone with silicate | |
Making silica ceramics in general | |
Making silicate ceramics in general | C04B 35/16 and subgroups |
Coating inorganic particles used for making ceramics with a silica or silicate layer | |
Coating of bulk ceramic objects with a silicate or silica coating | |
Coating of bulk ceramic objects with a clay or kaolin coating | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with silicon dioxide, silicic acids or their salts |
This place covers:
Fibers are coated with a layer that contains for the majority an alumina or aluminate phase. The aluminate can for instance be a spinel, calcium aluminate, lanthanum aluminate, etc.
This place does not cover:
Making fibers based on alumina | |
Alumino-silicate coating on inorganic fibers |
Attention is drawn to the following places, which may be of interest for search:
Making alumina ceramics in general | C04B 35/10 and subgroups |
Making aluminate ceramics in general | C04B 35/44 and subgroup |
Coating inorganic particles used for making ceramics with an alumina or aluminate layer | |
Coating of bulk ceramic objects with an alumina or aluminate coating | C04B 41/5031 and subgroup |
Coating of bulk ceramic objects with a spinel coating | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with oxides or hydroxides of elements of the third Group of the Periodic Table; Aluminates |
This place covers:
Fibers are coated with a layer that contains for the majority a refractory metal oxide phase. The refractory metal can for instance be chromium, vanadium, niobium, tungsten, titanium, zirconium etc., the oxide phase can also be a mixed oxide such as a chromate, niobate, tungstate, vanadate, titanate, zirconate, etc.
This place does not cover:
Making fibers based on zirconium oxide, e.g. zirconates such as PZT | |
Making fibers based on titanium oxide |
Attention is drawn to the following places, which may be of interest for search:
Making chromia based oxides in general | |
Making chromite based oxides in general | |
Making titania and titanate based oxides in general | C04B 35/46 and subgroups |
Making zirconia and zirconate based oxides in general | C04B 35/48 and subgroups |
Making ceramics based on the oxides of tantalum, niobium, tungsten, molybdenum and vanadium | C04B 35/495 and subgroups |
Coating of bulk ceramic objects with a chromium oxide coating | |
Coating of bulk ceramic objects with a titanium oxide or titanate coating | |
Coating of bulk ceramic objects with a zirconium oxide, hafnium oxide, zirconate or hafnate coating | C04B 41/5042 and subgroup |
Coating of bulk ceramic objects with a niobium oxide or niobate coating | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with oxides or hydroxides of elements of the fourth Group of the Periodic Table; titanates; tirconates; stannates; plumbates | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with oxides or hydroxides of elements of fifth Group of the Periodic Table; vanadates; niobates; tantalates; arsenates: antimonates; bismuthates | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with oxides of chromium, molybdenum or tungsten; chromates; dichromates; molybdates; tungstates |
This place covers:
Fibers are coated with a non-oxide layer, e.g. with a nitride, carbide, boride or silicide coating. The coating contains for the majority non-oxide material, but can also contain a minority of oxide material.
This place does not cover:
Making fibers based on non-oxide ceramics |
Attention is drawn to the following places, which may be of interest for search:
Coating inorganic particles used for ceramics with a non-oxide ceramic layer | C04B 35/62828 and subgroups |
Coating of bulk ceramic objects with a non-oxide coating | C04B 41/5072 and subgroups |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with boron, borides, boron nitrides | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with sulphur, selenium, tellurium, polonium or compounds thereof | D06M 11/51 and subgroups |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with boron or compounds thereof, e.g. borides |
This place covers:
Fibers are coated with a carbide layer, e.g. with titanium carbide, boron carbide, tungsten carbide, zirconium carbide, oxy carbides. The coating contains for the majority carbide material, but can also contain a minority other material such as oxides, nitrides, borides.
This place does not cover:
Making fibers based on carbides | C04B 35/62277 and subgroup |
Coating of fibers with carbo-nitrides | C04B 35/62865 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Making carbide ceramics in general | C04B 35/56 and subgroups |
Coating inorganic particles used for making ceramics with a carbide layer | |
Coating of bulk ceramic objects with a carbide coating | C04B 41/5057 and subgroups |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with boron carbide | |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with carbides | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with carbon or compounds thereof, e.g. with carbon or graphite; with carbides; with graphitic acids or their salts |
This place covers:
Fibers are coated with a silicon carbide layer, e.g. with silicon oxy-carbide, with alpha silicon carbide, beta silicon carbide. The coating contains for the majority silicon carbide material, but can also contain a minority other material such as oxides, nitrides, borides.
This place does not cover:
Making fibers based on silicon carbide | |
Coating of fibers with silicon carbo-nitrides |
Attention is drawn to the following places, which may be of interest for search:
Making silicon carbide ceramics in general | C04B 35/565 and subgroups |
Coating inorganic particles used for making ceramics with a silicon carbide layer | |
Coating of bulk ceramic objects with a silicon carbide coating |
This place covers:
Fibers are coated with a nitride layer, e.g. with titanium nitride, tungsten nitride, zirconium nitride, aluminium nitride, oxy nitrides, carbonitrides. The coating contains for the majority nitride material, but can also contain a minority other material such as oxides, carbides, borides.
This place does not cover:
Making fibers based on nitrides | C04B 35/62286 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Making nitride ceramics in general | C04B 35/58 and subgroups |
Coating inorganic particles used for making ceramics with a nitride layer | |
Coating of bulk ceramic objects with a nitride coating | C04B 41/5062, C04B 41/5063, C04B 41/5064, C04B 41/5066, C04B 41/5067, C04B 41/5068 |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with nitrides, nitrogen carbides | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with nitrogen or compounds thereof, e.g. with nitrides |
This place covers:
Fibers are coated with a boron nitride layer, e.g. with hexagonal or cubic boron nitride, boron oxy nitride, boron carbonitride. The coating contains for the majority boron nitride material, but can also contain a minority other material such as oxides, carbides, borides.
This place does not cover:
Making fibers based on boron nitrides |
Attention is drawn to the following places, which may be of interest for search:
Making boron nitride ceramics in general | C04B 35/583 and subgroup |
Coating inorganic particles used for making ceramics with a nitride layer | |
Coating of bulk ceramic objects with a boron nitride coating | |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with boron, borides, boron nitrides |
This place covers:
Fibers are coated with a silicon nitride layer, e.g. with alpha or beta silicon nitride, silicon oxy nitride, sialon, silicon carbonitride, silicon oxy carbonitride. The coating contains for the majority silicon nitride material, but can also contain a minority other material such as oxides, carbides, borides.
This place does not cover:
Making fibers based on silicon nitrides |
Attention is drawn to the following places, which may be of interest for search:
Making silicon nitride ceramics in general | C04B 35/584 and subgroups |
Coating inorganic particles used for making ceramics with a nitride layer | |
Coating of bulk ceramic objects with a silicon nitride coating | C04B 41/5066, C04B 41/5067 (sialon) |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with boron, borides, boron nitrides |
This place covers:
Fibers are coated with a carbon layer or carbon-like layer, e.g. with diamond, graphite, carbon black, pitch, tar, anthracene. The coating contains for the majority carbon material, but can also contain a minority other material such as oxides, carbides, borides, nitrides.
This place does not cover:
Coating of fibers with carbides | C04B 35/6286 and subgroup |
The synthesis of carbon fibers |
Attention is drawn to the following places, which may be of interest for search:
Making carbon based ceramics in general | C04B 35/52 and subgroups |
Coating inorganic particles used for making ceramics with a carbon layer | |
Coating of bulk ceramic objects with carbon or carbonisable material coating | C04B 41/5001 and subgroups |
Surface treatment of fibres or filaments from glass, minerals, or slags by coating with carbon, e.g. graphite | |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with carbon | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with carbon or compounds thereof, e.g. with carbon or graphite; with carbides; with graphitic acids or their salts |
This place covers:
Coating organic or inorganic fibers that are used for making ceramic objects with a metallic coating layer, e.g. with cobalt, nickel, iron, aluminium, titanium, silver, gold, platinum, palladium, chromium, copper. The coating contains for the majority metallic material, but can also contain a minority other material such as oxides, carbides, borides, nitrides, carbon.
This place does not cover:
Metalising fibers that are not used for making ceramics | C04B 41/51 and subgroups |
Making metallic fibers |
Attention is drawn to the following places, which may be of interest for search:
Coating inorganic particles, used for making ceramics with a metallic layer | |
Coating of bulk ceramic objects with a metallic coating | C04B 41/51 and subgroups |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with metals | D01F 11/127, D01F 11/16 (by electrolysis) |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with metals, with metal-generating compounds, e.g. metal carbonyls; Reduction of metal compounds on textiles |
This place covers:
The coating of fibers that are used in making ceramic objects with boron or silicon, e.g. coating a carbon fiber with silicon and reacting this fiber into a silicon carbide fiber, or coating a carbon fiber with boron and reacting this fiber into boron carbide
Attention is drawn to the following places, which may be of interest for search:
Coating inorganic particles used for making ceramics with boron or silicon | |
Coating of bulk ceramic objects with a boron coating | |
Coating of bulk ceramic objects with a silicon coating | |
Chemical after-treatment of artificial filaments or the like of carbon during manufacture with boron, borides, boron nitrides | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with silicon, with halides or oxyhalides of silicon, with fluorosilicates | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with boron, boron halides, fluoroborates |
This place covers:
Inorganic particles are coated with a metal salt, such as with a metal nitrate salt, a metal carbonate salt, a metal halide salt, a metal phosphate, or with organo-metallics such as metal acetate. The metal salts are not converted into an oxide, carbide, nitride, boride phase, but remain as a metal salt on the fiber.
This place does not cover:
Making fibers based on metal phosphorus oxides, e.g. phosphates | |
Coating inorganic fibers with a metal salt that is converted into an oxide layer | C04B 35/62847 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating of bulk ceramic objects with salts or salty compositions | C04B 41/5007 and subgroups |
Coating of bulk ceramic objects with phosphates | |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with halogens; with halogen acids or salts thereof; with oxides or oxyacids of halogens or salts thereof | D06M 11/07 and subgroups |
Treating fibres, threads, yarns, fabrics, or fibrous goods made from such materials, with phosphorus or compounds thereof, e.g. with chlorophosphonic acid or salts thereof | D06M 11/68 and subgroups |
This place covers:
The coating on the inorganic particles or on the fibers is applied by a gas phase technique, such as CVD (chemical vapour deposition), PVD (physical vapour deposition)
Making ceramic powders by gas phase techniques C01
This place does not cover:
Making ceramic fibers by gas phase techniques | C04B 35/62227 and subgroups |
Reacting an inorganic powder or fiber with a gas, other than oxygen, to create a new phase, e.g. reacting an oxide powder or carbide fiber with ammonia to make a nitride powder or carbo-nitride fiber | |
Coating metallic substrates by chemical coating by decomposition of gaseous compounds, without leaving reaction products of the surface material in the coating, e.g. chemical vapour deposition (CVD) processes | C23C 16/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating a bulk ceramic substrate applied from the gas phase | C04B 41/4529 and subgroups |
Gas infiltration of green bodies or pre-forms | |
PVD, CVD methods or coating in a gas-phase using a fluidized bed of preparing the interference pigments |
This place covers:
The coating on the inorganic particles or on the fibers is applied by using a suspension or solution, e.g. by dipping the fibers in the liquid, or by dispersing the powder in a solution and filtering the powder of
Making ceramic powders by gas phase techniques C01
This place does not cover:
Making ceramic fibers by wet chemical techniques | C04B 35/62227 and subgroups |
Chemically coating metallic substrates by decomposition of either liquid compounds or solutions | C23C 18/00 and subgroups |
Chemically coating metallic substrates by decomposition of either solid compounds or suspensions | C23C 20/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating a ceramic substrate applied as a solution, emulsion, dispersion or suspension | C04B 41/4535 and subgroups |
Liquid infiltration of green bodies or pre-forms | |
Wet methods, e.g. co-precipitation of preparing the interference pigments | C09C 2220/10 and subgroups |
This place covers:
The applied coating layer does not cover the whole surface of the particles or fibers
Attention is drawn to the following places, which may be of interest for search:
Partial coating of a bulk ceramic substrate | C04B 41/4572 and subgroups |
This place covers:
The coating layer on the particles or fibers consists out of individual particles
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating a ceramic substrate applied as a solution, emulsion, dispersion or suspension | |
Coating or impregnating a ceramic substrate with a powdery material | C04B 41/4545 and subgroups |
Coatings of catalysts comprising impregnated particles | |
Interference pigments comprising a layer or a core consisting of or comprising discrete particles, e.g. nanometric or submicrometer-sized particles | C09C 2200/50 and subgroups |
This place covers:
Coating the inorganic particles or fibers with at least two coating layers. The coating layers can be of the same material or of different material.
This place does not cover:
Multiple coatings of bulk ceramic substrates or multiple coatings of powders or fibers that are not used to make ceramic materials | C04B 41/52 and subgroups, C04B 41/89 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating fillers added to cement, concrete, mortars or artificial stone with multiple coatings | C04B 20/12 and subgroup |
Coatings of catalyst comprising several layer |
This place covers:
If the thickness of the coating layer on the inorganic particles or fibers is specified, this class is used.
Attention is drawn to the following places, which may be of interest for search:
Inorganic particles per se consisting of a mixture of two or more inorganic phases, one phase coated with the other: thin layer coatings, i.e. the coating thickness being less than 0.1 time the particle radius | |
Inorganic particles per se consisting of a mixture of two or more inorganic phases, one phase coated with the other: thick layer coatings | |
Interference pigments characterised by the thickness of the core or layers thereon or by the total thickness of the final pigment particle | C09C 2200/30 and subgroups |
This place covers:
The addition of additives that have a function in the shaping of the ceramic product, e.g. binders, plasticizers, lubricants, surfactants, seeds
This place does not cover:
Additives that are added to the ceramic material to create porosity after a heat treatment | C04B 38/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Binders for clay products | |
Addition of a binding agent for catalysts or of material, later completely removed a.o. as result of heat treatment, leaching or washing | |
Binders for refractory moulds | B22C 1/16 and subgroups |
Additional symbols (CCA) from the C04B 2235/00-scheme can be used to further specify the additive.
This place covers:
All inorganic additives that are added for influencing the shaping of the ceramic product, in praxis inorganic binders
This place does not cover:
Inorganic additives for clay products | |
Oxide ceramics having a carbon binder |
Attention is drawn to the following places, which may be of interest for search:
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder | C04B 28/00 and subgroups |
Inorganic binders for refractory moulds | B22C 1/18 and subgroups |
Additional symbols (CCA) from the C04B 2235/00-scheme can be used to further specify the inorganic additive.
This place covers:
Phosphoric acid or phosphates are added to ceramic mixtures specifically with the function as binder
This place does not cover:
Ceramics based on phosphate material | |
Adding phosphoric acid or phosphates for other purposes |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: containing phosphorus in the anion, e.g. phosphates | C04B 22/16 and subgroup |
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder: containing cold phosphate binders | C04B 28/34 and subgroups |
Inorganic additives for clay products | |
Inorganic phosphate binders for refractory moulds | |
Preparation of phosphates per se, e.g. phosphates powder, not preparative to making a phosphates ceramic | C01B 25/26 and subgroups |
This class can also be added in the case C04B 33/131 is given (inorganic additives for clay materials). No additional symbol (CCA) from the C04B 2235/00-scheme need to be used here.
If the phosphate binder phase forms the largest fraction of the ceramic, C04B 35/447 is used, rather than C04B 35/6306 or one of its subgroups.
This place covers:
The use of aluminium phosphate as binder for ceramic material
Attention is drawn to the following places, which may be of interest for search:
Alumina additives | C04B 2235/3217 and subgroups |
Preparation of aluminium phosphates per se, e.g. aluminium phosphates powder, not preparative to making an aluminium phosphate ceramic |
This class can also be added in the case C04B 33/131 is given (inorganic additives for clay materials). No additional symbol (CCA) from the C04B 2235/00-scheme need to be used here.
This place covers:
The use of alkali metal or alkaline earth metal phosphates as binder for ceramic material
Attention is drawn to the following places, which may be of interest for search:
Alkali oxides as additives for making ceramics or as secondary phase | C04B 2235/3201 and subgroup |
Alkaline earth oxides as additives for making ceramics or as secondary phase | C04B 2235/3205 and subgroups |
Preparation of alkali metal phosphates per se, e.g. alkali metal phosphates powder, not preparative to making an alkali metal phosphate ceramic | C01B 25/30 and subgroups |
Preparation of alkaline earth metal phosphates per se, e.g. alkaline earth metal phosphates powder, not preparative to making an alkaline earth metal phosphate ceramic | C01B 25/32 and subgroups |
This class can also be added in the case C04B 33/131 is given (inorganic additives for clay materials). The specific alkali metal or alkaline earth ions are indicated with additional symbols (CCA) from the C04B 2235/00-scheme.
This place covers:
Silicon compounds such as silica, silicates such as waterglass or clays, glass, silicon carbide (e.g. for diamond), silicon nitride, silicides, are used as binder for ceramic materials
This place does not cover:
Ceramics based on silica | |
Ceramics based on silicates | C04B 35/16 and subgroups |
Ceramics based on zircon (zirconium silicate) | |
Ceramics based on silicon carbide | C04B 35/565 and subgroups |
Ceramics based on silicides | C04B 35/58085 and subgroup |
Ceramics based on silicon nitride | C04B 35/584 and subgroups |
Ceramics based on sialon |
Attention is drawn to the following places, which may be of interest for search:
Alkali metal or ammonium silicate cements Alkyl silicate cements ; silica sol cements; soluble silicate cements | |
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder: containing mixtures of the silica-lime type | C04B 28/18 and subgroups |
Compositions of mortars, concrete or artificial stone, containing inorganic binders or the reaction product of an inorganic and an organic binder: containing alkyl, ammonium or metal silicates; containing silica sols | C04B 28/24 and subgroup |
Inorganic additives for clay products | |
Ceramics based on zirconia, containing a silica binder | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. water glass (Na2SiO3) | C04B 2235/3427 and subgroups |
Clays as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bentonites/smectites such as montmorillonite, kaolines such as halloysite, illite, talc, sepiolite and attapulgite, vermiculite | |
Glass starting materials for making ceramics, e.g. silica glass | C04B 2235/36 and subgroup |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic, i.e. chemical compounds between silicon and a one or more metals, e.g. chromium silicide (CrSi2), molybdenum disilicide (MoSi2), iron silicide (FeSi, FeSi2), cobalt silicide (Co2Si, CoSi, CoSi2) | |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Inorganic silica or silicate binders for refractory moulds | B22C 1/186 and subgroup |
Preparation of silica powders, sols, gels, dispersions and their after-treatments | C01B 33/113 and subgroups |
Preparation of silicate powders, sols, gels, dispersions and their after-treatments | C01B 33/20 and subgroups |
This class can also be added in the case C04B 33/131 is given (inorganic additives for clay materials). Additional symbols (CCA) from the C04B 2235/00-scheme normally need to be added as well, e.g. in the case of a silica binder: C04B 35/6316 and C04B 2235/3418. In the case of a silicon nitride binder, possibly formed in situ: C04B 35/6316 and C04B 2235/3873.
If the silicon compound binder phase forms the largest fraction of the ceramic, the respective class for the silicon binder compound is used, e.g. a silica binder that forms the largest fraction, then C04B 35/14 is used, rather than C04B 35/6316.
This place covers:
Organic additives such as binders, lubricants, flocculating agents, defoaming agents, dispersants, coupling agents, surfactants, photoinitiators, organics that are pyrolysed to form a ceramic material, the organic part becoming part of the ceramic
This place does not cover:
Organic additives for clay products | |
Using organic waste materials that become part of the ceramic, e.g. wood that is carbonised or rice bran | C04B 35/62204 and subgroups |
The use of organic materials in coatings of ceramic substrates | C04B 41/46 and subgroups, C04B 41/82 |
The addition of organic fibers |
Attention is drawn to the following places, which may be of interest for search:
Organics added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/00 and subgroups |
Impregnating a porous carbon product with organic material that is carbonised into carbon | |
Carbon products obtained from carbonaceous particles with a carbonisable binder | |
Metal organic salts used as starting materials for making ceramic | C04B 2235/44 and subgroups |
Organics compounds becoming part of a ceramic after heat-treatment | C04B 2235/48 and subgroups |
Organic binders for refractory moulds | B22C 1/20 and subgroups |
Organic additives used for shaping metallic powder | B22F 1/10 and subgroups |
In the case (metal)-organic additives are pyrolysed to form a ceramic material and the organic part becomes part of the ceramic, e.g. as carbon, boride, nitride or carbide, the (metal)-organic additives are classified with symbol from C04B 2235/48, and possibly also with EC classes from C04B 35/63404-C04B 35/6365, if this information is not already present in the main EC-class given, e.g. C04B 35/524 (carbon), C04B 35/571 (SiC), C04B 35/589 (Si3N4)
This place covers:
Organo-metallics used as additives for making ceramics, where the organic part is not an acid or alkoxide, e.g. an acetyl-acetonate
This place does not cover:
Metal-organic compounds used in coatings of ceramic substrates | C04B 41/49 and subgroups, C04B 41/84 |
Metal alkoxides used as additive for making ceramics | |
Metal organic acids used as additive for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Organometallics added as filler to cement, concrete, mortar or artificial stone | |
Organometallics added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/40 and subgroups, C04B 26/30 and subgroup |
Coating bulk ceramics with an organic or organo-metallic precursor of an inorganic material | |
Metal organic salts used as starting materials for making ceramic materials | C04B 2235/44 and subgroups |
Catalysts containing organo-metallic compounds or metal hydrides | B01J 31/12 and subgroups |
Binders for refractory mould or core materials based on organic silicon or metal compounds, other organometallic compounds |
This place covers:
Polymers that are used as additives in making ceramics, for instance binders that are burned away or polymers that are carbonised and become part of the ceramics.
Organic macromolecular compounds; their preparation or chemical working-up; compositions based thereon C08
This place does not cover:
Silicon carbide made from silicon containing polymers or pre-polymers | |
Silicon nitride made from silicon containing polymers or pre-polymers | |
Using polysaccharide or derivatives thereof as additive for making ceramics | C04B 35/636 and subgroup |
Macromolecular compounds that are added to the ceramic material to create porosity after a heat treatment | |
The use of polymers in coatings of ceramic substrates | C04B 41/48 and subgroups, C04B 41/83 |
Attention is drawn to the following places, which may be of interest for search:
Treatment of macromolecular material specially adapted to enhance its filling properties in mortars, concrete or artificial stone | |
Polymers added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/24 and subgroups |
Carbon ceramic obtained from polymer precursors | |
Polymers are carbonised and become part of the ceramics |
Polymers that are carbonised and become part of the ceramic product can be indicated by one of the subgroups of C04B 35/634, in combination with either C04B 2235/48 or a class such as C04B 35/524 that indicates that carbonisation takes place.
This place covers:
Polymerisation only by polymerising C=C bonds
Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds C08F
This place does not cover:
The use of other macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds in coatings of ceramic substrates | C04B 41/4857 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/26 and subgroups, C04B 26/04 and subgroups |
Binders for refractory mould or core materials based on resins or rosins obtained by reactions only involving carbon-to-carbon unsaturated bonds |
This place covers:
Polymerisation of (R1)(R2)-C=C-(R3)(R4), where R are alkyl groups or hydrogen atoms
This place does not cover:
The use of polyalkenes in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyalkenes added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained by reactions only involving carbon-to-carbon unsaturated bonds: polyalkenes |
This place covers:
Polymerised benzofuran (coumarone) 
This place does not cover:
The use of coumarone polymers in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Coumarone polymers added as active ingredient to cement, concrete, mortar or artificial stone | |
Coumarone-indene polymers | C08F 244/00 and subgroups, C08L 45/02, C09D 145/02, C09J 145/02, C09K 2200/064 |
This place covers:
Polyvinylalcohol
 Polyvinylacetate
Polyvinylacetate 
This place does not cover:
The use of polyvinylalcohols, polyvinylacetates in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyvinylalcohols or polyvinylacetates added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/2623 and subgroup |
Polyvinyl alcohol | C08F 16/06, C08F 116/06, C08F 216/06, C08L 29/04, C09D 129/04, C09J 129/04 |
This place covers:
Polyvinylbutyral is prepared from polyvinyl alcohol by reaction with butyraldehyde PVB
PVB
This place does not cover:
The use of polyvinylacetals in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyvinylacetals added as active ingredient to cement, concrete, mortar or artificial stone | |
Polyvinylacetals |
This place covers:
Acrylic acid  Polyacrylic acid
Polyacrylic acid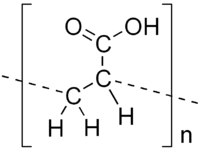
Poly(methylmethacrylate)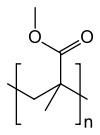
Methacrylates (CH2=CMeCOO-) are the salts or esters of methacrylic acid
This place does not cover:
The use of polyacrylates in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyacrylates or polymethacrylates added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/2641 and subgroup, C04B 26/06 |
Binders for refractory mould or core materials based on resins or rosins obtained by reactions only involving carbon-to-carbon unsaturated bonds: polyacrylates |
This place covers:
Polymers obtained by reactions only involving carbon-to carbon unsaturated bonds of ethylenically unsaturated dicarboxylic acid anhydre polymers, that are used as additives in making ceramics.
e.g.
Maleic acid is a dicarboxylic acid
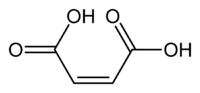
Maleic anhydride is hydrolysed maleic acid 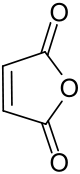
The C=C double bond can be used for polymerisation
Attention is drawn to the following places, which may be of interest for search:
Polymers of ethylenically unsaturated dicarboxylic acid polymers, e.g. maleic anhydride copolymers, added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/2664 and subgroup |
This place covers:
Polystyrene is poly(1-phenylethene-1,2-diyl)) also known as Thermocole. The only commercially important form of polystyrene is atactic, which means that the phenyl groups are randomly distributed on both sides of the polymer chain.
This place does not cover:
The use of polystyrenes in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polystyrenes added as active ingredient to cement, concrete, mortar or artificial stone | |
Polystyrene |
This place covers:
Polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds and containing halogen, that are used as additives in making ceramics.
e.g.
Poly(chloroethanediyl), or PVC
This place does not cover:
The use of halogenated polymers in coatings of ceramic substrates | C04B 41/4838 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Halogen containing polymers, e.g. PVC, added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained by reactions only involving carbon-to-carbon unsaturated bonds: halogen-containing polymers |
This place covers:
A heteropolymer or copolymer is a polymer derived from three (or more) monomeric species, as opposed to a homopolymer where only one monomer is used
Attention is drawn to the following places, which may be of interest for search:
Copolymers containing at least three different monomers added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/2688 and subgroup |
This place covers:
Polymers obtained by reactions only involving carbon-to-carbon unsaturated bonds and containing nitrogen, that are used as additives in making ceramics.
e.g.
Polyacrylamide
Polyacrylonitrile (PAN)
Polyvinylpyrrolidone (PVP),
polyethyleneimines (PEIs 
This place does not cover:
The use of polyacrylamides in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Nitrogen containing polymers, e.g. polyacrylamides, polyacrylonitriles added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/2652 and subgroup |
Polyalkylene polyamines; polyethylenimines; Derivatives thereof |
This place covers:
Obtained by polymerising C=N or C=O bonds, possibly also C=C bonds
Macromolecular compounds obtained otherwise than by reactions only involving unsaturated carbon-to-carbon bonds C08G
This place does not cover:
The use of other macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds in coatings of ceramic substrates | C04B 41/488 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Polymers obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/28 and subgroups, C04B 26/10 |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds |
This place covers:
Epoxy is a copolymer; that is, it is formed from two different chemicals. These are referred to as the "resin" or "compound" and the "hardener" or "activator". The resin consists of monomers or short chain polymers with an epoxide group at either end. Most common epoxy resins are produced from a reaction between epichlorohydrin and bisphenol-A, though the latter may be replaced by similar chemicals. The hardener consists of polyamine monomers, for example Triethylenetetramine (TETA). When these compounds are mixed together, the amine groups react with the epoxide groups to form a covalent bond. Each NH group can react with an epoxide group, so that the resulting polymer is heavily crosslinked, and is thus rigid and strong
Structure of unmodified epoxy prepolymer resin. n denotes the number of polymerized subunits and is in the range from 0 to about 25
Structure of TETA, a typical hardener. The amine (NH) groups react with the epoxide groups of the resin during polymerization
This place does not cover:
The use of polyepoxides in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyepoxides added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyepoxides | |
Polyepoxides |
This place covers:
A polyurethane (PUR and PU) is any polymer composed of a chain of organic units joined by carbamate (urethane) links. Polyurethane polymers are formed through step-growth polymerization, by reacting a monomer (with at least two isocyanate functional groups) with another monomer (with at least two hydroxyl or alcohol groups) in the presence of a catalyst
Polyurethane synthesis, wherein the urethane groups — NH-(C=O)-O- link the molecular units
Isocyanate is the functional group of elements –N=C=O (1 nitrogen, 1 carbon, 1 oxygen), not to be confused with the cyanate functional group which is arranged as –O–C≡N or with isocyanide, R-N≡C. Any organic compound which contains an isocyanate group may also be referred to in brief as an isocyanate. An isocyanate may have more than one isocyanate group. An isocyanate that has two isocyanate groups is known as a diisocyanate
 The isocyanate functional group
The isocyanate functional group
This place does not cover:
The use of polyurethanes in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyurethanes and polyisocyanates added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyurethanes; polyisocyanates | |
Polyurethanes | C08F 290/067, C08F 290/147, C08F 299/06, C08G 71/04, C08L 75/04, C08L 75/14, C09D 5/4465, C09D 175/00, C09D 175/04, C09J 175/00, C09J 175/04, C09K 3/1021, C09K 19/3885 |
Polyisocyanates |
This place covers:
Polyester is a category of polymers which contain the ester functional group (-C(O)O-) in their main chain. A common polyester is for instance polyethylene terephthalate (PET)

Polyethylene terephthalate
Polycaprolactone (PCL)
Polyglycolic acid (PGA)
poly-(R)-3-hydroxybutyrate (P3HB),
This place does not cover:
The use of polyesters in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyesters added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/283 and subgroup, C04B 26/18 |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyesters; polycarbonates |
This place covers:
Polycarbonates received their name because they are polymers containing carbonate groups (–O–(C=O)–O–). An example of a polycarbonate material is produced by the reaction of bisphenol A and phosgene COCl2
This place does not cover:
The use of polycarbonates in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polycarbonates added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyesters; polycarbonates |
This place covers:
The amide link is produced from the condensation reaction of an amino group and a carboxylic acid or acid chloride group. A small molecule, usually water, or hydrogen chloride, is eliminated.
The amino group and the carboxylic acid group can be on the same monomer, or the polymer can be constituted of two different bifunctional monomers, one with two amino groups, the other with two carboxylic acid or acid chloride groups.
Amino acids can be taken as examples of single monomer (if the difference between R groups is ignored) reacting with identical molecules to form a polyamide:
The reaction of two amino acids. Many of these reactions produce long chain proteins
Aramid (pictured below) is made from two different monomers which continuously alternate to form the polymer and is an aromatic polyamide:
The reaction of 1,4-phenyl-diamine (para-phenylenediamine) and terephthaloyl chloride to produce Aramid
This place does not cover:
The use of polyamides in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyamides added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyamides |
This place covers:
An aldehyde is an organic compound containing a formyl group. This functional group, with the structure R-CHO, consists of a carbonyl center bonded to hydrogen and an R group.[1] The group without R is called the aldehyde group or formyl group
a ketone is an organic compound with the structure RC(=O)R', where R and R' can be a variety of atoms and groups of atoms. It features a carbonyl group (C=O) bonded to two other carbon atoms.

This place does not cover:
The use of condensation polymers of aldehydes or ketones in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Condensation polymers of aldehydes or ketones added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: condensation polymers of aldehydes and ketones |
This place covers:
Ethers are a class of organic compounds that contain an ether group — an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R'.[1] A typical example is the solvent and anesthetic diethyl ether, commonly referred to simply as "ether" (CH3-CH2-O-CH2-CH3).
Polyether generally refers to polymers which contain the ether functional group in their main chain. The term glycol is reserved for low to medium range molar mass polymer when the nature of the end-group, which is usually a hydroxyl group, still matters. The term "oxide" or other terms are used for high molar mass polymer when end-groups no longer affect polymer properties.
PEG or PEO, depending on the n-number
This place does not cover:
The use of polyethers in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polyethers, e.g. alkylphenol polyglycolether added as active ingredient to cement, concrete, mortar or artificial stone | |
Binders for refractory mould or core materials based on resins or rosins obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds: polyethers |
This place covers:
Water-insoluble mixture of compounds derived from trees, especially conifers,
e.g. pine tar, pitch, gum, shellac.
Rosin is a solid form of resin obtained from pines and some other plants, mostly conifers
This place does not cover:
The use of wood waste material for making ceramics | |
The use of natural resins in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Natural resins, e.g. rosin added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/34, C04B 26/22 and subgroup |
Binders for refractory mould or core materials based on resins or rosins: natural polymers |
This place covers:
Bituminous materials such as tar or pitch are used as binder, either to be burned out later or to be carbonised and become part of the ceramic, as for instance carbon or carbide
bituminous materials C10
This place does not cover:
Impregnating a porous carbon product with tar or pitch that is carbonised into carbon | |
Carbon products obtained from carbonaceous particles with a carbonisable binder, such as tar or pitch | |
Using tar or pitch for joining ceramic with ceramic | |
Using tar or pitch for joining ceramic with metal | |
Using tar or pitch for joining ceramic with glass | |
Carbonaceous materials that are added to the ceramic material to create porosity after a heat treatment |
Attention is drawn to the following places, which may be of interest for search:
Bituminous materials, e.g. tar, pitch added as active ingredient to cement, concrete, mortar or artificial stone | |
Oxide ceramics containing carbon | |
Alumina refractories containing carbon | |
The use of bitumen, asphalt, e.g. paraffin in coatings of ceramic substrates | |
Carbonaceous additives to ceramics or secondary phases | C04B 2235/422 and subgroups |
Organic additives are carbonised to become part of the ceramic |
If bituminous materials are added as binder to oxide ceramics, C04B 35/013 and C04B 35/63496 both are given.
This place covers:
The use of polysaccharides such as xanthan, dextrin as binder, or for other uses, e.g. starch, glycogen, chitin, xylan, pectins
Polysaccharides; derivatives thereof C08B
This place does not cover:
Polysaccharides that are added to the ceramic material to create porosity after a heat treatment | |
The use of polysaccharides in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Polysaccharides or derivatives thereof added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/38 and subgroups, C04B 26/28 and subgroup |
This place covers:
Cellulose is an organic compound with the formula (C6H10O5)n, a polysaccharide consisting of a linear chain of several hundred to over ten thousand β(1→4) linked D-glucose units. It is often used as binder and does not leave carbon residue normally.
Production of cellulose D21
This place does not cover:
Cellulose materials that are added to the ceramic material to create porosity after a heat treatment | |
The use of cellulose in coatings of ceramic substrates |
Attention is drawn to the following places, which may be of interest for search:
Use of organic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of organic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: cellulose | |
Cellulose or derivatives thereof added as active ingredient to cement, concrete, mortar or artificial stone | C04B 24/383 and subgroup, C04B 26/24, C04B 26/285 |
The use of wood waste material for making ceramics | |
Preparatory treatment of cellulose for making derivatives thereof | C08B 1/00 and subgroups |
This place covers:
Burning out the organics of green shaped ceramics or of unshaped ceramic powder mixtures, or of ceramic-polymer fibers, e.g. barium titanate-PVP fibers. Removing the organics by using solvents.
This place does not cover:
Creating porous ceramics by dissolving-out added substances | |
Creating porous ceramics by burning-out added substances by burning natural expanding materials or by sublimating or melting out added substances | C04B 38/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Specific temperature used for heat treatment step of shaped ceramic | |
Heating rate of heat treatment step of shaped ceramic | |
Cooling rate of heat treatment step of shaped ceramic | |
Treatment time of heat treatment step of shaped ceramic | |
Multi step sintering | |
Removing of binder during sintering of metallic articles | B22F 3/1021 and subgroup |
General overview over methods for debinding | document XP004301883 |
If heating rate, cooling rate and heating time of the binder burn-out step are specified, C04B 2235/656 and subgroups can be used.
This place covers:
All specific burning and sintering methods used for shaped ceramic materials, e.g. using a specific heating or cooling rate, a specific furnace, a specific atmosphere
This place does not cover:
Burning methods for clay-wares | C04B 33/32 and subgroups |
Heat treatments of non-shaped powders that are used for making ceramics | C04B 35/62645 and subgroups |
Superficial sintering of clay or ceramic objects with the goal of creating a porous object | C04B 38/0038 and subgroup |
Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; Apparatus specially adapted therefore; Presses and furnaces | B22F 3/00 and subgroups |
Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression | B22F 7/00 and subgroups |
Mechanical aspects of sintering clay or ceramic objects | |
Chamber type furnaces | F27B 17/0016 and subgroups |
Travelling or movable supports or containers for the charge of furnaces, kilns, ovens, retorts in so far as they are of kinds occurring in more than one kind of furnace | F27D 3/12 and subgroup |
Attention is drawn to the following places, which may be of interest for search:
Heat treatment, e.g. precalcining, burning, melting; Cooling of hydraulic cements | C04B 7/43 and subgroups |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: making use of a rise in temperature, e.g. caused by an exothermic reaction | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: heating up to sintering temperatures | |
After-treatment of mortars, concrete, artificial stone or ceramics: heat treatment | |
Aspects relating to heat treatment of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes | C04B 2235/65 and subgroups |
Using setters during sintering | |
Sintering glass | C03B 19/06 and subgroups |
Abrasive particles per se obtained by division of a mass agglomerated by sintering | |
Shaft or like vertical or substantially vertical furnaces wherein no smelting of the charge occurs, e.g. calcining or sintering furnaces |
When giving this class, it should be checked if one of the heat treatment symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well.
In this place, the following terms or expressions are used with the meaning indicated:
Selective Laser Sintering (SLS) | A layer of ceramic powder is deposited on a support, and possibly compacted by a rolling device. A computer-controlled laser beam scans a two-dimensional cross-section of a part, selectively sintering the layer. A new layer of powder is deposited, compacted and sintered. After completion of the part, the unfused or unsintered powder, which helps hold the part during the process, is removed. This technique may allow local composition variations for gradient materials or manufacture of composites. |
This place covers:
Methods such as sinterforging, SPS (spark plasma sintering).
Presses in general B30B
This place does not cover:
Pressing and heating of the clay green compact at the same time at temperatures lower than the sintering temperature | |
Pressing clay at sintering temperatures | |
Pressure sintering to make silicon carbide based ceramics | |
Pressure sintering to make silicon nitride based ceramics | |
Using a pressurised atmosphere during sintering, e.g. an atmosphere of 2 bar nitrogen | |
Using constraining layers before or during sintering of ceramic laminates or ceramic substrates that are joined with other substrates | C04B 2237/56 and subgroups |
Processes using ultra high pressure, e.g. for the formation of diamonds; Apparatus therefore, e.g. moulds, dies | B01J 3/06 and subgroups |
Mechanical aspects of hot-pressing clay or ceramic materials |
Attention is drawn to the following places, which may be of interest for search:
Pressing at non-sintering temperatures of ceramic or refractory mixtures | |
Spark plasma sintering | |
Density of sintered ceramics | |
Both compacting and sintering of metallic articles | |
Both compacting and sintering of metallic articles by forging | |
Hot-pressing glass powder |
When giving this class, it should be checked if one of the symbol from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well. If SPS is used, C04B 2235/666 should be given as well.
This place covers:
Hot isostatic pressing, using normally a gas to transmit the pressure
This place does not cover:
Gas pressure sintering to make silicon carbide based ceramics | |
Gas pressure sintering to make silicon nitride based ceramics | |
Using a pressurised atmosphere during sintering, e.g. an atmosphere of 2 bar nitrogen |
Attention is drawn to the following places, which may be of interest for search:
Joints of implantable prostheses, made by hot isostatic pressing (HIP) | |
Hot isostatic pressing of workpieces or articles from metallic powder | B22F 3/15 and subgroup |
Hot isostatic pressing of metals or alloys |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well. Usually there is a pre-sintering step before HIP. The use of a presintering step can be indicated with C04B 2235/661.
This place covers:
A ceramic is made by reacting a metal or silicon, with oxygen (oxidising to make an oxide), with carbon (to make a carbide), with boron (to make a boride), with nitrogen (nitriding to make a nitride), or with either a free metal or free silicon to make a silicide. The majority of the ceramic has to be made by reaction sintering. Examples are the reaction of a metal such as molybdenum with silicon to make molybdenum silicide, reacting aluminium with nitrogen to make aluminium nitride, reacting nickel with oxygen to make nickel oxide, reacting titanium with carbon to make titanium carbide, reacting magnesium with boron to make magnesium boride.
This place does not cover:
Making silicon carbide by reaction sintering | |
Making silicon nitride by reaction sintering | |
Reaction sintering to make a material containing for the majority ceramic phases resulting from the reaction, but a minority of metallic phase, where the metallic phase is continuous, e.g. functions as a binder |
Attention is drawn to the following places, which may be of interest for search:
Reacting a material with a gas, other than oxygen, to create a ceramic phase, e.g. reacting with nitrogen to make a nitride | C04B 2235/46 and subgroup |
Manufacture of workpieces or articles from metallic powder involving a self-propagating high-temperature synthesis or reaction sintering step |
This place covers:
Thermite is a pyrotechnic composition of a metal powder and a metal oxide that produces an exothermic oxidation-reduction reaction known as a thermite reaction. If aluminium is the reducing agent it is called an aluminothermic reaction. Thermites can be a diverse class of compositions. Some "fuels" that can be used include aluminium, magnesium, calcium, titanium, zinc, silicon, and boron and others. One commonly-used fuel in thermite mixtures is aluminium, because of its high boiling point. The oxidizers can be boron(III) oxide, silicon(IV) oxide, chromium(III) oxide, manganese(IV) oxide, iron(III) oxide, iron(II,III) oxide, copper(II) oxide, and lead(II,III,IV) oxide and others.
The aluminium reduces the oxide of another metal, most commonly iron oxide, because aluminium is highly reactive:
Fe2O3 + 2Al → 2Fe + Al2O3
This place does not cover:
Heating methods that result in the oxidation or reduction of powders, preparatory to the making of a ceramic material | |
Pyrolysis, carbonisation or auto-combustion reactions for making ceramic powder |
Attention is drawn to the following places, which may be of interest for search:
Reductive heat treatment for making a ceramic | |
Oxidative annealing | |
Reductive annealing |
This place covers:
The Lanxide process, also known as pressureless metal infiltration, is a way of producing metal-matrix composite materials by a process of partial reaction; the process involves a careful choice of initial alloy (usually aluminium with about 3% magnesium and about 10% silicon), and then the maintenance of conditions in which the polycrystalline reaction product has a mechanical composition such that metal is drawn up through it towards the oxidiser by capillary action, so the composite material grows downwards. The normal application is to produce alumina-reinforced aluminium; the process also allows the growth of ceramic layers inside metal encasements, providing pre-stressing. A metal melt is simultaneously both oxidised and solidified, in a directional way, meaning the oxidation and solidification start at one end of the material and progress towards the other end. This can also be done with a powder bed, in which case there is only directional oxidation.
This place does not cover:
Porous ceramic starting from inorganic materials only, e.g. metal foam; Lanxide type products |
Attention is drawn to the following places, which may be of interest for search:
Making ceramics by making use of a melting process | C04B 35/653 and subgroup |
Porous mortars, concrete, artificial stone or ceramic ware obtained by a chemical conversion or reaction other than those relating to the setting or hardening of cement-like material or to the formation of a sol or a gel, e.g. by carbonising or pyrolysing preformed cellular materials based on polymers, organo-metallic or organo-silicon precursors: starting from inorganic materials only, e.g. metal foam; Lanxide type products | |
Directionally solidified metal castings | |
Directionally-solidified crystalline structure | F05C 2253/083 and subgroup |
This place covers:
Making a bulk ceramic object by melting at least the largest part of a badge of material
This place does not cover:
Melting clay materials to make a clay ceramic object | |
Melting material in order to make ceramic powder |
Attention is drawn to the following places, which may be of interest for search:
Artificial stone from molten metallurgical slag | C04B 5/00 and subgroups |
Artificial stone obtained by melting at least part of the composition, e.g. metal | |
Porous clay ceramics obtained by generating pores in the ceramic material while in the molten state | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: involving melting of at least part of the composition | |
Thermally activated mortars, e.g. by melting ingredients | |
Coating or impregnating "in situ", e.g. impregnating of artificial stone by subsequent melting of a compound added to the artificial stone composition | |
Coating or impregnating applied from the molten state; Thermal spraying, e.g. plasma spraying | C04B 41/4523 and subgroup |
Superficial melting of the ceramic substrate before or during the coating or impregnating step | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
Casting non-ferrous metals with a high melting point, e.g. metallic carbides | |
Shaping methods specially adapted for producing clay or ceramic articles from molten material, e.g. slag refractory ceramic materials | |
Abrasive particles per se obtained by division of a mass agglomerated by melting, at least partially, e.g. with a binder | |
Making hard metals based on borides, carbides, nitrides, oxides, silicides starting from a melt |
When giving this class, it should be checked if one of the symbols from the range C04B 2235/65-C04B 2235/668 is applicable. If so, this symbol should be given as well.
This place covers:
Making a bulk ceramic refractory object by melting at least the largest part of a badge of material
This place does not cover:
Magnesia-based refractories made by fusion casting | C04B 35/05 and subgroup |
Alumina-based refractories made by fusion casting | C04B 35/107 and subgroup |
Zirconia-based refractories made by fusion casting |
Attention is drawn to the following places, which may be of interest for search:
Melting clay materials to make a clay ceramic object | |
Melting material in order to make ceramic powder | |
Applying ceramic coatings by spraying the coating material in the molten state, e.g. by flame, plasma or electric discharge | C23C 4/10 and subgroup |
This place covers:
All refractory compositions that do not have as largest fraction magnesia, alumina or zirconia, e.g. a refractory composition with titania or silicon carbide as largest fraction
This place does not cover:
Grain-sized magnesia-based refractories | C04B 35/043 and subgroups |
Grain-sized alumina-based refractories | C04B 35/101 and subgroups |
Grain-sized zirconia-based refractories | |
Making refractory metal carbides | C04B 35/5607 and subgroups |
Making refractory metal nitrides | C04B 35/58007 and subgroups |
Making refractory metal borides | C04B 35/58064 and subgroups |
Making refractory metal silicides |
Attention is drawn to the following places, which may be of interest for search:
Re-using refractory waste for making clay ceramics | |
Grain-sized titania-based refractories | C04B 35/46 and C04B 35/66 |
Grain-sized silicon carbide based refractories | |
Cement, concrete, mortar or artificial stone being refractory | |
Cement, concrete, mortar or artificial stone as a refractory coating, e.g. for tamping | |
Cement, concrete, mortar or artificial stone composition being cement free, being calciumaluminate-free refractories | |
Using insulating materials or refractories in chemical or physical processes | |
Molecular sieve catalysts supported in or on refractory materials | |
Compositions of refractory mould or core materials; Grain structures thereof | B22C 1/00 and subgroups |
Hot tops from refractory material for ingot moulds | |
Linings for casting melt-holding vessels, e.g. ladles, tundishes, cups or the like | B22D 41/02 and subgroups |
Refractory plugging masses for melt-holding vessels, e.g. ladles, tundishes, cups or the like | B22D 41/46 and subgroup |
Soldering or welding materials comprising refractory compounds, e.g. carbides | |
Fireproof paints including high temperature resistant paints | C09D 5/18 and subgroup |
Miscellaneous materials being Fire-resistant, heat-resistant materials | |
Fireproofing materials | C09K 21/00 and subgroups |
Blast furnaces with special refractories, e.g. linings | C21B 7/04 and subgroup |
Opening or sealing the tap holes of blast furnaces with refractory plugging mass | |
Refractory linings for carbon-steel converters | C21C 5/44 and subgroups |
Refractory coated lances; Immersion lances for carbon-steel converters | |
Coating metal with enamels or vitreous layers: with refractory materials | |
Refractory bricks or blocks specially shaped for burner openings | |
Making or repairing of linings | F27D 1/16 and subgroups |
Cooling of furnaces the cooling medium passing through a pattern of tubes integrated with refractories in a panel |
This place covers:
A ceramic matrix contains fibers, whiskers, platelets, nanofibers, nanotubes
This place does not cover:
Reinforced clay wares | |
Making SiC by reactive infiltration of carbon body with Si | |
Making Si3N4 by reactive infiltration of carbon body with nitrogen or nitrogen containing materials | |
Monolithic refractories or refractory mortars | |
Ceramics containing macroscopic reinforcements that are removed to create porosity | C04B 38/06 and subgroups |
Infiltration of a porous ceramic matrix with a material forming a non-ceramic phase | C04B 41/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Arrangements specially adapted for the production of shaped ceramic articles with elements wholly or partly embedded in the moulding material; Production of reinforced objects | B28B 23/00 and subgroups |
Metallic alloys containing fibers and filaments | C22C 47/00, C22C 49/00 and subgroups |
The symbols from the range C04B 2235/5208-C04B 2235/5296 can be used to indicate which macroscopic reinforcement is being used.
In this place, the following terms or expressions are used with the meaning indicated:
CMC | ceramic matrix composite |
This place covers:
A ceramic matrix containing shaped metallic material, where the shaped material is not a fiber, whisker, platelet, filament.
Attention is drawn to the following places, which may be of interest for search:
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi | C04B 2235/40 and subgroups |
This place covers:
A ceramic matrix containing for instance steel fibers, aluminium platelets, titanium nanofibers, etc.
This place does not cover:
Mechanical aspects of shaping ceramic objects containing metallic fibers |
Attention is drawn to the following places, which may be of interest for search:
Metallic fibers or whiskers added as filler to concrete, cement, mortar or artificial stone | |
Fiber or whisker reinforced substrate joined with another substrate or being part of a ceramic laminate | |
Metallic fibers per se | |
Manufacture of articles essentially made from metallic fibres | |
Making alloys containing metallic or non-metallic fibres or filaments | C22C 47/00 and subgroups |
Alloys containing metallic or non-metallic fibres or filaments | C22C 49/00 and subgroups |
Non-woven fabrics formed wholly or mainly of staple fibres or like relatively short fibre: metal fibers | |
Sealings containing metallic fibers |
This place covers:
Ceramic matrix with ceramic, glass reinforcement
This place covers:
The ceramic matrix is reinforced with ceramic fibers, ceramic whiskers, ceramic nanotubes, silicon fibers
Attention is drawn to the following places, which may be of interest for search:
Making ceramic fibers per se | |
Coating the ceramic fibers or inorganic fibers used in ceramics | C04B 35/62844 and subgroups |
Carbon reinforced with carbon fibers | |
Using inorganic fibers for ceramics | C04B 2235/5216 and subgroups |
Using inorganic whiskers, spindles, needles, pins for ceramics | |
Using hollow fibers for ceramics, e.g. nanotubes | C04B 2235/5284 and subgroup |
Using flakes, platelets, plates for ceramics | |
Fiber or whisker reinforced substrate joined with another substrate or being part of a ceramic laminate | |
Composition of friction linings based on metals or inorganic oxides, containing fibres |
The symbols from the range C04B 2235/5208-C04B 2235/5296 can be used to indicate which macroscopic reinforcement is being used.
The material of the matrix phase is classified with one of the groups C04B 35/01 - C04B 35/597.
This place covers:
The ceramic matrix is reinforced with asbestos, glass fibers or fused silica fibers or whiskers. The matrix can be both oxide and non-oxide.
Attention is drawn to the following places, which may be of interest for search:
Asbestos used as fillers, e.g. pigments, for mortars, concrete or artificial stone | C04B 14/40 and subgroup |
Glass fibers or whiskers added as filler to concrete, cement, mortar or artificial stone | C04B 14/42 and subgroup |
Hazardous waste used for making clay materials, the waste not being a combustion residue | |
Melting of material to make a ceramic powder | |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Glass starting materials for making ceramics, e.g. silica glass | C04B 2235/36 and subgroup |
Silicate other than alumino-silicate or silica fibers used as starting material for making ceramics, e.g. quartz fibers | |
Filtering material for liquid or gaseous fluids, containing inorganic material, e.g. asbestos fibres, glass beads or fibres | |
Disposal of asbestos | |
Layered products essentially comprising sheet glass, or glass, slag, or like fibres | B32B 17/00 and subgroups |
Layered products essentially comprising natural mineral fibres or particles, e.g. asbestos | |
Processes specially adapted for the production of quartz or fused silica articles | |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Glass fibre or filament compositions | C03C 13/00 and subgroups |
Use of asbestos fibers as ingredient for polymers | |
Yarns or threads made from asbestos | |
Woven glass fibers | |
Fleeces or layers composed of fibres that are not woven, glass fibers | D04H 1/4218 and subgroup, D04H 5/12, D04H 13/008 |
Treating fibers made of asbestos | |
Inorganic fibres based on oxides or oxide ceramics, e.g. silicates, Asbestos | |
Composition of friction linings containing asbestos | F16D 69/021 and subgroup |
Insulators mainly consisting out of asbestos | H01B 3/06 and subgroup |
Insulators containing quartz; glass; glass wool; slag wool; vitreous enamels | H01B 3/08 and subgroups |
This place covers:
A matrix consisting for the largest part out of carbon phase, e.g. amorphous carbon, graphite, diamond, which contains carbon fibers, carbon nanotubes. The carbon fibers or nanotubes can contain a non-carbon coating, although usually such an intermediate coating will be made of carbon.
This place does not cover:
A carbide matrix containing carbon fibers | |
A carbon matrix containing carbide fibers | |
A carbon matrix containing non-carbon fibers having a carbon coating |
Attention is drawn to the following places, which may be of interest for search:
Carbon fibers or whiskers added as filler to concrete, cement, mortar or artificial stone | |
Carbon-based ceramics | C04B 35/52 and subgroups |
Coating inorganic fibers with a carbon coating | |
Carbon fibers used in ceramics | |
Carbon nanotubes used in ceramics | |
Carbon fiber or whisker reinforced carbon substrate joined with another substrate or being part of a ceramic laminate | |
Prosthesis containing Carbon reinforced with carbon fibres | |
Friction linings; Attachment thereof; Selection of co-acting friction substances or surfaces, the lining made of composite materials containing carbon and carbon fibres or fibres made of carbonizable material |
In patent documents, the following abbreviations are often used:
C/C, CFC | Carbon fibres in a carbon matrix |
This place covers:
Joining ceramic articles with other ceramic articles through heating is in the groups C04B 37/001 - C04B 37/008. In principle the head-group C04B 37/00 should not be used for joining a ceramic substrate with another ceramic substrate, since the joining is either direct (C04B 37/001) or through an interlayer (C04B 37/003 or C04B 37/008), but there are no other options. The joining of ceramic articles through heating with articles that are not metal nor glass nor ceramic is also in C04B 37/003 and C04B 37/008. This can be for instance the joining of a ceramic substrate with a silicon substrate, a wood substrate, etc. If the joining of the ceramic substrate with for instance the silicon would be direct joining, C04B 37/001 is not applicable, since this is only for ceramic-ceramic joining. In this case C04B 37/00 is used.
A ceramic article or other article is any pre-shaped from. This also includes pre-shaped films or foils that are joined to another object, e.g. first making a diamond thin film by CVD, lifting this film from its substrate and joining the film with a carbide substrate. If a substrate is coated with two coatings, and on top of the two coatings a foil is applied, the foil is seen as a substrate, which means this is coded in C04B 37/00.
The joining can also mean joining two objects through spacers that actually keep the two objects apart. The two objects do not make direct contact, but are joined through the spacer(s).
Normally a joint between a cermet and a metal substrate is not classified in C04B 37/00, but in the case that a porous ceramic is joined to a metal through a metal infiltrated in the porous ceramic, this is classified in C04B 37/00(using the code C04B 2237/61).
This place does not cover:
Joining individual ceramic particles with other ceramic particles | C04B 35/6303 (inorganic binders) C04B 35/632 (organic binders) |
Coating a ceramic substrate with a preformed sheet-like element | |
Joining plastics material to carbon | |
Joining plastics material to ceramics | B29C 66/7461 and subgroup |
Application of procedures in order to connect objects or parts, e.g. coating with sheet metal otherwise than by plating | |
Joining a ceramic layer with a polymer/plastic layer through heating, unless the polymer/plastic is an adhesive that functions as an interlayer for two other substrates, of which at least one is ceramic |
Attention is drawn to the following places, which may be of interest for search:
Clay-wares | C04B 33/00 and subgroups |
Porous ceramic products | |
Honeycomb structures assembled from subunits | |
Coating ceramic substrates | C04B 41/00 and subgroups |
Aspects relating to ceramic starting mixtures or sintered ceramic products | C04B 2235/00 and subgroups |
Aspects relating to ceramic laminates or to joining of ceramic articles with other articles by heating | C04B 2237/00 and subgroups |
Application of procedures in order to connect objects or parts, e.g. coating with sheet metal otherwise than by plating | |
Friction heat forging | |
Riveting | |
Uniting components to form integral members, e.g. turbine wheels and shafts, caulks with inserts, with or without shaping of the components | |
Soldering or unsoldering; welding; cladding or plating by soldering or welding; cutting by applying heat locally e.g. flame cutting; working by laser beam | |
Soldering and welding materials | |
Connecting metal parts or objects by metal-working techniques, not covered wholly by either B21J or B23K | |
Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefore | B29C 65/00 and subgroups |
Laminated products | |
Laminated products composed mainly of ceramics, e.g. refractory materials | |
Printing on laminates | B32B 38/14 and subgroup |
Uniting glass pieces by fusing without substantial reshaping | |
Joining pieces of glass to pieces of other inorganic material; Joining glass to glass other than by fusing | |
Coating a metallic substrate with a ceramic coating | |
Structural elements; building materials | |
Joining constructional elements in general | |
Connecting constructional elements or machine parts by sticking or pressing them together, e.g. cold pressure welding | |
Seals between parts of vessels of electric discharge tubes or discharge lamps |
In C04B 37/00 and subgroups the emphasis is on how the articles are joined. If the emphasis is not on the joining aspect, classification in other technical fields, mainly B32B, should be considered. If two ceramic layers are joined, but the emphasis is not on how they are joined, classification in B32B 18/00 takes place.
Secondary aspects of making ceramic laminates (B32B 18/00) and of joining ceramic articles with other articles through heating (C04B 37/00 and subgroups) are classified in C04B 2237/00 and subgroups, e.g. the composition of the layers or articles that are laminated or joined, the composition of the interlayers that are used for joining, processing aspects such as surface treatments to the layers-to-be-joined and also the geometrical configuration of the articles that are joined, e.g. joining both layers on their small side or one layer on the largest surface with one layer on the shortest surface.
Aspects regarding the heat treatments that are used are classified in C04B 35/64 and subgroups and coded in C04B 2235/65 and subgroups, where the heat treatment of the joining step should be seen as a sintering step. If for instance pressure is exerted during heating to join the articles, C04B 35/645 is given. Aspects regarding the atmosphere of the heating step, possible annealing steps, heating rate, cooling rate, etc. are classified in C04B 2235/65 and subgroups.
If much detail regarding the composition and/or synthesis of one or more ceramic layers or articles is given, classification in C04B 35/00 and subgroups should be considered. If much detail regarding the composition and/or synthesis of one or more metallic layers or articles is given, classification in B22F 3/00 or B22F 5/00 and subgroups should be considered.
In this place, the following terms or expressions are used with the meaning indicated:
A ceramic article | an article made of material that is classified in C04B 33/00(clay materials), C04B 35/00- C04B 35/597 (ceramic materials), C04B 35/62204 (ceramic materials made out of waste material) and C04B 35/71- C04B 35/83 (ceramic materials containing macroscopic reinforcing agents). |
In patent documents, the following words/expressions are often used with the meaning indicated:
"oxides, phosphates, carbon-based materials, carbides, nitrides, borides, silicides, fluorides, sulphides, selenides | "ceramic materials". |
This place covers:
Joining the two ceramic articles without the use of an interlayer. The surface of one or more of the ceramic articles to-be-joined can be treated, e.g. by cleaning or oxidising, leading to an oxidised surface, or can for instance be wetted, but no external layer is applied to any of the surfaces to-be-bonded. An interlayer could be formed by bonding.
In the case the direct bonding results in the in-situ formation of an interlayer, the interlayer is indicated with a symbol from C04B 2237/02-C04B 2237/16.
Non-bonding electrode layers do not count as interlayer. If two substrates contain only a non-bonding electrode in between, these substrates are regarded to be directly bonded.
This place covers:
A layer/coating is externally applied on at least one of the two substrates, or a foil or sheet is laid in between the two substrates, e.g. the interlayer is Si. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
This place does not cover:
The creation of an internal layer within the substrate before bonding, e.g. by oxidising the surface or otherwise treating the surface | C04B 37/001 (direct bonding of ceramics) |
Ceramic substrates containing a non-bonding electrode layer in between. |
Attention is drawn to the following places, which may be of interest for search:
Honeycomb structures characterised by the material used for sealing or plugging (some of) the channels of the honeycombs | |
Honeycomb structures assembled from subunits characterised by the material used for joining separate subunits | |
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: interlayers, transition pieces for metallurgical bonding of workpieces | B23K 35/001 and subgroups |
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: sheets or foils for use in soldering or brazing | B23K 35/0233 and subgroup |
Selection of soldering or welding materials proper | B23K 35/24 and subgroups |
Although a pre-treated substrate with an internal layer (e.g. oxidised surface) that is joined is not seen as an interlayer for classification in C04B 37/00, the internal layer is seen as an interlayer for classification in C04B 2237/00.
Documents classified in C04B 38/0019 should normally also be classified in C04B 37/003, as most honeycombs are made from ceramic material.
Electrode and electrodes layers that are inserted between ceramic substrate layers are normally not seen as interlayer, since they normally do not have the function of joining the two ceramic substrates. They therefore do not receive a C04B 2237/12 code. Only if it is clear that the electrode does have a joining effect, it is regarded as interlayer, and C04B 2237/12 or a subgroup is allocated.
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/008 is also attributed, since for bonding a polymeric adhesive is used. The ceramic interlayer formed through the bonding is coded with either C04B 2237/08 or C04B 2237/083.
This place covers:
The adhesive is normally a resin, but could also be tar, pitch. The bonding material in principle does not contain inorganic matter. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
Adhesives; non-mechanical aspects of adhesive processes in general; adhesive processes not provided for elsewhere; use of materials as adhesives |
Attention is drawn to the following places, which may be of interest for search:
Coating a ceramic substrate with a preformed sheet-like element, using an adhesive layer |
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/005 is also attributed, since after bonding a ceramic interlayer has been formed. This interlayer is classified with either C04B 2237/08 or C04B 2237/083.
The classes from the range C04B 35/63404-C04B 35/6365 can be used to indicate the polymer adhesive.
This place covers:
Binding a ceramic substrate with a metallic substrate. All layers/objects based on metallic phases as well as ceramic layers/objects having a metallic binder (cermets) are regarded as metallic. If the layer/object has a continuous metallic phase, it is regarded as metallic, even if the amount of metal is as low as for instance 5 wt%.
This place does not cover:
A second metal layer/object that is joined to a first metal layer/object, which itself is joined to a ceramic layer/object. Only the first metal layer/object, that is joined directly or through an interlayer with the ceramic layer/object, is classified. | B32B 15/00 and subgroups (Layered products essentially comprising metal) |
Attention is drawn to the following places, which may be of interest for search:
Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; Apparatus specially adapted therefore; Presses and furnaces | B22F 3/00 and subgroups |
Manufacture of workpieces or articles from metallic powder characterised by the special shape of the product | B22F 5/00 and subgroups |
This place covers:
Joining the two articles without the use of an interlayer. The surface of one or more of the articles to-be-joined can be treated, e.g. by cleaning or oxidising, leading to an oxidised surface, or can for instance be wetted, but no external layer is applied to any of the surfaces to-be-bonded.
In the case the direct bonding results in the in-situ formation of an interlayer, the interlayer is indicated with a symbol from C04B 2237/02-C04B 2237/16
This place covers:
A layer/coating is externally applied on at least one of the two substrates, or a foil or sheet is laid in between the two substrates. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
This place does not cover:
The creation of an internal layer within the substrate before bonding, e.g. by oxidising the surface or otherwise treating the surface | C04B 37/021 (direct bonding of ceramic with metal) |
Joining a ceramic article with a metal article though heating, using an adhesive |
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: interlayers, transition pieces for metallurgical bonding of workpieces | B23K 35/001 and subgroups |
Although a pre-treated substrate with an internal layer (e.g. oxidised surface) that is joined is not seen as an interlayer for classification in C04B 37/00, the internal layer is seen as an interlayer for classification in C04B 2237/00.
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/028 is also attributed, since for bonding a polymeric adhesive is used. The ceramic interlayer formed through the bonding is classified with either C04B 2237/08 or C04B 2237/083.
This place covers:
The adhesive is normally a resin, but could also be tar, pitch. The bonding material in principle does not contain inorganic matter. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
Attention is drawn to the following places, which may be of interest for search:
Coating a ceramic substrate with a preformed sheet-like element, using an adhesive layer |
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/005 is also attributed, since after bonding a ceramic interlayer has been formed. This interlayer is classified with either C04B 2237/08 or C04B 2237/083.
The classes from the range C04B 35/63404-C04B 35/6365 can be used to indicate the polymer adhesive.
This place covers:
joining a ceramic with a glass article or glass-ceramic article
Attention is drawn to the following places, which may be of interest for search:
Layered products essentially comprising sheet glass, or glass, slag, or like fibres | B32B 17/00 and subgroups |
Joining pieces of glass to pieces of other inorganic material; joining glass to glass other than by fusing | C03C 27/00 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
Glass-ceramic | a crystallised glass or a mixture of glass particles and ceramic particles, in which the glass forms a continuous matrix phase |
This place covers:
Joining the two articles without the use of an interlayer. The surface of one or more of the articles to-be-joined can be treated, e.g. by cleaning or oxidising, leading to an oxidised surface, or can for instance be wetted, but no external layer is applied to any of the surfaces to-be-bonded.
Attention is drawn to the following places, which may be of interest for search:
Fusing glass directly to metal |
In the case the direct bonding results in the in-situ formation of an interlayer, the interlayer is indicated with a symbol from C04B 2237/02-C04B 2237/16
This place covers:
A layer/coating is externally applied on at least one of the two substrates, or a foil or sheet is laid in between the two substrates. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
This place does not cover:
Joining a ceramic article with a glass article though heating, using an adhesive |
Attention is drawn to the following places, which may be of interest for search:
Joining glass to metal by means of an interlayer | C03C 27/04 and subgroups |
Joining glass to glass with the aid of intervening metal | |
Joining metals with the aid of glass |
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/047 is also attributed, since for bonding a polymeric adhesive is used. The ceramic interlayer formed through the bonding is classified with either C04B 2237/08 or C04B 2237/083.
This place covers:
The adhesive is normally a resin, but could also be tar, pitch. The bonding material in principle does not contain inorganic matter. Bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive.
Attention is drawn to the following places, which may be of interest for search:
Joining glass to another inorganic material using an adhesive | |
Joining glass to glass using an adhesive |
In the case of bonding through the use of pre-ceramic polymers, such as polycarbosilane, polysiloxane, polysilazane, as adhesive, C04B 37/005 is also attributed, since after bonding a ceramic interlayer has been formed. This interlayer is classified with either C04B 2237/08 or C04B 2237/083.
The classes from the range C04B 35/63404-C04B 35/6365 can be used to indicate the polymer adhesive.
This place covers:
This part of C04B relates to porous or lightweight cement-, mortar-, concrete-, and artificial stone compositions and porous or lightweight ceramics.
Subdivision of C04B 38/00 is largely based on the methods used for obtaining the porosity or the reduction in weight.
e.g. melting ice;
e.g. by electrolysing;
e.g. expansion of air by reducing pressure takes C04B 40/0089 code in a C-set;
e.g. evaporation of solvent without expansion;
e.g. applying vacuum to draw gas out of gas permeable hollow particles
Porous or lightweight ceramics are always classified in C04B 38/00. When the ceramic composition and/or its precursors are described also in detail, classification is also made in C04B 33/00 or C04B 35/00.
- melting ice- classify also in C04B 40/0683 and/or C04B 38/061
This place does not cover:
Treating slag with gases or gas generating material to make porous slag | |
Expanded graphite | |
Reaction sintered ceramics | |
Catalyst supports by co-precipitation |
Officially in main group C04B 38/00, there is no LPR. Nonetheless when porosity is obtained by a combination of methods, as a general rule, classification is made in the last appropriate place. Classification in two places can be made when all methods are considered to represent invention information. In the case of combination methods the method that provides simply additional information and is not identified by the classification is given as C04B symbol in the C-set. Other aspects of interest can identified further with C04B 38/00 symbols e.g. C04B 38/0054, C04B 38/0074.
The central idea for classification/C-set in C04B 38/00 is: classification according to the method and identifying the nature of the material that is made porous or lightweight by a symbol in the C-set. These symbols can be chosen from C04B 26/00 - C04B 35/00. For a stone substrate C04B 14/00 symbols are used.
This place does not cover:
Honeycomb structures from one or more corrugated sheets by winding or stacking | |
Extrusion of honeycomb structures |
Attention is drawn to the following places, which may be of interest for search:
Filters i.e. particle separators or filtering processes specially modified for separating dispersed particles from gases or vapours; | |
Honeycombs | |
Honeycombs used for filtering exhaust gases of an internal combustion engine |
The C04B 38/0019 set contains only information of the binder or the ceramic material of the adhesive.
Example: (C04B 38/0019; C04B 28/24
The honeycomb ceramic itself has to be given in a different set bearing class C04B 38/0006.
e.g. (C04B 38/0006; C04B 35/565)
Additional class in C04B 28/24 (for above example) with symbols for the specific fillers /additives)
This place covers:
e.g. reaction sintering
This place covers:
e.g. microporous < 2 nm;
e.g. mesoporous 2-50 nm;
e.g. macroporous > 50 nm
This place does not cover:
Use of expanded clay as fillers for mortars, concrete or artificial stone | |
Use of fired or melted materials as fillers for mortars, concrete or artificial stone | |
Use of lightweight materials as fillers for mortars, concrete or artificial stone | |
Use of hollow or porous granular materials as fillers for mortars, concrete or artificial stone | |
Expanding clay, perlite, vermiculite or like granular materials as fillers for mortars, concrete or artificial stone | |
Coating or impregnating of particulate or fibrous ceramic material | |
Catalysts characterised by their shape, spheres |
-Documents classified in this group receive further C-set symbols for the method of making the porosity
- takes precedence over C04B 38/02 or C04B 38/04
This place does not cover:
Preparing or treating the raw materials for obtaining porous material by burning out of a substance e.g. coating of burnable material to give coated pores | |
Physical aspects of the porous material obtained by burning out a substance |
This place covers:
Porous mortars, concrete, artificial stoneir ceramic ware prepared by the addition of blowing agents
e.g. foaming by evaporation of solvent (involves expansion);
e.g. foaming by evaporation of crystal water;
e.g. foaming by using Ca-carbide (+ water --> acetylene) or Si
This place does not cover:
Evaporation of solvent without expansion | |
Porous or hollow ceramic granular materials |
C04B 38/009 takes precedence
This place covers:
Porous mortars, concrete, artificial stoneir ceramic ware prepared by dissolving-out added substances,
e.g. with gaseous HF or by etching
This place does not cover:
Porous or hollow ceramic granular materials |
C04B 38/009 takes precedence
This place does not cover:
Porous or hollow ceramic granular materials |
C04B 38/009 takes precedence
This place covers:
Porous mortars, concrete, artificial stoneir ceramic ware prepared by using foam agents or by using mechanical means
e.g. waterglass is a well-known deflocculant for these compositions;
e.g. with sulfate or sulfonate product
This place does not cover:
Porous or hollow ceramic granular materials | |
Porous mortars, concrete, artificial or ceramic ware prepared by adding chemical blowing agents | |
Foam producing agents |
-C04B 38/009 takes precedence;
This place covers:
Processes for influencing or modifying the properties of mortars, concrete or artificial stone compositions occurring before the shaping of the composition.
Attention is drawn to the following places, which may be of interest for search:
Documents are classified in C04B 40/00 and subgroups when the preparation or characteristics of the mixture are the main aspect of the invention. If the mixture per se or its ingredients are considered new or unusual, classification is made for these aspects also in C04B 22/00 - C04B 32/00 and C04B 38/00. Process steps that are not the main aspect of the invention are classified as part of C-Sets of a mixture using entries from C04B 40/00.
For processes occurring after shaping or moulding of the composition that concern hardening, setting, pre-curing or curing, it should be classified in C04B 40/02.
C04B 40/0082 and C04B 40/0263 contain older documents related to rise in temperature by microwaves.
This place covers:
Mixing steps or sequence of mixing steps, e.g. dry mixing, later adding water or aqueous solution or granulated mortar compacted in a mould.
The presence of a C04B 28/00 or C04B 26/00 symbol in the C-set indicates that the premixture is intended for use with mineral or organic binder systems.
This place covers:
An active ingredient that is a premixture or admixture on its own.
Attention is drawn to the following places, which may be of interest for search:
Sequence of mixing steps |
This group is used as the main classification entry when the invention relates to an active ingredient that is a mixture on its own. The specific ingredients that are part of the mixture are classified in the C-Sets.
This place covers:
The active ingredient is a premixture (admixture) on its own, in the form of a powder.
This place covers:
High shear mixing of the mortar preparation.
Processes for obtaining macro-defect free materials.
Non-porous materials with macro-defect free are also classified in C04B 2111/00301.
In patent documents, the following abbreviations are often used:
MDF | Macro-defect free |
This place covers:
The mixing takes place in a CO2 atmosphere or environment.
This place covers:
Provisional binders, mortars or concrete, i.e. materials intended to be destroyed or removed after hardening, used in the processes for influencing or modifying the properties of mortars, concrete or artificial stone compositions, e.g. processes resulting in concretes with decreasing mechanical properties.
Attention is drawn to the following places, which may be of interest for search:
Control of cementation level in oil wells | |
Investigating or analysing concrete by specific methods |
This place covers:
Processes occurring after the shaping or moulding of the composition that concern hardening, setting, pre-curing or curing.
See Special rules under C04B 40/00.
This place covers:
Steam hardening of mortars, concrete or artificial stone compositions, e.g. as applied to "sand-lime" mixtures or lightweight concrete.
This place does not cover:
Permanent coverings |
Attention is drawn to the following places, which may be of interest for search:
Protecting building materials against unfavourable weather influence |
This place covers:
Inhibiting the setting or inhibiting the action of active ingredients of mortars, concrete or artificial stone compositions, e.g. coating cements with retarder.
Attention is drawn to the following places, which may be of interest for search:
Coating cement powders with retarder | |
Producing shaped articles from dry mixtures containing fibres to which a setting agent is applied after forming | |
Encapsulated ingredients for macromolecular compositions | |
Ground anchors for anchoring structural elements or bulkheads | |
Anchoring-bolts for roof, floor or shaft-lining protection |
This place covers:
Inhibiting the setting with dry ready-made mixture.
Factory made dry mixtures of sands, binders and additives, transported in containers or sacks to the place of use where water is added to make them workable.
The complete dry ready-made cementitious or cement composition is considered as a whole for classification.
Premixture of ingredients in dry form added to the cementitious composition is classified in C04B 40/0042.
Attention is drawn to the following places, which may be of interest for search:
Chemical plugs in general |
This place covers:
Inhibiting the setting by freezing or cooling, e.g. by adding snow or ice.
Attention is drawn to the following places, which may be of interest for search:
Creating porosity by melting ice | |
Creating porosity by melting out added substances |
This place covers:
After-treatment of materials covered by C04B, i.e. artificial stones, mortars, concrete and ceramics, e.g. coating of impregnation of green concrete or unsintered ceramics after primary shaping.
Non-mechanical treatment of natural stone.
While other kinds of after-treatment are not excluded, C04B 41/00 relate to after- treatment of substrate, mainly to :
- coating or impregnation of the substrates: C04B 41/45 and subgroups
- removing material from the substrates: C04B 41/53 and subgroups.
In main group C04B 41/00, no distinction is made between coating or impregnation. Therefore, the terms coating, impregnation and layer are considered equivalent.
This place does not cover:
Coating of fillers for mortars, concrete or artificial stone | |
Infiltration with silicon resulting in reaction bonded silicon carbide | |
Coating of ceramic fibres or powders used in the manufacture of monolithic ceramics | |
Impregnation processes, which lead to fibre-reinforced composites with ceramic matrix | |
Removal of material by burning out added substances | |
Working by laser beam | |
Glazes other than cold glazes | |
Coating of class-ceramics | |
Pigments | |
Ceramic compositions containing free metal bonded to carbides, diamond, oxides, borides, nitrides, silicides, e.g. cermets, or other metal compounds, e.g. oxynitrides or sulfides, other than macroscopic reinforcing agents | |
Infiltration of preforms containing free metal, e.g. cermets | |
After-treatment of materials containing free metal bonded to carbides, diamond, oxides, borides, nitrides, silicides, e.g. cermets, other than as macroscopic reinforcing agents | |
After-treatment of single crystals, e.g. silicon wafers | |
Drying by electro-osmosis | |
Etching of semiconductor bodies |
Attention is drawn to the following places, which may be of interest for search:
Conditioning of the materials prior to shaping | |
Preparations for dentistry | |
Prostheses | |
Filters, membranes | |
Catalysts | |
Applying liquids or other fluent materials to surfaces in general | |
Grinding or polishing | |
Impregnating wood | |
Apparatus or processes for treating or working shaped articles of clay or other ceramic compositions, slag or mixtures containing cementitious material | |
Working stone or stone-like materials | |
Layered products | |
Producing decorative effects by removing surface material | |
Surface treatment of glass | |
Coating compositions | |
Etching, surface-brightening or pickling compositions | |
Covering materials with metals in general | |
Thermal spraying | |
Coating by vacuum evaporation of the coating material, e.g. sputtering | |
Chemical vapour deposition | |
Chemical coating by decomposition of either liquid compounds or solutions of the coating forming compounds, e.g. sol-gel processing | |
Anodic or cathodic protection | |
After-treatment of single crystals | |
Building materials | |
Gas turbines | |
Filters for internal combustion engines | |
Friction materials | |
Fuel cells | |
Processes of apparatus for the manufacture of semiconductor devices |
In this group the C-set system is used. See details under C04B
In group C04B 41/45 and subgroups, as a general rule, classification is made according to the end products present in the coating. However, in C04B 41/49 and subgroups classification is made according to the nature of the starting materials in the coating composition.
- As a general rule subdivision of main group C04B 41/00 is based on aspects relating to the method of after-treatment, such as the selection of the method for applying the coating material on the substrate, e.g. by CVD (C04B 41/4531) or the selection of the coating or impregnation material with which the substrate is treated, e.g. coating with carbon (C04B 41/5001).
For further classifying only the range C04B 41/00 - C04B 41/5392 is used . Documents classified in the range C04B 41/60 - C04B 41/91 always get also a class in C04B 41/00 - C04B 41/5392, which may be combined with one or more C-sets.
- To identify the substrate that is after-treated, the class C04B 41/009 is given and C-sets are created using complementary codes chosen from:
- C04B 14/02 - C04B 14/36 when natural stone is treated
- C04B 26/00 - C04B 32/005 when artificial stone, e.g. concrete is treated
- C04B 33/00 - C04B 35/83 when ceramics are treated
- C04B 38/00 - C04B 38/106 when porous materials are treated
- C04B 14/38 - C04B 14/48 when ceramic fibres are treated, i.e. only when classifying in C04B 41/4584.
When the substrate is further defined e.g. a wood fiber/particle board, which in itself is information that does not require classification in the substrate class itself e.g. C04B 28/02, then the C04B 41/009 set will be:
If a class in C04B 28/00 is also required because the mixture per se is interesting and is part of the invention information, then the C04B 41/009 set will comprise only the C04B 28/02 symbol.
- When the same substrate is coated with two or more layers, classification is made in C04B 41/52. If one of the layers as such might be new in the field, classification for this layer as such is made too.
For each layer a separate C-set is made, each starting with C04B 41/52, the first set relating to the first layer, the second set relating to the second layer etc.
- When, in the case of multiple coating, alternatives are mentioned, the following procedure is followed.
If, e.g. for layer 2 an alternative is to be identified, the third C-set will represent this alternative layer, with at the end the code C04B 41/522. [This symbol is not to be used for classification.] So in this case, a possible third layer will be identified by the fourth C-set, because the third one refers to an alternative of the second layer (represented by the second set).
- For the sake of classification/indexing in C04B, treatment of "green" concrete or ceramics, i.e. concrete that has not hardened yet, resp. ceramic products that are not fired yet, is considered to be covered by C04B 41/00. Such documents will receive C04B 41/4578 as an extra code in the C-set. Only in exceptional cases, classification can be made in this group.
- Group C04B 41/53 relates to the removal of part of the materials of the treated article. A coating process including a step like polishing, roughening or etching is however not classified in C04B 41/53 or a subgroup (what could be expected applying the last place rule), but is classified applying the general rules for coatings above and adding C04B 41/53 or a subgroup to the C-set. If however the removal is the essential step of the invention, classification in C04B 41/53 is (also) made.
- In the same way as when classifying in the other parts of C04B, mentioned above, symbols of the series C04B 2111/00 can be used to identify uses or characteristics of the products obtained.
In this place, the following terms or expressions are used with the meaning indicated:
Green ceramics | unsintered ceramics |
In patent documents, the following abbreviations are often used:
Physical vapour deposition | PVD |
Chemical vapour deposition | CVD |
This scheme is associated mainly with groups C04B 22/00 - C04B 24/00, but also other C04B groups and is used to indicate the function or property of the (active) ingredients. When used in a C-set it shows the presence of an ingredient characterised by its function.
Example:
When used as the base class of a C-set followed by a number of C04B symbols, it shows that all these symbols represent alternative ingredients having the same function.
Example:
In patent documents, the following words/expressions are often used as synonyms:
- "repellent" and "repellant"
This scheme is associated mainly with groups C04B 26/00 - C04B 32/00, C04B 38/00 and C04B 41/00 and is used to indicate the function, property or use of the mortar, concrete, artificial stone or porous material.
In patent documents, the following words/expressions are often used as synonyms:
- "repellent" and "repellant"
Attention is drawn to the following places, which may be of interest for search:
Compositions or ingredients of mortars, concrete or artificial stone characterised by the absence or the very low content of chromium VI, e.g. for avoiding chromium eczema |
Attention is drawn to the following places, which may be of interest for search:
Sprayable mixtures, i.e. concrete-like, materials able to be shaped by spraying instead of by casting, e.g. gunite |
Attention is drawn to the following places, which may be of interest for search:
Materials containing photocatalysts, e.g. TiO2, for avoiding staining by air pollutants or the like |
Attention is drawn to the following places, which may be of interest for search:
Aspects relating to the protection of the health, e.g. materials containing special additives to afford skin protection |
Attention is drawn to the following places, which may be of interest for search:
Materials containing photocatalysts, e.g. TiO2, for avoiding staining by air pollutants or the like |
Attention is drawn to the following places, which may be of interest for search:
Self-cleaning materials, e.g. using lotus effect |
This scheme is associated mainly with groups C04B 26/00 - C04B 32/00, C04B 38/00 or C04B 41/00 and is used to indicate mortars, concrete or artificial stone characterised by specific physical values
This place covers:
Additional aspects that cannot be indicated with CPC groups regarding the starting materials for making a ceramic, the methods of shaping a green ceramic, the heat treatments that are given to green, melted or already sintered ceramics, aspects that regard the sintered ceramic, it's properties, it's use.
Attention is drawn to the following places, which may be of interest for search:
Clay materials | C04B 33/00 and sub-classes |
Ceramic materials | C04B 35/00 and sub-classes |
joining of a ceramic layer to another layer | C04B 37/00 and sub-classes |
porous ceramic products | |
Aspects relating to ceramic laminates or to joining of ceramic articles with other articles by heating | C04B 2237/00 and sub-codes |
Layered products essentially comprising ceramics , e.g. refractory products |
The symbols from the C04B 2235/00-scheme are meant mainly for the fields C04B 33/00 and C04B 35/00, but can also be used for the fields B32B 18/00 and C04B 37/00.
This place covers:
The aspects relate either to the starting materials that can be used for making a ceramic or to the secondary phases of ceramic objects.
This place does not cover:
Aspects relating to the preparation, properties or mechanical treatment of green bodies or pre-forms | C04B 2235/60 and subgroups |
Aspects relating to heat treatment of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes | C04B 2235/65 and subgroups |
Aspects relating to sintered or melt-casted ceramic products, other than the specific secondary phases that are present | C04B 2235/70 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on oxide ceramics | C04B 35/01 and subgroups |
Additives to cement, concrete, mortar or artificial stone, characterised by their function, e.g. dispersant, oxidising agent, pigment | C04B 2103/00 and subgroups |
This place covers:
The starting materials are defined by their chemical composition, and can be a powder, suspension, solution, but not a fiber. The secondary phases of the shaped ceramics are also defined by their composition and can have any grain size or shape.
This place does not cover:
Coatings around inorganic particles that are used as starting material for making a ceramic | C04B 35/62802 and subgroups |
Binders based on phosphoric acid or phosphates | C04B 35/6306 and subgroups |
Polymer additives | C04B 35/634 and subgroups |
Polysaccharide additives | C04B 35/636 and subgroups |
Constituents or additives of the starting mixture chosen for their shape or used because of their shape or their physical appearance | C04B 2235/50 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Inorganic additives for clay mixtures | |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Ceramic interlayer used for joining a ceramic with another substrate | C04B 2237/04 and subgroups |
Ceramic substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/32 and subgroups |
This place covers:
All metal oxides or metal salts that convert to oxide upon heating, used as starting material for making a ceramic or present as secondary phase in a sintered ceramic.
This place does not cover:
Glass, asbestos or fused silica fibers added to a ceramic | |
Non-metal oxide starting material or secondary phase, e.g. silica, silicates, boron oxide | C04B 2235/34 and subgroups |
Metal oxide starting material or secondary phase present in a glass phase | C04B 2235/36 and subgroup |
Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate | C04B 2235/44 and subgroups |
Oxide fibers added to ceramics | C04B 2235/522 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on beryllium oxide | |
Ceramics based on actinide oxides, e.g. uranium or plutonium oxides | |
Inorganic additives used for making ceramics | C04B 35/6303 and subgroups |
Non-oxide ceramic constituents or additives, non-oxide phases present as secondary phase in a sintered ceramic | C04B 2235/38 and subgroups |
Metallic constituents or additives not added as binding phase, or present as secondary phase in a sintered ceramic | C04B 2235/40 and subgroups |
Non metallic elements added as constituents or additives, or present as secondary phase in a sintered ceramic, e.g. silicon, boron, carbon, sulphur, phosphor, selenium or tellurium | C04B 2235/42 and subgroups |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Oxide interlayer used for joining a ceramic with another substrate | C04B 2237/06 and subgroups |
Oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/34 and subgroups |
Oxides used as filler for polymers | C08K 3/20 and subgroup |
The C04B 35/6303 class and sub-classes are in praxis only used for inorganic binders, not for all inorganic additives.
The code C04B 2235/32 is given only to the actinide oxides (e.g. uranium, plutonium, thorium). Arsenic oxide, selenium oxide and tellurium oxide receive C04B 2235/34, since Ar, Se and Te are not metals.
If certain metal salts are used, the metal salt can be classified with a C04B 2235/32 symbol for the cation and a C04B 2235/44 symbol for the anion. Cations present in mixed oxide additives are also individually classified, e.g. if a magnesium ferrite is used as starting powder, both C04B 2235/3274 for the ferrite and C04B 2235/3206 for the MgO are added. If barium titanate is used as additive, both C04B 2235/3236 for alkaline earth titanate additives and C04B 2235/3215 for barium salt or oxide additives are used. This also accounts for additives from the C04B 2235/34 range. If for instance an alkali silicate is used as additive, both C04B 2235/3427 and C04B 2235/3201 are given.
The C04B 2235/32 and C04B 2235/34 are not combined with the C04B 2235/38 or C04B 2235/40 symbols for one and the same additive. If for instance magnesium nitride is added, just C04B 2235/3852 is used, but not C04B 2235/3206. A mixed non-oxide such as aluminium silicon carbide will be classified with C04B 2235/3826 for the silicon carbide and C04B 2235/3817 for the aluminium carbide. If a non-oxide additive is made starting from a metal salt, however, for instance titanium boride additive is first made from titanium acetate and boron, then not only the boride symbol, C04B 2235/3813, but also the symbols for the first starting materials are given, thus C04B 2235/3232 for the Ti-salt, C04B 2235/421 for the boron and C04B 2235/449 for the acetate.
This place covers:
Alkali metal oxides, e.g. Na2O, K2O, alkali metal containing mixed oxides, e.g. sodium niobate (NaNbO3), alkali metal oxide containing clay, alkaline metal oxide containing silicates, e.g. sodium feldspar (NaAlSi3O8) or waterglass (Na2SiO3), alkali metal salts, e.g. potassium chloride (K2Cl), alkali metal containing salts, all used as starting material for making ceramics.
Alkali metal oxide containing secondary phases of sintered ceramic, e.g. K2O or Na2O containing mixed oxides, e.g. potassium tantalate (KTaO3).
compounds of alkali metals, i.e. lithium, sodium, potassium, rubidium, caesium, or francium C01D
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on beta alumina (normally contains alkali oxides) | |
Alkali metal aluminosilicates based ceramics | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead | |
Other ferrites containing alkali metals | |
Alkali metal phosphate added as binder | |
Alkali aluminate starting material or secondary phase | |
Alkali titanate starting material or secondary phase | |
Alkali chromate starting material or secondary phase | |
Alkali zirconate starting material or secondary phase | |
Alkali niobate starting material or secondary phase | |
Alkali molybdate starting material or secondary phase | |
Alkali manganate starting material or secondary phase | |
Alkali ferrite starting material or secondary phase | |
Alkali cobaltate starting material or secondary phase | |
Alkali cuprate starting material or secondary phase | |
Alkali zincate starting material or secondary phase | |
Alkali stannate starting material or secondary phase | |
Alkali bismuthate starting material or secondary phase | |
Alkali borate starting material or secondary phase | |
Alkali silicate starting material or secondary phase | |
Alkali alumino-silicate starting material or secondary phase | |
Alkali glass starting material or secondary phase | |
Alkali nitride starting material or secondary phase | |
Alkali hydroxide starting material | |
Alkali iodide starting material | |
Alkali sulphide starting material |
In this place, the following terms or expressions are used with the meaning indicated:
Alkali metal oxides | lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), francium (Fr) |
This place covers:
Lithium oxide, Li2O, lithium containing mixed oxides, e.g. lithium niobate (LiNbO3), lithium oxide containing clay, lithium oxide containing silicates, e.g. spodumene(LiAl(SiO3)2), lithium salts, e.g. lithium bromide (Li2Br) or lithium hydroxide (LiOH), lithium containing salts, all used as starting material for making ceramics.
Lithium oxide containing secondary phases of a sintered ceramic, Li2O or Li2O containing mixed oxides, e.g. lithium titanate (LiTiO3).
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and lithium | |
Lithium aluminate (LiAlO2) starting material or secondary phase | |
Lithium gallate (LiGaO2) starting material or secondary phase | |
Preparation of lithium salts, e.g. oxides, nitrates, sulphates | C01D 15/00 and subgroups |
This place covers:
Alkaline earth metal oxides, e.g. BeO, MgO, RaO, alkaline earth metal containing mixed oxides, e.g. calcium zirconate (CaZrO3), alkaline earth oxide containing clay, alkaline earth oxide containing silicates, e.g. wollastonite (CaSiO3), alkaline earth salts, e.g. barium carbonate (BaCO3), alkaline earth metal containing salts, all used as starting material for making ceramics.
Alkaline earth metal oxide containing secondary phases of sintered ceramic, e.g. SrO or alkaline earth metal oxide containing mixed oxides, e.g. barium titanate (BaTiO3.)
Attention is drawn to the following places, which may be of interest for search:
Obtaining lime, magnesia or dolomite | C04B 2/00 and subgroups |
Alkaline earth metal alumino-silicate based ceramics | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead | |
Other ferrites containing alkaline earth metals or lead | |
Ceramics based on alkaline earth titanates | C04B 35/465 and subgroups |
Alkaline earth metal phosphate added as binder | |
Alkaline earth aluminate starting material or secondary phase | |
Alkaline earth titanate starting material or secondary phase | |
Alkaline earth chromate starting material or secondary phase | |
Alkaline earth zirconate starting material or secondary phase | |
Alkaline earth niobate starting material or secondary phase | |
Alkaline earth molybdate starting material or secondary phase | |
Alkaline earth manganate starting material or secondary phase | |
Alkaline earth ferrite starting material or secondary phase | |
Alkaline earth cobaltate starting material or secondary phase | |
Alkaline earth cuprate starting material or secondary phase | |
Alkaline earth zincate starting material or secondary phase | |
Alkaline earth stannate starting material or secondary phase | |
Alkaline earth bismuthate starting material or secondary phase | |
Alkaline earth borate starting material or secondary phase | |
Alkaline earth silicate starting material or secondary phase | |
Alkaline earth alumino-silicate starting material or secondary phase | |
Alkaline earth glass starting material or secondary phase | |
Alkaline earth carbide starting material or secondary phase | |
Alkaline earth metal starting material or secondary phase | |
Alkaline earth hydroxide starting material | |
Alkaline earth bromide starting material | |
Alkaline earth sulphate starting material | |
Alkaline earth oxalate starting material | |
treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of alkaline earth metals or magnesium | C09C 1/02 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic alkaline earth metal compounds |
The symbol C04B 2235/3205 is used only for BeO, and RaO and in the case alkaline earth metal oxides are used without specifying which. As soon as one of MgO, CaO, SrO or BaO is mentioned, the respective symbol is used. Also if all 4 are mentioned, all 4 symbols are used, not C04B 2235/3205.
This place covers:
MgO, MgO containing mixed oxides, e.g. spinel (MgAl2O4), Mg containing clay, MgO containing silicates, e.g. cordierite (Mg2Al4Si5O18), Mg salts, e.g. magnesium carbonate (MgCO3), Mg containing salts, e.g. magnesium calcium nitrate (Mg0.5Ca0.5NO3), all used as starting material for making ceramics.
MgO containing secondary phases of sintered ceramic, e.g. MgO or MgO containing mixed oxides, e.g. forsterite (Mg2SiO4)
This place does not cover:
MgO containing glass additive for making ceramics | |
Mg containing non-oxide additives for making ceramics, e.g. magnesium boride, magnesium nitride | C04B 2235/38 and subgroups |
Metallic Mg used as additive for making ceramics | |
MgO containing fiber additives for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: magnesia | |
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone: Magnesia; Magnesium hydroxide | |
Magnesium oxide or magnesium carbonate cements | C04B 28/105, C04B 28/30 and subgroup |
Ceramics based on magnesium oxide | C04B 35/04 and subgroups |
Ceramics based on oxide mixtures derived from dolomite (containing both CaO and MgO) | |
Mixed oxides of MgO with both alumina and silica, e.g. cordierite | |
Mixed oxides of MgO with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and magnesium | |
Mixed oxides of MgO with iron oxides, e.g. ferrites | |
Mixed oxides of MgO with chromium oxide, e.g. chromites | |
Mixed oxides of MgO with alumina, without silica, e.g. magnesium aluminate, spinel | |
Magnesium based phosphates | |
Mixed oxides of MgO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of MgO with zinc oxide and/or bismuth oxide, e.g. magnesium bismuthate | |
Mixed oxides of MgO with tin oxide, e.g. magnesium stannate | |
Mixed oxides of MgO with titanium oxides, such as magnesium titanate | |
Mixed oxides of MgO with zirconium oxide, e.g. magnesium zirconate | C04B 35/48 and subgroups |
Mixed oxides of MgO with zirconium oxide and titanium oxide, e.g. magnesium titanate zirconate (MgTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of MgO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. magnesium tantalum niobate (MgNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Making fibres based on magnesium oxide | |
Coating or impregnating ceramic substrates with magnesium oxide | C04B 41/5029, C04B 41/5084 (cementitious) |
Spinel starting material or secondary phase | |
Sr and Mg doped lanthanum gallate (La0.90Sr0.10Ga0.80Mg0.2)O3 starting material or secondary phase | C04B 2235/3286, C04B 2235/3206, C04B 2235/3213, C04B 2235/3227 (La) |
Magnesium silicate starting material or secondary phase | |
Magnesium alumino-silicate starting material or secondary phase | |
Magnesium boride starting material or secondary phase | |
Magnesium starting material or secondary phase | |
Magnesium phosphate starting material or secondary phase | |
Materials for prostheses based on magnesia or magnesium oxide | |
Catalysts comprising the elements, oxides, or hydroxides of magnesium | |
Preparation of magnesium compound powders, e.g. magnesium oxide powder | C01F 5/00 and subgroups |
treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds containing only magnesium as metal |
This place covers:
CaO, CaO containing mixed oxides, e.g. calcium zirconate (CaZrO3), CaO containing clay, calcium oxide containing silicates, e.g. wollastonite (CaSiO3), calcium salts, e.g. calcium carbonate (CaCO3), calcium containing salts, all used as starting material for making ceramics.
Calcium oxide containing secondary phases of sintered ceramic, e.g. CaO or calcium oxide containing mixed oxides, e.g. calcium alumino ferrite (Ca2(Al,Fe)2O5).
This place does not cover:
Rendering lime in clay mixtures harmless |
Attention is drawn to the following places, which may be of interest for search:
Hydraulic lime | |
Eliminating lime or iron from clay mixtures | |
Ceramics based on calcium oxide | |
Ceramics based on oxide mixtures derived from dolomite (containing both CaO and MgO) | |
Mixed oxides of CaO with both alumina and silica, e.g. cordierite | |
Mixed oxides of CaO with silica without alumina, e.g. wollastonite (CaSiO4) | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and calcium, strontium or barium | |
Mixed oxides of CaO with chromium oxide, e.g. chromites | |
Mixed oxides of CaO with alumina, without silica, e.g. calcium aluminate | |
Calcium based phosphates | |
Mixed oxides of CaO with copper oxide, e.g. cuprates | C04B 35/45 and subgroups |
Mixed oxides of CaO with zinc oxide and/or bismuth oxide, e.g. calcium bismuthate | |
Mixed oxides of CaO with tin oxide, e.g. calcium stannate | |
Mixed oxides of CaO with titanium oxides, such as calcium titanate | |
Mixed oxides of CaO with zirconium oxide, e.g. calcium zirconate | C04B 35/48 and subgroups |
Mixed oxides of CaO with zirconium oxide and titanium oxide, e.g. calcium titanate zirconate (CaTi0.5Zr0.5O3) | C04B 35/49 and subgroups |
Mixed oxides of CaO with vanadium oxide and/or niobium oxide and/or molybdenum oxide and/or tungsten oxide and/or tantalum oxide, e.g. calcium tantalum niobate (CaNb0.5Ta0.5O3) | C04B 35/495 and subgroups |
Calcium zirconate starting material or secondary phase | |
Calcium silicate starting material or secondary phase | |
Calcium alumino-silicate starting material or secondary phase | |
Calcium nitride starting material or secondary phase | |
Calcium ethoxide starting material or secondary phase | |
Calcium nitrate starting material or secondary phase | |
Materials for prostheses based on calcia or calcium oxide CaO | |
The preparation of compounds of calcium, barium and strontium in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01F 11/00 and subgroups |
treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcium carbonates | C09C 1/021 and subgroups |
treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: calcium sulphates |
This place covers:
Mixed calcium magnesium carbonate or mixed calcium magnesium oxide, either as starting material or secondary phase in a sintered ceramic.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on oxide mixtures derived from dolomite (containing both CaO and MgO) |
In this place, the following terms or expressions are used with the meaning indicated:
Dolomite | (CaMg)(CO3)2 |
This place covers:
Calcium phosphates such as hydroxyapatite used as starting material for making ceramics or as secondary phase in a sintered ceramic.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on phosphates | |
Phosphate based binders for ceramic materials | C04B 35/6306 and subgroups |
Phosphate starting materials for making ceramics or phosphate secondary phases of sintered ceramics |
This place covers:
SrO, SrO containing mixed oxides, e.g. lanthanum strontium chromite (La1-xSrxCrO3), SrO containing clay, strontium oxide containing silicates, e.g. SrSiO3, strontium salts, e.g. strontium fluoride (SrF2), strontium containing salts, all used as starting material for making ceramics.
Strontium oxide containing secondary phases of sintered ceramic, e.g. SrO or strontium oxide containing mixed oxides, e.g. strontium aluminate (Sr2Al2O5).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on strontium titanate | |
Strontium manganate starting material or secondary phase | |
Sr and Mg doped lanthanum gallate (La0.90Sr0.10Ga0.80Mg0.2)O3 starting material or secondary phase | C04B 2235/3286, C04B 2235/3206, C04B 2235/3213, C04B 2235/3227 (La) |
Strontium silicate starting material or secondary phase | |
Strontium alumino-silicate starting material or secondary phase | |
Strontium silicide starting material or secondary phase | |
Strontium acetylacetonate starting material or secondary phase | |
Strontium phosphate starting material or secondary phase |
This place covers:
BaO, barium containing mixed oxides, e.g. barium molybdate (BaMoO4), barium oxide containing clay, barium oxide containing silicates, e.g. celsian (BaAl2Si2O8), barium salts, e.g. barium carbonate (BaCO3), barium containing salts, all used as starting material for making ceramics.
Barium oxide containing secondary phases of sintered ceramic, e.g. BaO or barium oxide containing mixed oxides, e.g. barium titanate (BaTiO3.)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on barium titanate | C04B 35/468 and subgroups |
Barium titanate starting material or secondary phase | |
Barium gallate (BaGa2O4) starting material or secondary phase | |
Barium silicate starting material or secondary phase | |
Barium alumino-silicate starting material or secondary phase | |
Barium carbide starting material or secondary phase | |
Barium phosphide starting material or secondary phase | |
Barium selenide starting material or secondary phase | |
treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: barium sulphates |
This place covers:
Al2O3, Al2O3 containing mixed oxides, e.g. aluminum chromate Al2(CrO4)3, Al salts, e.g. aluminium nitrite(Al(NO2)3), Al containing salts, e.g. aluminium calcium nitrate, all used as starting material for making ceramics.
Al2O3 containing secondary phases of sintered ceramic, e.g. Al2O3 or Al2O3 containing mixed oxides, e.g. calcium alumino ferrite (Ca2(Al,Fe)2O5).
This place does not cover:
Aluminium phosphate added as binder |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: alumina | |
Clay wares | C04B 33/00 and sub-classes |
Alumina based ceramics | C04B 35/10 and sub-classes |
Alumino-silicate based ceramics | C04B 35/18 and sub-classes |
Aluminate based ceramics | C04B 35/44 and sub-class |
Ceramics based on aluminium titanate | |
Zirconia fine ceramics containing also alumina | |
Making fibres based on aluminium oxide | |
Coating or impregnating ceramic substrates with alumina | |
Aluminum titanate starting material or secondary phase | |
aluminum chromate Al2(CrO4)3 starting material or secondary phase | |
Aluminum niobate (AlNbO4) starting material or secondary phase | |
Aluminum tungstate (Al2W2O9) starting material or secondary phase | |
Aluminum containing ferrite (e.g. Co1−xZnxFe2−xAlxO4) starting material or secondary phase | C04B 2235/3274, C04B 2235/3217, C04B 2235/3275, C04B 2235/3284 |
Aluminum borate (Al2B2O6) starting material or secondary phase | |
Alumino-silicate starting material or secondary phase | C04B 2235/3463 and subgroups |
Aluminum glass starting material or secondary phase | |
Aluminum carbide starting material or secondary phase | |
Aluminum nitride starting material or secondary phase | C04B 2235/3865 and subgroup |
Aluminum metal starting material or secondary phase | |
Aluminum nitrite (Al(NO2)3) starting material | |
Aluminum methoxide starting material | |
Aluminum sulphide starting material | |
Aluminum citrate starting material | |
Alumina or aluminate fibers used in ceramic compositions | |
Alumina or aluminate interlayer used for joining a ceramic with another substrate | |
Alumina or aluminate substrate joined with another substrate or being part of a ceramic laminate | |
Materials for prostheses or coatings of prostheses based on aluminium oxides | |
alumina-based membranes | |
Catalysts comprising alumina | |
Preparation of aluminium compound powders, e.g. aluminium oxide powder | C01F 7/00 and sub-classes |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of aluminium | C09C 1/40 and subgroups |
The code C04B 2235/3217 is not given to alumino-silicates, the alumino-silicates just receive the C04B 2235/3463 code.
This place covers:
All hydrated alumina starting materials, aluminum hydroxide, alumina sol.
Attention is drawn to the following places, which may be of interest for search:
Inorganic binders for ceramics | |
Metal hydroxides as starting materials for making ceramics |
This place covers:
Alumina phases that are not stable and convert to alpha alumina upon heating at high temperature, e.g. delta or gamma alumina
This place does not cover:
beta-alumina as starting material for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on beta-alumina |
This place covers:
All mixed oxides in which alumina is mixed with alkali metal oxides, alkaline earth metal oxides or rare earth metal oxides, used as starting material for making ceramics or present as secondary phase in sintered ceramics, e.g. spinel (MgAl2O4), yttrium aluminium garnet (YAG, Y3Al5O15)
This place does not cover:
Aluminum titanate starting material or secondary phase | |
aluminum chromate Al2(CrO4)3 starting material or secondary phase | |
Aluminum niobate (AlNbO4) starting material or secondary phase | |
Aluminum tungstate (Al2W2O9) starting material or secondary phase | |
Aluminum containing ferrite (e.g. Co1−xZnxFe2−xAlxO4) starting material or secondary phase | C04B 2235/3274, C04B 2235/3217, C04B 2235/3275, C04B 2235/3284 |
Aluminum borate (Al2B2O6) starting material or secondary phase | |
Alumino-silicate starting material or secondary phase | C04B 2235/3463 and sub-classes |
Alumina or aluminate fibers used in ceramic compositions |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators: aluminates | |
Ceramics based on aluminates | C04B25/44 and subgroups |
Hydraulic aluminate cements | C04B 28/06 and subgroups, C04B 7/323 |
Clay wares | C04B 33/00 and subgroups |
Ceramics based on beta-alumina | |
Ceramics based on silico-aluminates | C04B 35/18 and subgroups |
Coating or impregnating ceramic substrates with aluminate | |
Coating or impregnating ceramic substrates with spinels | |
Aluminate catalysts or catalysts carrier | |
Preparation of alkali metal aluminates powders | C01F 7/04 and sub-classes |
Preparation of alkaline earth metal aluminates powders | C01F 7/16 and sub-classes |
Purification of aluminates | C01F 7/47 and sub-classes |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing refractory metal aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth aluminates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth aluminates | C09K 11/77062, C09K 11/77212, C09K 11/77342, C09K 11/77492, C09K 11/7758, C09K 11/7764, C09K 11/77742, C09K 11/77922 |
This place covers:
Rare earth metal oxides, e.g. Sc2O3, Lu2O3, Nd2O3, rare earth metal containing mixed oxides, e.g. erbium manganite ErMnO3, rare earth oxide containing clay, rare earth oxide containing silicates, e.g. apatite type rare earth silicate (Sr2RE2)(RE6)(SiO4)6O2, rare earth salts, e.g. dysprosium sulphide (Dy2S3), rare earth metal containing salts, all used as starting material for making ceramics.
Rare earth metal oxide containing secondary phases of sintered ceramic, e.g. Yb2O3 or rare earth metal oxide containing mixed oxides, e.g. rare earth niobate (RENbO3.)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on beta alumina (often contains rare earth oxides) | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead | |
Other ferrites containing rare earth metals, e.g. rare earth ferrite garnets | |
Ceramics based on rare earth oxide containing cuprates. | C04B 35/4504 and subgroups |
Ceramics based on rare-earth compounds | |
Coating or impregnating ceramic substrates with rare earth oxides | |
Rare earth aluminate starting material or secondary phase | |
Rare earth titanate starting material or secondary phase | |
Rare earth chromate starting material or secondary phase | |
Rare earth zirconate starting material or secondary phase | |
Rare earth niobate starting material or secondary phase | |
Rare earth molybdate starting material or secondary phase | |
Rare earth manganate starting material or secondary phase | |
Rare earth ferrite starting material or secondary phase | |
Rare earth cobaltate starting material or secondary phase | |
Rare earth cuprate starting material or secondary phase | |
Rare earth zincate starting material or secondary phase | |
Rare earth stannate starting material or secondary phase | |
Rare earth bismuthate starting material or secondary phase | |
Rare earth borate starting material or secondary phase | |
Rare earth silicate starting material or secondary phase | |
Rare earth alumino-silicate starting material or secondary phase | |
Rare earth glass starting material or secondary phase | |
Rare earth boride starting material or secondary phase, e.g. dysprosium boride (DyB2) | |
Rare earth hydroxide starting material | |
Rare earth chloride starting material, e.g. ytterbium chloride YbCl3 | |
Rare earth sulphide starting material, e.g. dysprosium sulphide Dy2S3 | |
Rare earth oxalate starting material | |
Rare earth oxide interlayer used for joining a ceramic with another substrate | |
Catalysts comprising metals or metal oxides or hydroxides of rare earths | |
The preparation of rare earth compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01F 17/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth germinates | C09K 11/7707, C09K 11/7735, C09K 11/775, C09K 11/7775, C09K 11/7793 |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth Antimonates; Arsenates |
The class C04B 2235/3224 is used for the compounds of scandium (Sc), lutetium (Lu), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb), and in the case rare earths in general are mentioned.
In this place, the following terms or expressions are used with the meaning indicated:
rare earth oxides | The oxides of scandium (Sc), yttrium (Y), lutetium (Lu), lanthanum (La), cerium (Ce), praseodymium (Pr), neodymium (Nd), promethium (Pm), samarium (Sm), europium (Eu), gadolinium (Gd), terbium (Tb), dysprosium (Dy), holmium (Ho), erbium (Er), thulium (Tm), ytterbium (Yb) |
This place covers:
Yttrium oxide, Y2O3, yttrium containing mixed oxides, e.g. yttrium doped zirconia (YSZ), yttrium containing clay, yttrium containing silicates, e.g. yttrium silicate (Y2SiO5), yttrium salts, e.g. yttrium chloride (YCl3), yttrium containing salts, all used as starting material for making ceramics.
Yttrium oxide containing secondary phases of sintered ceramic, e.g. Y2O3 or yttrium oxide containing mixed oxides, e.g. yttrium aluminium garnet (YAG, Y3Al5O15)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on yttrium aluminium garnet (YAG, Y3Al5O15) | C04B 35/44, C04B 2235/3225, C04B 2235/764 (garnets) |
Ceramics based on yttrium stabilised zirconia | |
Ceramics based on yttrium oxide | |
Yttrium aluminate (YAG) starting material or secondary phase | |
Yttrium doped zirconia starting material or secondary phase | |
Yttrium ferrite starting material or secondary phase | |
Yttrium silicate starting material or secondary phase | |
Yttrium alumino-silicate starting material or secondary phase | |
Yttrium glass starting material or secondary phase | |
Yttrium boride starting material or secondary phase |
This place covers:
Lanthanum oxide, La2O3, lanthanum containing mixed oxides, e.g. lanthanum chromite (LaCrO3), lanthanum containing clay, lanthanum containing silicates, e.g. lanthanum gallium silicate (LGS), also known as langasite, with the chemical formula A3BC3D2O14, lanthanum salts, e.g. lanthanum chloride (LaCl3), lanthanum containing salts, all used as starting material for making ceramics.
Lanthanum oxide containing secondary phases of sintered ceramic, e.g. La2O3 or lanthanum oxide containing mixed oxides, e.g. lanthanum niobate vanadate (LaNb1−xVxO4).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on lanthanum chromite (LaCrO3) | |
Ceramics based on lanthanum niobate vanadate (LaNb1−xVxO4) | C04B 35/495, C04B 2235/3227, C04B 2235/3251 (Nb), C04B 2235/3239 (V) |
Ceramics based on lanthanum oxide | |
lanthanum chromite (LaCrO3) starting material or secondary phase | |
lanthanum niobate vanadate (LaNb1−xVxO4) starting material or secondary phase | |
Sr and Mg doped lanthanum gallate (La0.90Sr0.10Ga0.80Mg0.2)O3 starting material or secondary phase | C04B 2235/3286, C04B 2235/3206, C04B 2235/3213, C04B 2235/3227 |
lanthanum gallium silicate starting material or secondary phase | |
Lanthanum alumino-silicate starting material or secondary phase | |
Lanthanum glass starting material or secondary phase | |
Lanthanum boride (LaB6) starting material or secondary phase |
This place covers:
Cerium oxide, Ce2O3 or CeO2, cerium containing mixed oxides, e.g. cerium gadolium oxide (CGO, Ce1-xGdxO2), cerium containing clay, cerium containing silicates, e.g. Ce6[Si4O13][SiO4]2, cerium salts, e.g. cerium nitrate (Ce(NO3)3), cerium containing salts, all used as starting material for making ceramics.
Cerium oxide containing secondary phases of sintered ceramic, e.g. Ce2O3 or cerium oxide containing mixed oxides, e.g. cerium stabilised zirconia.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on cerium stabilised zirconia | |
Ceramics based on cerium gadolium oxide (CGO, Ce1-xGdxO2) | |
Ceramics based on cerium oxide | |
cerium gadolium oxide (CGO, Ce1-xGdxO2) starting material or secondary phase | |
cerium stabilised zirconia starting material or secondary phase | |
cerium silicate starting material or secondary phase | |
Cerium alumino-silicate starting material or secondary phase | |
Cerium glass starting material or secondary phase | |
Cerium carbide (CeC2) starting material or secondary phase |
This place covers:
Refractory metal oxides, e.g. TiO2, WO6, refractory metal containing mixed oxides, e.g. calcium zirconate (CaZrO3), refractory oxide containing clay, refractory oxide containing silicates, e.g. barium titanium silicate (BaTiSi3O9), refractory salts, e.g. vanadium chloride (VCl3), refractory metal containing salts, all used as starting material for making ceramics.
Refractory metal oxide containing secondary phases of sintered ceramic, e.g. CrO3 or refractory metal oxide containing mixed oxides, e.g. barium titanate (BaTiO3.)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Refractory metal boride starting material or secondary phase | |
Refractory metal carbide starting material or secondary phase | C04B 2235/3839 and subgroups |
Refractory metal nitride starting material or secondary phase | |
Refractory metal silicide starting material or secondary phase | |
Refractory metal starting material or secondary phase | |
Refractory metal oxide interlayer used for joining a ceramic with another substrate | |
Refractory metal oxide substrate joined with another substrate or being part of a ceramic laminate |
In this place, the following terms or expressions are used with the meaning indicated:
refractory oxides | titanium oxide, vanadium oxide, chromium oxide, zirconium oxide, niobium oxide, molybdenum oxide, hafnium oxide, tantalum oxide, tungsten oxide |
This place covers:
Titanium oxides (titania), e.g. TiO2, titanium containing mixed oxides, e.g. lanthanum titanate (LaTiO3), titanium oxide containing clay, titanium oxide containing silicates, e.g. barium titanium silicate (BaTiSi3O9), titanium salts, e.g. titanium hydroxide (TiO(OH)2), titanium metal containing salts, all used as starting material for making ceramics.
Titanium metal oxide containing secondary phases of sintered ceramic, e.g. rutile (TiO2) or titanium metal oxide containing mixed oxides, e.g. strontium titanate (SrTiO3.)
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: titania, e.g. titanates | |
Ceramics based on titanium oxide | |
Making fibres based on titanium oxide | |
Coating or impregnating ceramic substrates with titanium oxides or titanates | |
titanium ferrite (TiFe2O4) starting material or secondary phase | |
barium titanium silicate (BaTiSi3O9) starting material or secondary phase | |
Titanium containing glass starting material or secondary phase | |
Titanium diboride (TiB2) starting material or secondary phase | |
Titanium carbide (TiC) starting material or secondary phase | |
Titanium nitride (TiN) starting material or secondary phase | |
Titanium disilicide (TiSi2) starting material or secondary phase | |
Titanium starting material or secondary phase | |
titanium hydroxide (TiO(OH)2) starting material or secondary phase | |
Titania or titanate substrate joined with another substrate or being part of a ceramic laminate | |
Catalysts or catalyst carriers comprising titanium; Oxides or hydroxides thereof | |
The preparation of titanium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 23/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of titanium | C09C 1/36 and subgroups |
This place covers:
Titanates, e.g. aluminium titanate (Al2TiO5) or mixed niobate-titanates, used as starting material for making ceramics
Titanate containing secondary phases of sintered ceramic, e.g. lead titanate (PbTiO3).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on titanates | C04B 35/462 and subgroups |
Ceramics based on zirconates-titanates | C04B 35/49 and subgroups |
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates, based on solid solutions with lead, containing also titanates | |
Ceramics or ceramic mixtures based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates containing also lead and also titanates | |
Zirconates or hafnates containing also titanium oxide or titanates as starting material for making ceramics or as secondary phase in a ceramic | |
The preparation of titanium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides titanium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of titanate compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 23/003 and subgroups |
This place covers:
Alkaline earth titanates, e.g. magnesium titanate (MgTiO3), used as starting material for making ceramics.
Titanate containing secondary phases of sintered ceramic, e.g. barium titanate (BaTiO3).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on alkaline earth titanates | C04B 35/465 and subgroups |
Alkaline earth oxides or salts as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3205 and subgroups |
barium titanium manganite (BaTi1/2Fe1/2O3) starting material or secondary phase | |
The preparation of alkaline earth metal titanate compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates |
This place covers:
Titanium oxide with the formula TiOx, where x < 2, e.g. Ti2O3 or TiO, used as starting material for making a ceramic or present as a secondary in a sintered ceramic.
This place does not cover:
A sintered ceramic having as the main phase a sub-stoichiometric titanium oxide | |
A sintered ceramic having as the main phase a sub-stoichiometric titanate phase, e.g. BaTiO2.9 | C04B 35/462 and subgroups ( C04B 35/4682 for barium titanate) and C04B 2235/79 |
Attention is drawn to the following places, which may be of interest for search:
The preparation titanium sub-oxide compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
Compositional and structural details of pigments exhibiting interference colours the core consisting of an inorganic suboxide or a mixture thereof, e.g. SiOx, TiOx | C09C 2200/1037 and sub-classes |
This place covers:
Vanadium oxides, e.g. V2O5, vanadium containing mixed oxides, e.g. yttrium vanadate (YVO4), vanadium oxide containing clay, vanadium oxide containing silicates, e.g. cavansite (Ca(VO)Si4O10(H2O)4), vanadium salts, e.g. ammonium vanadate (NH4VO3), vanadium metal containing salts, all used as starting material for making ceramics.
Vanadium metal oxide containing secondary phases of sintered ceramic, e.g. VO2 or vanadium metal oxide containing mixed oxides, e.g. magnesium vanadate (Mg2V2O7).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
vanadium containing ferrite (Li0.5+tZn0.2Ti0.2VtFe2.1−2tO4) starting material or secondary phase | C04B 2235/3239, C04B 2235/3274 (ferrite), C04B 2235/3203 (Li), C04B 2235/3284 (Zn), C04B 2235/3232 (Ti) |
vanadium oxide containing silicates, e.g. cavansite (Ca(VO)Si4O10(H2O)4) starting material or secondary phase | C04B 2235/3454 (Ca-silicate), C04B 2235/3208 (Ca), C04B 2235/3239 |
Vanadium containing glass starting material or secondary phase | |
Vanadium diboride (VB2) starting material or secondary phase | |
Vanadium carbide (VC) starting material or secondary phase | |
Vanadium nitride (VN) starting material or secondary phase | |
Vanadium disilicide (VSi2) starting material or secondary phase | |
Vanadium starting material or secondary phase | |
vanadium carbonyl (V(CO)6) starting material or secondary phase | |
Catalysts comprising metals or metal oxides or hydroxides of vanadium | |
The preparation of vanadium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 31/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of bismuth and vanadium | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium | C09K 11/69 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
This place covers:
Chromium oxides, e.g. Cr2O3, chromium containing mixed oxides, e.g. cobalt chromite (CoCr2O4), chromium oxide containing clay, chromium oxide containing silicates, e.g. uvarovite (Ca3Cr2(SiO4)3), chromium salts, e.g. chromium perchlorate (Cr(ClO4)3), chromium metal containing salts, all used as starting material for making ceramics.
Chromium metal oxide containing secondary phases of sintered ceramic, e.g. CrO3 or chromium metal oxide containing mixed oxides, e.g. cobalt chromate (CoCrO4)
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone Chromium oxide | |
Magnesia-based refractories containing chromia | C04B 35/047 and subgroups C04B 35/051 |
Alumina-based refractories containing chromia | |
Ceramics based on chromium oxide | |
Ceramics based on chromites | |
Coating or impregnating ceramic substrates with chromium oxide | |
chromium containing ferrite (NiCrxFe2–xO4) starting material or secondary phase | C04B 2235/3241, C04B 2235/3274 (ferrite), C04B 2235/3279 (Ni) |
chromium oxide containing silicates, e.g. uvarovite (Ca3Cr2(SiO4)3) starting material or secondary phase | C04B 2235/3454 (Ca-silicate), C04B 2235/3208 (Ca), C04B 2235/3241 |
Chromium containing glass starting material or secondary phase | |
Chromium boride (CrB or CrB2) starting material or secondary phase | |
Chromium carbide (Cr3C2) starting material or secondary phase | |
Chromium nitride (CrN) starting material or secondary phase | |
Chromium silicide (CrSi2) starting material or secondary phase | |
Chromium starting material or secondary phase | |
chromium perchlorate (Cr(ClO4)3) starting material or secondary phase | |
Materials for prostheses based on chromium oxides | |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 37/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of chromium | C09C 1/34 and subgroups |
This place covers:
Chromites and chromates, e.g. aluminum chromate Al2(CrO4)3. All starting powders or secondary phases of sintered ceramics containing mixed oxides of chromium with alkali metals, alkaline earth metals and rare earth metals, not containing other transition or post-transition metal oxides, or mixed oxides of chromium with other transition or post-transition metal oxides, in which the amount of chromium is larger than of any other transition or post-transition metal oxide, e.g. a mixture with titanium oxide, containing more Cr, e.g. Cr0.6Ti0.4O2.
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as active ingredients for mortars, concrete or artificial stone, e.g. accelerators | |
Ceramics based on chromites | |
Chromite containing catalysts | B01J 23/26, B01J 23/86 and subgroups |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides chromium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of chromium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being chromates or bichromates | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: zinc chromate | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: lead chromate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten | C09K 11/68 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
In this place, the following terms or expressions are used with the meaning indicated:
chromate | Chromate salts contain the chromate anion, CrO42−, with Cr(VI) |
chromite | Chromite salts contain the chromite anion, Cr2O42−, with Cr(III) |
This place covers:
Zirconium or hafnium oxides, e.g. ZrO2 or HfO2, zirconium or hafnium containing mixed oxides, e.g. bismuth zirconate (2Bi2O3·3ZrO2), zirconium or hafnium oxide containing clay, zirconium or hafnium oxide containing silicates, e.g. hafnium silicate (HfSiO4), zirconium or hafnium salts, e.g. zirconium iodide (ZrI4), zirconium or hafnium containing salts, all used as starting material for making ceramics.
Zirconia or hafnia containing secondary phases of a sintered ceramic, e.g. yttrium stabilised zirconia (YSZ) or zirconium or hafnium oxide containing mixed oxides, e.g. lithium zirconate (Li2ZrO3).
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: Zirconium oxide | |
Alumina based refractories containing zirconia | |
Alumina refractories containing zirconia, made by melt-casting | |
fine alumina ceramics containing zirconia | |
Ceramics based on zirconia or zirconates, hafnia or hafnates | C04B 35/48 and subgroups |
Making fibres based on zirconium oxide | |
Coating or impregnating ceramic substrates with zirconium oxides or zirconates, hafnium oxides or hafnates | C04B 41/5042 and subgroups |
Zirconium of hafnium containing glass starting material or secondary phase | |
Zirconium of hafnium diboride (ZrB2 or HfB2) starting material or secondary phase | |
Zirconium of hafnium carbide (ZrC or HfC) starting material or secondary phase | |
Zirconium of hafnium nitride (ZrN or HfN) starting material or secondary phase | |
Zirconium of hafnium disilicide (ZrSi2 or HfSi2) starting material or secondary phase | |
Zirconium of hafnium starting material or secondary phase | |
zirconium of hafnium phosphide (ZrP2 or HfP2) starting material or secondary phase | |
Zirconia fibers as starting material for making ceramics | |
Zirconia, hafnia, zirconate or hafnate substrate joined with another substrate or being part of a ceramic laminate | |
Catalysts comprising Zirconium or hafnium; Oxides or hydroxides thereof | |
The preparation of zirconium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 25/00 and subgroups |
If the class C04B 35/119 is given, it's not necessary anymore to give the symbol C04B 2235/3244.
This place covers:
Zirconia or hafnia, which without additives have a monoclinic lattice, are stabilised in a tetragonal or cubic phase through the dissolution in the lattice of a stabilising cation, either an alkaline earth metal oxide, e.g. MgO, or a rare earth oxide (yttria, ceria).
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth metal oxides as starting material or secondary phase | C04B 2235/3205 and subgroups |
Rare earth metal oxides as starting material or secondary phase | C04B 2235/3224 and subgroups |
Ceramic material having a monoclinic lattice | |
Ceramic material having a cubic lattice | |
Ceramic material having a tetragonal lattice |
In patent documents, the following abbreviations are often used:
YSZ | Yttria-stabilised zirconia |
3Y-TZP | Zirconia partially stabilised in the tetragonal phase by 3 mol% yttria |
This place covers:
Zirconates or hafnates, e.g. bismuth zirconate (2Bi2O3·3ZrO2), used as starting material for making ceramics.
Zirconate or hafnate containing secondary phases of a sintered ceramic, e.g. magnesium zirconate or hafnate (MgHfO3), zirconium silicate (zircon ZrSiO4).
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: zircon | |
Alumina based refractories containing zircon | |
Alumina based refractories containing zircon, made by melt-casting | |
Ceramics based on silicates | C04B 35/16 and subgroups |
Ceramics based on zircon | |
Silicate starting material for making ceramics or present as a secondary phase in a sintered ceramic | C04B 2235/3427 and subgroups |
The preparation of zirconium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates: the compounds containing, besides zirconium, two or more other elements, with the exception of oxygen or hydrogen |
Zircon is in principle the only silicate that is not classified as a silicate, but is classified according to the other metal cation(s) present in the silicate.
This place covers:
Mixed zirconate-titanates (or hafnates-titanates) used as starting material for making a ceramic or present as secondary phase in a sintered ceramic, e.g. lead zirconate titanate (PZT).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on zirconates-titanates | C04B 35/49 and subgroups |
Titanate starting material or secondary phase not containing zirconium | C04B 2235/3234 and subgroups |
This place covers:
Niobium or tantalum oxides, e.g. Nb2O5 or Ta2O5, niobium or tantalum containing mixed oxides, e.g. lithium niobate (LiNbO3), niobium or tantalum oxide containing clay, niobium or tantalum oxide containing silicates, e.g. murmanite (Na2(Ti,Nb)2Si2O9-n(H2O)), niobium or tantalum salts, e.g. tantalum selenide (TaSe2), niobium or tantalum containing salts, all used as starting material for making ceramics.
Niobium oxide or tantalum oxide containing secondary phases of a sintered ceramic, e.g. Nb2O5 or Ta2O5 or niobium or tantalum oxide containing mixed oxides, e.g. strontium bismuth niobate (SrBi2Nb2O9).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Coating or impregnating ceramic substrates with niobium oxides or niobates | |
murmanite (Na2(Ti,Nb)2Si2O9-n(H2O)) starting material or secondary phase | C04B 2235/3427, C04B 2235/3201 (Na), C04B 2235/3232 (Ti), C04B 2235/3251 |
Niobium or tantalum containing glass starting material or secondary phase | |
Niobium or tantalum diboride (NbB2 or TaB2) starting material or secondary phase | |
Niobium or tantalum carbide (NbC or TaC) starting material or secondary phase | |
Niobium or tantalum nitride (NbN or TaN) starting material or secondary phase | |
Niobium or tantalum disilicide (NbSi2 or TaSi2) starting material or secondary phase | |
Niobium or tantalum starting material or secondary phase | |
niobium or tantalum telluride (TaTe2) starting material or secondary phase | |
The preparation of niobium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 33/00 and subgroups |
The preparation of tantalum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 35/00 and subgroups |
This place covers:
Niobium or tantalum oxide with the formula NbOx or TaOx, where x < 2, e.g. Ta2O3 or NbO, used as starting material for making a ceramic or present as a secondary in a sintered ceramic.
This place does not cover:
A sintered ceramic having as the main phase a sub-stoichiometric niobium or tantalum oxide (not having used sub-stoichiometric niobium or tantalum oxide as starting material). | |
A sintered ceramic having as the main phase a sub-stoichiometric niobate or tantalate phase, e.g. KNbiO2.9 | C04B 35/495 and subgroups, C04B 2235/3251 and C04B 2235/79 |
Attention is drawn to the following places, which may be of interest for search:
A sintered ceramic having as the main phase a stoichiometric niobium or tantalum oxide, or niobate or tantalate |
This place covers:
Niobates or tantalates, e.g. silver niobate (AgNbO3), used as starting material for making ceramics.
Niobate or tantalate containing secondary phases of a sintered ceramic, e.g. potassium niobate or tantalate (KTaO3).
Attention is drawn to the following places, which may be of interest for search:
The preparation of niobium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides niobium, two or more other elements, with the exception of oxygen or hydrogen | |
The preparation of tantalum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides tantalum, two or more other elements, with the exception of oxygen or hydrogen |
This place covers:
Molybdenum oxides, e.g. Mo2O3, molybdenum containing mixed oxides, e.g. bismuth molybdate (Bi2MoO6 or Bi2(MoO4)3), molybdenum oxide containing clay, molybdenum oxide containing silicates, molybdenum salts, e.g. molybdenum oxy trichloride (MoOCl3), molybdenum metal containing salts, all used as starting material for making ceramics.
Molybdenum metal oxide containing secondary phases of sintered ceramic, e.g. MoO2 or molybdenum metal oxide containing mixed oxides, e.g. cadmium molybdate (CdMoO4).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
molybdenum containing ferrite Fe2-xZnxMoO4 (0.0<=x<=1.0) starting material or secondary phase | C04B 2235/3251, C04B 2235/3274 (ferrite), C04B 2235/3284 (Zn) |
Molybdenum containing glass starting material or secondary phase | |
Molybdenum boride (Mo2B or Mo2B5) starting material or secondary phase | |
Molybdenum carbide (MoC or Mo2C) starting material or secondary phase | |
Molybdenum nitride (MoN) starting material or secondary phase | |
Molybdenum disilicide (MoSi2) starting material or secondary phase | |
Molybdenum starting material or secondary phase | |
molybdophosphoric acid (H3P(Mo3O10)4 starting material or secondary phase | |
Catalysts comprising metals or metal oxides or hydroxides of molybdenum | |
The preparation of molybdenum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 39/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of molybdenum | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten | C09K 11/68 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
This place covers:
Tungsten oxides, e.g. WO2, tungsten containing mixed oxides, e.g. barium strontium tungstate (Ba2SrWO6), tungsten oxide containing clay, tungsten oxide containing silicates, tungsten salts, e.g. tungsten bromide (WBr5), tungsten metal containing salts, all used as starting material for making ceramics.
Tungsten metal oxide containing secondary phases of sintered ceramic, e.g. WO3 or tungsten metal oxide containing mixed oxides, e.g. scheelite (CaWO4) or huebnerite (MnWO4)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates | C04B 35/495 and subgroups |
Tungsten containing glass starting material or secondary phase | |
Tungsten boride (W2B, WB or W2B5) starting material or secondary phase | |
Tungsten carbide (WC) starting material or secondary phase | |
Tungsten nitride (W2N or WN2) starting material or secondary phase | |
Tungsten silicide (WSi2) starting material or secondary phase | |
Tungsten starting material or secondary phase | |
tungsten ethanolate (W(C2H5O)5) starting material or secondary phase | |
Catalysts comprising metals or metal oxides or hydroxides of tungsten | |
The preparation of tungsten compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 41/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten | C09K 11/68 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth vanadates; Chromates; Molybdates; Tungstates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth vanadates; Chromates; Molybdates; Tungstates | C09K 11/7708, C09K 11/7722, C09K 11/7736, C09K 11/7751, C09K 11/7765, C09K 11/7776, C09K 11/7794 |
This place covers:
Tungstates, e.g. copper tungstate (CuWO4), or iron tungstate (FeWO4) used as starting material for making ceramics.
Tungstate containing secondary phases of a sintered ceramic, e.g. zirconium tungstate (Zr(WO4)2).
Attention is drawn to the following places, which may be of interest for search:
The preparation of tungsten compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides tungsten, two or more other elements, with the exception of oxygen or hydrogen |
This place covers:
Manganese or rhenium oxides, e.g. MnO or Re2O7, manganese or rhenium containing mixed oxides, e.g. lithium manganite (Li2MnO3), manganese or rhenium oxide containing clay, manganese or rhenium oxide containing silicates, e.g. tephroite (Mn2SiO4), manganese or rhenium salts, e.g. rhenium dioxydifluoride(ReO2F2), manganese or rhenium containing salts, all used as starting material for making ceramics.
Manganese oxide or rhenium oxide containing secondary phases of a sintered ceramic, e.g. MnO3 or ReO3 or manganese or rhenium oxide containing mixed oxides, e.g. barium manganate (BaMnO4).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on manganites | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing manganese or zinc, e.g. Mn-Zn ferrites | |
Coating or impregnating ceramic substrates with manganates | |
manganese aluminate (Al2MnO4) starting material or secondary phase | |
manganese titanate (MnTiO3) starting material or secondary phase | |
manganese vanadate (MnV2O6) starting material or secondary phase | |
manganese chromate (MnCrO4) starting material or secondary phase | |
manganese zirconate (MnZrO3) starting material or secondary phase | |
manganese niobate (MnNb2O6) starting material or secondary phase | |
manganese molybdate (MnMnO4) starting material or secondary phase | |
manganese tungstate, hubnerite, (MnWO4) starting material or secondary phase | |
manganese ferrite (MnFe2O4) starting material or secondary phase | |
manganese cobaltite (MnCo2O4) starting material or secondary phase | |
manganese stannate (MnSnO3) starting material or secondary phase | |
manganese tetraborate (MnB4O7) starting material or secondary phase | |
manganese silicate (tephroite, Mn2SiO4 or MnSiO3) starting material or secondary phase | |
Manganese oxide containing glass starting material or secondary phase | |
Manganese boride (Mn2B, MnB or MnB2) starting material or secondary phase | |
Manganese carbide (Mn3C) starting material or secondary phase | |
Manganese silicide (MnSi2) starting material or secondary phase | |
Manganese starting material or secondary phase | |
manganese sulphate MnSO4 starting material or secondary phase | |
The preparation of manganese compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
The preparation of rhenium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing manganese or rhenium | C09K 11/57 and subgroups |
Electrolytic production of manganese oxides |
This place covers:
Mn3O4 as starting material for making ceramics or as secondary phase in sintered ceramics.
This place covers:
Mn2O3 as starting material for making ceramics or as secondary phase in sintered ceramics.
This place covers:
MnO2 as starting material for making ceramics or as secondary phase in sintered ceramics.
This place covers:
Manganites, e.g. lithium manganite (Li2MnO3), or manganates, e.g. barium manganate (BaMnO4) used as starting material for making ceramics or present as secondary phase in a sintered ceramic.
Attention is drawn to the following places, which may be of interest for search:
The preparation of manganese compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being manganates or permanganates | |
The preparation of manganese compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, compounds containing, besides manganese, two or more other elements, with the exception of oxygen or hydrogen |
In this place, the following terms or expressions are used with the meaning indicated:
manganate | Any negatively charged molecular entity with manganese as the central atom. However, the name is usually used to refer to the tetraoxidomanganate(2−) anion, MnO42-, also known as manganate(VI) because it contains manganese in the +6 oxidation state. Manganates are the only known manganese(VI) compounds |
manganite | A MnO33- ion containing Mn(III) |
This place covers:
Iron group oxides, e.g. FeO, Co3O4, iron group containing mixed oxides, e.g. lithium cobaltite (LiCoO2), iron group oxide containing clay, iron group oxide containing silicates, e.g. garnierite ((Ni,Mg)3Si2O5(OH)4), iron group salts, e.g. ferrous chloride (FeCl2), iron group containing salts, all used as starting material for making ceramics.
Iron group oxide containing secondary phases of a sintered ceramic, e.g. Fe2O3 or iron group oxide containing mixed oxides, e.g. barium hexaferrite (BaFe12O19.)
Attention is drawn to the following places, which may be of interest for search:
Iron group metal starting material or secondary phase | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt | C09K 11/60 and subgroups |
The symbol C04B 2235/327 is little used, since normally symbols of one or more of the subgroups can be attributed. Only if it is mentioned that iron group oxides are used without specifying which ones, this symbol is used.
This place covers:
Iron oxides, e.g. Fe3O4 (magnetite) or FeO (wüstite), iron oxide containing mixed oxides, e.g. cobalt ferrite (CoFe2O4), iron oxide containing clay, iron oxide containing silicates, e.g. fayalite Fe2SiO4), iron salts, e.g. iron sulphate (FeSO4), iron metal containing salts, all used as starting material for making ceramics.
Iron metal oxide containing secondary phases of sintered ceramic, e.g. Fe2O3 (hematite) or iron metal oxide containing mixed oxides, e.g. Ni-Zn ferrite (MnaZn(1-a)Fe2O4).
This place does not cover:
The use of unburned red mud for making clay objects |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: iron oxide | |
Eliminating lime or iron from clay mixtures | |
Ceramics based on iron oxide | |
iron aluminate (Fe(AlO2)2 starting material or secondary phase | |
iron titanate (FeTiO3) starting material or secondary phase | |
iron chromate (FeCrO3) starting material or secondary phase | |
iron chromite (called chromite, FeCr2O4) starting material or secondary phase | |
iron zirconate (Fe2ZrO5) starting material or secondary phase | |
lead iron niobate (PbFe1/2Nb1/2O3) starting material or secondary phase | |
iron molybdate (Fe2(MoO4))3) starting material or secondary phase | |
iron tungstate, ferberite, (FeWO4) starting material or secondary phase | |
iron manganite (FeMn2O4) starting material or secondary phase | |
iron cobaltite (CoxFe3-xO4) starting material or secondary phase | |
iron borate (Fe3BO6) starting material or secondary phase | |
iron silicate (fayalite Fe2SiO4) starting material or secondary phase | |
Iron oxide containing glass starting material or secondary phase | |
Iron boride (Fe2B, FeB) starting material or secondary phase | |
Iron carbide (Fe3C) starting material or secondary phase | |
Iron silicide (FeSi, FeSi2) starting material or secondary phase | |
Iron starting material or secondary phase | |
iron nitrate (Fe(NO3)3) starting material or secondary phase | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 49/00 and subgroups |
This place covers:
All oxidic ferrites, combinations between Fe2O3 and other oxides, such as FeO, ZnO, MnO, BaO, NiO, CoO, Co3O4, CuO, MgO, SrO, CaO
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: ferrites | |
Ceramics based on ferrites | C04B 35/26 and subgroups |
Coating or impregnating ceramic substrates with ferrite | |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds being mixed oxides or hydroxides, e.g. ferrites | C01G 49/0018 and subgroups |
The preparation of iron compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides iron, two or more other elements, with the exception of oxygen or hydrogen |
This place covers:
Cobalt oxides, e.g. Co2O3 or CoO, cobalt oxide containing mixed oxides, e.g. cobalt ferrite (CoFe2O4), cobalt oxide containing clay, cobalt oxide containing silicates, Co2SiO4), cobalt salts, e.g. cobalt oxalate (CoC2O4), cobalt metal containing salts, all used as starting material for making ceramics.
Cobalt metal oxide containing secondary phases of sintered ceramic, e.g. CoO or cobalt metal oxide containing mixed oxides, e.g. cobalt perrhenate (Co(ReO4)4).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on cobalt oxide or cobaltates | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing nickel, copper or cobalt | |
cobalt orthoarsenate (Co3(AsO4)2) starting material or secondary phase | |
cobalt selenate (CoSeO4) starting material or secondary phase | |
cobalt aluminate, thenard's blue, (CoAl2O4) starting material or secondary phase | |
cobalt titanate (Co2TiO4) starting material or secondary phase | |
cobalt vanadate (Co2V2O7) starting material or secondary phase | |
cobalt chromate (CoCrO4) starting material or secondary phase | |
cobalt chromite (CoCr2O4) starting material or secondary phase | |
cobalt zirconate (CoZrO3) starting material or secondary phase | |
cobalt niobate (CoNb2O6) starting material or secondary phase | |
cobalt molybdate (CoMoO4) starting material or secondary phase | |
cobalt tungstate, (CoWO4) starting material or secondary phase | |
cobalt manganite (CoMn2O4) starting material or secondary phase | |
cobalt perrhenate (Co(ReO4)4) starting material or secondary phase | |
cobalt ferrite (CoFe2O4) starting material or secondary phase | |
cobalt orthostannate (Co2SnO4) starting material or secondary phase | |
cobalt silicate (Co2SiO4) starting material or secondary phase | |
Cobalt oxide containing glass starting material or secondary phase | |
Cobalt boride (CoB) starting material or secondary phase | |
Cobalt carbide (CoC) starting material or secondary phase | |
Cobalt silicide (Co2Si, CoSi, CoSi2) starting material or secondary phase | |
Cobalt starting material or secondary phase | |
cobalt selenide (CoSe) starting material or secondary phase | |
cobalt phosphate (Co3(PO4)2) starting material or secondary phase | |
The preparation of cobalt compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 51/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt | C09K 11/60 and subgroups |
This place covers:
Co3O4 as starting material for making ceramics or as secondary phase in sintered ceramics.
This place covers:
Nickel oxides, e.g. NiO, nickel oxide containing mixed oxides, e.g. nickel ferrite (NiFe2O4), nickel oxide containing clay, nickel oxide containing silicates, Ni2SiO4), nickel salts, e.g. nickel fluosilicate (NiSiF6), nickel metal containing salts, all used as starting material for making ceramics.
Nickel metal oxide containing secondary phases of sintered ceramic, e.g. NiO or nickel metal oxide containing mixed oxides, e.g. nickel orthoarsenate (Ni3(AsO4)2)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on nickel or nickelates | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing nickel, copper or cobalt | |
nickel orthoarsenate (Ni3(AsO4)2) starting material or secondary phase | |
nickel selenate (NiSeO4) starting material or secondary phase | |
nickel aluminate, (NiAl2O4) starting material or secondary phase | |
nickel titanate (NiTiO3) starting material or secondary phase | |
nickel vanadate (Ni2V2O7) starting material or secondary phase | |
nickel chromate (NiCrO4) starting material or secondary phase | |
nickel chromite (NiCr2O4) starting material or secondary phase | |
nickel zirconate (NiZrO3) starting material or secondary phase | |
nickel niobate (NiNb2O6) starting material or secondary phase | |
nickel molybdate (NiMoO4) starting material or secondary phase | |
nickel tungstate, (NiWO4) starting material or secondary phase | |
nickel manganite (NiMn2O4) starting material or secondary phase | |
nickel perrhenate (Ni(ReO4)2) starting material or secondary phase | |
nickel ferrite (NiFe2O4) starting material or secondary phase | |
sodium nickel cobaltate (Na0.9Co0.99Ni0.01O2) starting material or secondary phase | |
nickel cuprate (NiCuO2) starting material or secondary phase | |
nickel germanate (Ni2GeO4) starting material or secondary phase | |
nickel stannate (NiSnO3) starting material or secondary phase | |
nickel antimonate (NiSb2O6) starting material or secondary phase | |
nickel silicate (Ni2SiO4) starting material or secondary phase | |
Nickel oxide containing glass starting material or secondary phase | |
Nickel boride (NiB) starting material or secondary phase | |
Nickel carbide (Ni3C) starting material or secondary phase | |
Nickel silicide (Ni2Si) starting material or secondary phase | |
Nickel starting material or secondary phase | |
nickel selenide (NiSe) starting material or secondary phase | |
nickel phosphate (Ni3(PO4)2) starting material or secondary phase | |
The preparation of nickel compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 53/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt | C09K 11/60 and subgroups |
This place covers:
Copper oxides, e.g. Cu2O, copper oxide containing mixed oxides, e.g. copper dichromate (CuCr3O7), copper oxide containing clay, copper oxide containing silicates, CuSiO3), copper salts, e.g. copper formate (CuCHO2), copper metal containing salts, all used as starting material for making ceramics.
Copper metal oxide containing secondary phases of sintered ceramic, e.g. CuO or copper metal oxide containing mixed oxides, e.g. copper chromite (Cu2(Cr2O4)).
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: copper oxides | |
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing nickel, copper or cobalt | |
Ceramics based on copper oxide | |
Making fibres based on copper oxide | |
Coating or impregnating ceramic substrates with copper oxide ceramic material | C04B 41/5074 and subgroups |
copper orthoarsenate (Cu3(AsO4)2) starting material or secondary phase | |
copper selenate (CuSeO4) starting material or secondary phase | |
copper aluminate, (CuAl2O4) starting material or secondary phase | |
copper titanate (CuTiO3) starting material or secondary phase | |
copper vanadate (CuV2O6) starting material or secondary phase | |
copper chromate (CuCrO4) starting material or secondary phase | |
copper chromite (CuCr2O4) starting material or secondary phase | |
copper zirconate (CuZrO3) starting material or secondary phase | |
copper niobate (CuNb2O6) starting material or secondary phase | |
copper molybdate (CuMoO4) starting material or secondary phase | |
copper tungstate, (CuWO4) starting material or secondary phase | |
copper manganite (CuxMn3-xO4) starting material or secondary phase | |
Lanthanum Copper Manganate (La2CuMnO6) starting material or secondary phase | |
copper perrhenate (Cu(ReO4)2) starting material or secondary phase | |
copper ferrite (CuFe2O4) starting material or secondary phase | |
Copper cobaltite Cux(Co3 − xO4) starting material or secondary phase | |
copper germanate (CuGeO3) starting material or secondary phase | |
copper stannate (CuSnO3) starting material or secondary phase | |
copper silicate (CuSiO3) starting material or secondary phase | |
Copper oxide containing glass starting material or secondary phase | |
Copper boride (Cu3B2) starting material or secondary phase | |
Copper carbide (Cu2C) starting material or secondary phase | |
Copper nitride (Cu3N) starting material or secondary phase | |
Copper silicide (Cu4Si) starting material or secondary phase | |
Copper starting material or secondary phase | |
copper selenide (CuSe) starting material or secondary phase | |
copper phosphate (Cu3(PO4)2) starting material or secondary phase | |
The preparation of copper compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 3/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing copper, silver or gold | C09K 11/58 and subgroups |
This place covers:
Cuprates, e.g. YBa2Cu3O7 (YBCO) cuprate used as starting material for making ceramics.
Cuprate containing secondary phases of a sintered ceramic, e.g. nickel cuprate (NiCuO2).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on rare earth oxide containing cuprates. | C04B 35/4504 and subgroups |
Ceramics based on thallium oxide containing cuprates. | C04B 35/4512 and subgroups |
Ceramics based on bismuth oxide containing cuprates | C04B 35/4521 and subgroups |
The preparation of copper compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates, the compounds containing, besides copper, two or more other elements, with the exception of oxygen or hydrogen |
This place covers:
Zinc oxide, cadmium oxide or mercury oxides, e.g. CdO, HgO, zinc oxide, cadmium oxide or mercury oxide containing mixed oxides, e.g. mercury tungstate (Hg2WO4), zinc oxide, cadmium oxide or mercury oxide containing clay, zinc oxide, cadmium oxide or mercury oxide containing silicates, e.g. CdSiO3, zinc, cadmium or mercury salts, e.g. zinc tetrabromide (ZnBr4), zinc, cadmium or mercury containing salts, all used as starting material for making ceramics.
Zinc, cadmium or mercury oxide containing secondary phases of sintered ceramic, e.g. ZnO or zinc, cadmium or mercury oxide containing mixed oxides, e.g. zinc tellurate (Zn3TeO6).
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising manganese, zinc and one or more ferrites of the group comprising nickel, copper or cobalt | |
Other ferrites containing manganese or zinc, e.g. Mn-Zn ferrites | |
Ceramics based on zinc oxides | |
Cadmium hydrogen arsenate (CdH(AsO4)) starting material or secondary phase | |
cadmium selenate (CdSeO4), zinc selenate (ZnSeO4) starting material or secondary phase | |
Mercury tellurate (Hg3TeO6), zinc tellurate (Zn3TeO6) starting material or secondary phase | |
Zinc aluminate, gahnite (ZnAl2O4) starting material or secondary phase | |
Zinc titanate (ZnTiO3) starting material or secondary phase | |
Zinc vanadate (ZnV2O6) starting material or secondary phase | |
Mercury chromate (HgCrO4), zinc chromate (ZnCrO4), starting material or secondary phase | |
Cadmium chromite (CdCr2O4) starting material or secondary phase | |
zinc zirconate (ZnZrO3) starting material or secondary phase | |
zinc niobate (ZnNb2O6) starting material or secondary phase | |
Cadmium molybdate (CdMoO4) starting material or secondary phase | |
cadmium tungstate, (CdWO4), mercury tungstate, (HgWO4), starting material or secondary phase | |
Cadmium permanganate (Cd(MnO4)2), zinc permanganate (Zn(MnO4)2) starting material or secondary phase | |
zinc ferrite (ZnFe2O4) starting material or secondary phase | |
Zinc cobaltite (ZnCo2O4) starting material or secondary phase | |
Zinc gallate (ZnGa2O4) starting material or secondary phase | |
Zinc stannate (Zn2SnO4) starting material or secondary phase | |
zinc antimonate (ZnSb2O6) starting material or secondary phase | |
zinc bismuthate (Zn(BiO3)2) starting material or secondary phase | |
Cadmium borate (Cd(BO)3)2, zinc borate (ZnO)3(B2O3)2 starting material or secondary phase | |
cadmium metasilicate (CdSiO3), zinc metasilicate (ZnSiO3), zinc orthosilicate, willemite (Zn2SiO4), starting material or secondary phase | |
Zinc, cadmium or mercury oxide containing glass starting material or secondary phase | |
Zinc carbide (ZnC) starting material or secondary phase | |
Mercury nitride (Hg3N2), zinc nitride (Zn3N2) starting material or secondary phase | |
Zinc starting material or secondary phase | |
Zinc selenide (ZnSe) starting material or secondary phase | |
Cadmium orthophosphate (Cd3(PO4)2), zinc orthophosphate (Zn3(PO4)2) starting material or secondary phase | |
Cadmium acetate (Cd(C2H3O2)2) starting material or secondary phase | |
The preparation of zinc compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 9/00 and subgroups |
The preparation of cadmium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
The preparation of mercury compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of cadmium | C09C 1/10 and subgroup |
This place covers:
Gallium oxide, indium oxide or thallium oxides, e.g. Ga2O3, gallium oxide, indium oxide or thallium oxide containing mixed oxides, e.g. gallium selenate (Ga2(SeO4)3), gallium oxide, indium oxide or thallium oxide containing clay, gallium oxide, indium oxide or thallium oxide containing silicates, e.g. lanthanum gallium silicate, La3Ga5SiO14, gallium, indium or thallium salts, e.g. gallium nitrate (Ga(NO3)3), gallium, indium or thallium containing salts, all used as starting material for making ceramics.
Gallium, indium or thallium oxide containing secondary phases of sintered ceramic, e.g. InO, Tl2O2 or gallium, indium or thallium oxide containing mixed oxides, e.g. thallium molybdate (Tl2MoO4).
Attention is drawn to the following places, which may be of interest for search:
Gallium, indium or thallium based ceramics | |
Ceramics based on thallium oxide containing cuprates. | C04B 35/4512 and subgroups |
Gallium selenate (Ga2(SeO4)3), indium selenate (In2(SeO4)3), thallium selenate (Tl2SeO4) starting material or secondary phase | |
Indium titanate (In2TiO5) starting material or secondary phase | |
Thallium metavanadate (TlVO3) starting material or secondary phase | |
Thallium chromate (Tl2CrO4), starting material or secondary phase | |
Lead indium-niobate Pb(In1/2Nb1/2)O3 starting material or secondary phase | |
Thallium molybdate (Tl2MoO4) starting material or secondary phase | |
Indium Tungstate, In2(WO4)3 starting material or secondary phase | |
nickel-zinc-indium ferrite (NZIFO)(Ni0.58Zn0.42InxFe2−xO4)) starting material or secondary phase | C04B 2235/3286, C04B 2235/3274, C04B 2235/3279 (Ni), C04B 2235/3284 (Zn) |
Gallium ferrite (GaFeO3) starting material or secondary phase | |
1222-Type Thallium-Indium Layered Cuprates (Tl,In)Sr2(Nd,Ce)2Cu2Oz starting material or secondary phase | C04B 2235/3286, C04B 2235/3282, C04B 2235/3213 (Sr), C04B 2235/3224 (Nd), C04B 2235/3229 (Ce) |
GaSr2YCu2Oz cuprate starting material or secondary phase | C04B 2235/3286, C04B 2235/3282, C04B 2235/3213 (Sr), C04B 2235/3225 (Y) |
Gallium stannate (Ga4SnO8) starting material or secondary phase | |
Ba3Ga2Ge4O14 germanate starting material or secondary phase | |
Gallium or indium antimonate (GaSbO4 or InSbO4) starting material or secondary phase | |
Bismuth gallium oxide (Bi2Ga4O9) starting material or secondary phase | |
Gallium borate (GaBO3), indium borate (InBO3) starting material or secondary phase | |
lanthanum gallium silicate, La3Ga5SiO14 starting material or secondary phase | |
Gallium, indium or thallium oxide containing glass starting material or secondary phase | |
Gallium boride (GaB12) starting material or secondary phase | |
Gallium indium nitride (Ga1-xInxN) starting material or secondary phase | |
Gallium silicide (Ga3Si) starting material or secondary phase | |
Gallium, indium or thallium starting material or secondary phase | |
Thallium nitrite (Tl(NO2)2) starting material or secondary phase | |
Thallium sulphite (Tl2SO3) starting material or secondary phase | |
The preparation of gallium, indium or thallium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 15/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing gallium, indium or thallium | C09K 11/62 and subgroups |
This place covers:
Germanium oxides, e.g. GeO, germanium oxide containing mixed oxides, e.g. nickel germanate (Ni2GeO4), germanium oxide containing clay, germanium oxide containing silicates, germanium salts, e.g. germanium sulphide (GeS2), germanium metal containing salts, all used as starting material for making ceramics.
Germanium metal oxide containing secondary phases of sintered ceramic, e.g. GeO2 or germanium metal oxide containing mixed oxides, e.g. copper germanate (CuGeO3)
Attention is drawn to the following places, which may be of interest for search:
Barium germanium aluminate. BaGeAl6O12 starting material or secondary phase | |
Barium germanium titanate (Ba2Ge2TiO8) starting material or secondary phase | |
Ni1.25Fe1.5Ge0.25O4 starting material or secondary phase | |
Germanium cuprate CuGeO3 starting material or secondary phase | |
Barium germanium gallate BaGeGa6O12 starting material or secondary phase | |
Bismuth germanium oxide (Bi4Ge3O12) starting material or secondary phase | |
Germanium oxide containing glass starting material or secondary phase | |
molybdenum germanium boride (Mo1.7Ge0.3B) starting material or secondary phase | |
Germanium carbide (GeC) starting material or secondary phase | |
Germanium nitride (Ge3N4) starting material or secondary phase | |
germanium silicide (Si1–xGex) starting material or secondary phase | |
Germanium starting material or secondary phase | |
germanium iodide (GeI4) starting material or secondary phase | |
germanium selenide (GeSe2) starting material or secondary phase | |
The preparation of germanium compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 17/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead | C09K 11/66 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth germanates | C09K11/74E |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth germinates | C09K 11/7707, C09K 11/7735, C09K 11/775, C09K 11/7775, C09K 11/7793 |
This place covers:
Noble metal oxides, e.g. Au2O3, OsO4, PtO, RuO4, noble metal containing mixed oxides, noble metal oxide containing clay, noble metal oxide containing silicates, noble metal salts, e.g. iridium fluoride (IrF6), rhodium nitrate Rh(NO3)3, noble metal containing salts, all used as starting material for making ceramics.
Noble metal oxide containing secondary phases of sintered ceramic, e.g. IrO2, PdO, RhO2 or noble metal oxide containing mixed oxides.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on noble metal oxides | |
Palladium selenate (PdSeO4) starting material or secondary phase | |
Scandium Iridium Boride (Sc3Ir5B2) starting material or secondary phase | |
Gold carbide (Au2C2) starting material or secondary phase | |
Ruthenium nitride (RuN) starting material or secondary phase | |
Osmium silicide (Os2Si3) starting material or secondary phase | |
Noble metal starting material or secondary phase | |
Ruthenium hydroxide (Ru(OH)2) starting material or secondary phase | |
Gold cyanide (AuCN) starting material or secondary phase | |
Osmium telluride (OsTe2) starting material or secondary phase | |
Platinum pyrophosphate (PtP2O7) starting material or secondary phase | |
The preparation of gold compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 7/00 and subgroups |
The preparation of ruthenium, rhodium, palladium, osmium, iridium, or platinum compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 55/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing copper, silver or gold | C09K 11/58 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing platinum group metals |
In this place, the following terms or expressions are used with the meaning indicated:
noble metals | ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), Osmium (Os), iridium (Ir), Platinum (Pt), gold (Au) |
This place covers:
Silver oxides, e.g. Ag2O2, silver oxide containing mixed oxides, e.g. silver tellurite (Ag2TeO3), silver oxide containing clay, silver oxide containing silicates (Ag2SiO3), silver salts, e.g. silver bromate (AgBrO3), silver metal containing salts, all used as starting material for making ceramics.
Silver metal oxide containing secondary phases of sintered ceramic, e.g. Ag2O or silver metal oxide containing mixed oxides, e.g. silver selenate (Ag2SeO4)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on silver metal oxides | |
silver selenate (Ag2SeO4) starting material or secondary phase | |
silver tellurite (Ag2TeO3) starting material or secondary phase | |
silver aluminate, (AgAlO2) starting material or secondary phase | |
silver vanadate (AgVO3) starting material or secondary phase | |
silver chromate (Ag2CrO4) starting material or secondary phase | |
silver niobate (AgNbO3) starting material or secondary phase | |
silver molybdate (Ag2MoO4) starting material or secondary phase | |
silver tungstate (Ag2WO4) starting material or secondary phase | |
Silver permanganate (AgMnO4) starting material or secondary phase | |
silver perrhenate (AgReO4) starting material or secondary phase | |
silver ferrite (AgFeO2) starting material or secondary phase | |
silver cuprate (AgCuO2) starting material or secondary phase | |
silver germanate (Ag2Ge2O5) starting material or secondary phase | |
silver stannate (Ag2SnO3) starting material or secondary phase | |
silver antimonate (AgSbO3) starting material or secondary phase | |
silver plumbate (Ag5Pb2O6) starting material or secondary phase | |
silver bismuthate (AgBiO3) starting material or secondary phase | |
silver tetraborate (Ag2B4O7) starting material or secondary phase | |
silver silicate (Ag2SiO3) starting material or secondary phase | |
Silver oxide containing glass starting material or secondary phase | |
Silver boride (AgB2) starting material or secondary phase | |
Silver carbide (Ag2C2) starting material or secondary phase | |
Silver nitride (AgN3) starting material or secondary phase | |
Silver starting material or secondary phase | |
silver carbonate (Ag2CO3) starting material or secondary phase | |
Silver chlorite (AgClO2) starting material or secondary phase | |
Silver metaphosphate (AgPO3) starting material or secondary phase | |
The preparation of silver compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 5/00 and subgroups |
This place covers:
Tin oxides, e.g. SnO, tin oxide containing mixed oxides, e.g. manganese stannate (MnSnO3), tin oxide containing clay, tin oxide containing silicates, e.g. SnSi2O6, tin salts, e.g. tin fluoride (SnF4), tin metal containing salts, all used as starting material for making ceramics.
Tin metal oxide containing secondary phases of sintered ceramic, e.g. SnO2 or tin metal oxide containing mixed oxides, e.g. silver stannate (Ag2SnO3)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on tin oxides | |
tin arsenate (Sn2As2O7) starting material or secondary phase | |
tin titanate (Sn2TiO4) starting material or secondary phase | |
tin chromate (Sn(CrO4)2) starting material or secondary phase | |
tin molybdate (SnMo2O8) starting material or secondary phase | |
tin tungstate (SnWO4) starting material or secondary phase | |
Nickel tin ferrite (Ni1+xSnxFe2-2xO4) starting material or secondary phase | |
tin silicate (SnSi2O6) starting material or secondary phase | |
Tin oxide containing glass starting material or secondary phase | |
Tin nitride (SnN) starting material or secondary phase | |
Tin starting material or secondary phase | |
Tin monophosphide (SnP) starting material or secondary phase | |
tin sulphide (SnS) starting material or secondary phase | |
Tin metaphosphate (Sn(PO3)2) starting material or secondary phase | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead | C09K 11/66 and subgroups |
This place covers:
Antimony oxides, e.g. Sb2O3, antimony oxide containing mixed oxides, e.g. nickel antimonate (NiSb2O6), antimony oxide containing clay, antimony oxide containing silicates, antimony salts, e.g. antimony iodosulfide (SbSI), antimony metal containing salts, all used as starting material for making ceramics.
Antimony metal oxide containing secondary phases of sintered ceramic, e.g. Sb2O5 or antimony metal oxide containing mixed oxides, e.g. gallium or indium antimonate (GaSbO4 or InSbO4)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on antimony oxide | |
antimony aluminate, (Sb3Al2O4) starting material or secondary phase | |
antimony vanadate (Sb(VO3)3) starting material or secondary phase | |
Doped PZT Pb(Sb2/3Mn1/3)0.08(Zr0.52Ti0.48)0.92O3 starting material or secondary phase | C04B 2235/3294, C04B 2235/3249 (ZT), C04B 2235/3296 (Pb), C04B 2235/3262 (Mn) |
antimony niobate (SbNbO4) starting material or secondary phase | |
antimony molybdate (KSbMo2O8) starting material or secondary phase | |
antimony tungstate, (CuWO4) starting material or secondary phase | |
antimony germanate (Sb2Ge3O9) starting material or secondary phase | |
Antimony oxide containing glass starting material or secondary phase | |
Antimony carbide (SbC) starting material or secondary phase | |
Antimony nitride (SbN) starting material or secondary phase | |
Antimony starting material or secondary phase | |
antimony selenide (Sb2Se3) starting material or secondary phase | |
antimony oxysulphate (Sb2O2SO4) starting material or secondary phase | |
The preparation of antimony compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 30/00 and subgroups |
Antimony oxide used as filler for polymers | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of antimony | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing antimonates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth | C09K 11/74 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth Antimonates; Arsenates |
This place covers:
Lead oxides, e.g. Pb2O3, lead oxide containing mixed oxides, e.g. silver plumbate (Ag5Pb2O6), lead oxide containing clay, lead oxide containing silicates, e.g. lead orthosilicate, barysilite (Pb2Si2O7), lead salts, e.g. lead carbonate, cerussite (PbCO3), lead containing salts, all used as starting material for making ceramics.
Lead oxide containing secondary phases of sintered ceramic, e.g. PbO2 or lead oxide containing mixed oxides, e.g. lead iron niobate (PbFe1/2Nb1/2O3).
Attention is drawn to the following places, which may be of interest for search:
Compositions containing one or more ferrites of the group comprising manganese, zinc, nickel, copper or cobalt and one or more ferrites of the group comprising rare earth metals, alkali metals, alkaline earth metals or lead | C04B 35/2608 and subgroups |
Compositions containing one or more ferrites of the group comprising rare earth metals and one or more of the group comprising alkali metals, alkaline earth metals or lead | |
Other ferrites containing alkaline earth metals or lead | |
Ceramics based on thallium oxide containing cuprates, also containing lead oxide | |
Ceramics based on bismuth oxide containing cuprates, also containing lead oxide | |
Ceramics based on barium titanate perovskite, containing also lead | |
Ceramics based on barium titanate non-perovskite, containing also lead | |
Ceramics based on lead titanates | |
Ceramics based on zirconates-titanates, containing also lead | C04B 35/491 and subgroups |
Ceramics based on vanadium, niobium, tantalum, molybdenum or tungsten oxides or solid solutions thereof with other oxides, e.g. vanadates, niobates, tantalates, molybdates or tungstates, based on solid solutions with lead | C04B 35/497 and subgroups |
lead manganate (PbMnO3) starting material or secondary phase | |
lead iron niobate (PbFe1/2Nb1/2O3) starting material or secondary phase | |
Doped PZT Pb(Sb2/3Mn1/3)0.08(Zr0.52Ti0.48)0.92O3 starting material or secondary phase | C04B 2235/3294 (Sb), C04B 2235/3249 (ZT), C04B 2235/3296, C04B 2235/3262 (Mn) |
lead orthoarsenate (Pb3(AsO4)2) starting material or secondary phase | |
lead selenate (PbSeO4) starting material or secondary phase | |
lead aluminate, (PbAl2O4) starting material or secondary phase | |
lead metatitanate (PbTiO3) starting material or secondary phase | |
lead vanadate (PbV2O6) starting material or secondary phase | |
lead chromate, crocoite (PbCrO4) starting material or secondary phase | |
lead molybdate, wulfenite (PbMoO4) starting material or secondary phase | |
lead tungstate, (PbWO4) starting material or secondary phase | |
lead ferrite (PbFe2O4) starting material or secondary phase | |
Lead cobaltate (PbCoO3, PbCo2O4) starting material or secondary phase | |
Lead cuprate (Pb2Sr2NdCu3O8) starting material or secondary phase | C04B 2235/3296, C04B 2235/3282, C04B 2235/3213 (Sr), C04B 2235/3224 (Nd) |
lead germanate (Pb5Ge3O11) starting material or secondary phase | |
lead stannate (PbSnO3) starting material or secondary phase | |
lead diantimonate (Pb2Sb2O7) starting material or secondary phase | |
lead metaborate (Pb(BO2)2) starting material or secondary phase | |
lead metasilicate, alamosite (PbSiO3) starting material or secondary phase | |
Lead oxide containing glass starting material or secondary phase | |
Lead nitride (Pb(N3)2) starting material or secondary phase | |
Lead starting material or secondary phase | |
lead selenide, clausthalite (PbSe) starting material or secondary phase | |
lead orthophosphate (Pb2(PO4)2) starting material or secondary phase | |
The preparation of lead compounds in powder form, e.g. oxides, carbonates, halides, nitrates, sulphates | C01G 19/00 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of lead | C09C 1/14 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead | C09K 11/66 and subgroups |
This place covers:
Bismuth oxides, e.g. Bi2O3, bismuth oxide containing mixed oxides, e.g. zinc bismuthate (Zn(BiO3)2), bismuth oxide containing clay, bismuth oxide containing silicates, e.g. bismuth silicate, eulytite (2Bi2O3 3SiO2), bismuth salts, e.g. bismuth hydroxide (Bi(OH)3), bismuth containing salts, all used as starting material for making ceramics.
Bismuth oxide containing secondary phases of sintered ceramic, e.g. Bi2O5 or bismuth oxide containing mixed oxides, e.g. silver bismuthate (AgBiO3).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on bismuth oxide containing cuprates | C04B 35/4521 and subgroups |
Ceramics based on bismuth oxides | |
Ceramics based on bismuth titanates | |
barium strontium bismuth niobate (Ba0.1Sr0.9Bi2Nb2O9) starting material or secondary phase | C04B 2235/3213 (Sr), C04B 2235/3215 (Ba), C04B 2235/3255, C04B 2235/3298 |
bismuth manganate (Bi2.4MnO3) starting material or secondary phase | |
bismuth manganite (BiMnO3) starting material or secondary phase | |
Bismuth gallium oxide (Bi2Ga4O9) starting material or secondary phase | |
Bismuth germanate (Bi4Ge3O12) starting material or secondary phase | |
bismuth orthoarsenate (BiAsO4) starting material or secondary phase | |
bismuth tellurate, montanite (Bi2TeO6) starting material or secondary phase | |
bismuth aluminate, (BiAlO3) starting material or secondary phase | |
bismuth titanate (Bi4Ti3O12) starting material or secondary phase | |
bismuth vanadate, pucherite (Bi2O3 V2O5)) starting material or secondary phase | |
bismuth dichromate ((BiO2)2Cr2O7) starting material or secondary phase | |
bismuth zirconate (Bi2Zr2O7) starting material or secondary phase | |
bismuth molybdate (Bi2(MoO4)3) starting material or secondary phase | |
bismuth tungstate, (Bi2WO6) starting material or secondary phase | |
bismuth ferrite (BiFeO3) starting material or secondary phase | |
Bismuth cobaltate (BiCoO3) starting material or secondary phase | |
Bismuth cuprate (Bi2CuO4) starting material or secondary phase | |
bismuth stannate (Bi2Sn3O9) starting material or secondary phase | |
bismuth metaborate (Pb(BO2)2) starting material or secondary phase | |
bismuth silicate, eulytite (2Bi2O3 3SiO2) starting material or secondary phase | |
Bismuth oxide containing glass starting material or secondary phase | |
Nickel bismuth boride (Ni23-xBixB6) starting material or secondary phase | |
Bismuth nitride (BiN) starting material or secondary phase | |
Bismuth starting material or secondary phase | |
bismuth selenide, juanajuatite (Bi2Se3) starting material or secondary phase | |
bismuth orthophosphate (BiPO4) starting material or secondary phase | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth | C09K 11/74 and sub-classes |
This place covers:
All oxides of non-metals, such as B, Ar, Se, Te or Si, mixed oxides based on these non-metals, e.g. arsenates such as cobalt orthoarsenate (Co3(AsO4)2), selenates such as zinc selenate (ZnSeO4), tellurates such as bismuth tellurate, montanite (Bi2TeO6), or metal salts that convert to these oxides upon heating, used as starting material for making a ceramic or present as secondary phase in a sintered ceramic.
This place does not cover:
The oxides of the non-metal phosphor | |
Oxide compounds of the non-metal sulphur |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on arsenates, selenates or tellurates | |
Ceramics based on phosphates | |
Ceramics based on metal-phosphor compounds without oxygen, the phosphides | |
Ceramics based on sulfides, selenides or tellurides | |
Metal arsenides, e.g. GaAs, as starting material for making ceramics | |
Metal phosphides as starting material for making ceramics | |
Metal sulphides, selenides or tellurides as starting material for making ceramics | |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Oxide interlayer used for joining a ceramic with another substrate | C04B 2237/06 and subgroups |
Oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/34 and subgroups |
This place covers:
Boron oxides, e.g. B2O3, boric acids, e.g. HBO2, boron oxide containing mixed oxides, e.g. boron arsenate (BAsO4), boron oxide containing clay, boron oxide containing silicates, e.g. Danburite, CaB2Si2O8, boron salts, e.g. boron bromide (BBr3), boron containing salts, all used as starting material for making ceramics.
Boron oxide containing secondary phases of a sintered ceramic, e.g. B2O3 or boron oxide containing mixed oxides, e.g. silver tetraborate (Ag2B4O7).
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on boron oxide | |
boron arsenate (BAsO4) starting material or secondary phase | |
boron aluminate • Al4B2O9 starting material or secondary phase | |
boron titanate (BTi3O9) starting material or secondary phase | |
Borotungstic acid, (H5BW12O40) starting material or secondary phase | |
boron ferrite series B2O3 · Fe2O3 · 4MeO(Me = Mg, Ni, Co, Cu) starting material or secondary phase | |
Danburite, CaB2Si2O8, calcium boron silicate starting material or secondary phase | |
Boron oxide containing glass starting material or secondary phase | |
Boron oxide and silica containing glass starting material or secondary phase | |
Boride starting material or secondary phase | C04B 2235/3804 and subgroups |
Boron carbide (B4C) starting material or secondary phase | |
Boron nitride (BN) starting material or secondary phase | |
Boron silicide (B6Si, B2Si) starting material or secondary phase | |
Boron starting material or secondary phase | |
boron hydride (B2H6) starting material or secondary phase | |
boron phosphide (BP) starting material or secondary phase | |
Making compounds containing boron and oxygen | C01B 35/10 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing borates | C09K 11/0877, C09K 11/647, C09K 11/667, C09K 11/678, C09K 11/687, C09K 11/708, C09K 11/7485, C09K 11/765, C09K 11/7712, C09K 11/7726, C09K 11/774, C09K 11/7755, C09K 11/778, C09K 11/7797, C09K 11/888 |
This place covers:
Silicon oxides, e.g. crystalline silica such as quartz, cristobalite, tridymite, amorphous silica such as silica sol, silica fume, fused silica, silicic acids, e.g. H2Si2O5, silicon salts, e.g. silicon bromide (Si2Br6), silicon containing salts, all used as starting material for making ceramics.
Silicon oxide containing secondary phases of a sintered ceramic.
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: granular materials: quartz; sand | |
Silica fume waste material used for making clay wares | |
Lean materials, e.g. grog, quartz | |
Alumina based refractories containing zircon | |
Ceramic silica-based materials | |
Ceramic silicate-based materials | |
Making fibres based on silica | |
Inorganic binders based on silicon compounds | |
Asbestos; Glass; Fused silica | |
Coating or impregnating ceramic substrates with silica | |
Silicates other than clay, e.g. water glass | |
Clays, e.g. bentonites, smectites such as montmorillonite, vermiculites or kaolines, e.g. illite, talc or sepiolite | |
Silicon oxide containing glass starting material or secondary phase | |
Silicon boride (SiBn) starting material or secondary phase | |
Boron silicide = silicon boride | |
Silicon carbide (SiC) starting material or secondary phase | |
Silicon nitride (Si3N4) starting material or secondary phase | |
Silicon starting material or secondary phase | |
Silicon fluoride (SiF4) starting material or secondary phase | |
Silicon sulphide (SiS2) starting material or secondary phase | |
Silica or silicates other than aluminosilicates, e.g. quartz | |
Silica or silicate interlayer used for joining a ceramic with another substrate | |
Silica or silicate substrate joined with another substrate or being part of a ceramic laminate | |
Silica-based membranes | |
Catalysts comprising silica | |
Preparation of silica powders, sols, gels, dispersions and their after-treatments | |
Processes specially adapted for the production of quartz or fused silica articles | |
Glass compositions with more than 90% silica by weight, e.g. quartz | |
Catalysts used in the preparation of hydrocarbons comprising silica | |
Silica used as filler for polymers | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: compounds of silicon | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing silicon |
This place covers:
All silicates that are not clay (see C04B 2235/349 for the definition of clays). A silicate is a compound containing a silicon bearing anion. The great majority of silicates are oxides, but hexafluorosilicate ([SiF6]2−) and other anions are also included. Silicate compounds, including the minerals, consist of silicate anions whose charge is balanced by various cations. Myriad silicate anions can exist, and each can form compounds with many different cations. Hence this class of compounds is very large. Both minerals and synthetic materials fit in this class. Silicates are mainly a mixed oxide phase of SiO2 with at least one other metal oxide, e.g. Willemite - Zn2SiO4, Fayalite - Fe2SiO4, Ferrosilite - FeSiO3, Aegirine (Acmite) - NaFe3+Si2O6, Rhodonite - MnSiO3.
This place does not cover:
Zirconium or hafnium containing silicates, e.g. zircon (ZrSiO4) | |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint, e.g. silicic acid H2Si2O5 | |
Clay silicate starting materials or secondary phase of a sintered ceramic, e.g. illite | |
Silica or silicate fibers used in ceramic compositions |
Attention is drawn to the following places, which may be of interest for search:
Use of silica-rich materials or silicates as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone | C04B 14/04 and subgroups |
Clay wares | C04B 33/00 and subgroups |
Ceramic silica based materials | |
Ceramic silicate based materials | C04B 35/16 and subgroups |
Ceramics based on zircon | |
Inorganic binders based on silicon compounds | |
Silica or silicate interlayer used for joining a ceramic with another substrate | |
Silica or silicate substrate joined with another substrate or being part of a ceramic laminate | |
Preparation of silicate powders, sols, gels, dispersions and their after-treatments | C01B 33/20 and subgroups C01B 37/005 |
polysilicate macromolecular compounds | |
Coating compositions, e.g. paints, varnishes or lacquers, based on alkali metal silicates | C09D 1/02 and subgroups |
Adhesives based on water-soluble alkali silicate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing iron, nickel and cobalt as silicate | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic halogen silicate compounds | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing germanium, tin or lead silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing refractory silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing chromium, molybdenum or tungsten silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing vanadium silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth silicates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth silicates | C09K 11/7758,C09K 11/77062, C09K 11/77212, C09K 11/77342, C09K 11/77492, C09K 11/77742, C09K 11/77922C09K 11/7764 |
The presence of the metal cations that combine with the silicate anion is indicate with symbols from the C04B 2235/32 scheme, e.g. C04B 2235/3284 to indicate the Zn of willemite, C04B 2235/3272 to indicate the Fe of fayalite.
In this place, the following terms or expressions are used with the meaning indicated:
Silicate mineral | Mineralogically, silicate minerals are divided according to structure of their silicate anion into the following groups: Nesosilicates (lone tetrahedron) - [SiO4]4−, e.g. olivine . Sorosilicates (double tetrahedra) - [Si2O7]6−, e.g. epidote, melilite group. Cyclosilicates (rings) - [SinO3n]2n−, e.g. tourmaline group. Inosilicates (single chain) - [SinO3n]2n−, e.g. pyroxene group. Inosilicates(double chain) - [Si4nO11n]6n−, e.g. amphibole group. Phyllosilicates (sheets) - [Si2nO5n]2n−, e.g. micas and clays . Tectosilicates (3D framework) - [AlxSiyO2(x+y)]x−, e.g. quartz, feldspars, zeolites . Note that tectosilicates can only have additional cations if some of the silicon is replaced by a lower-charge cation such as aluminium . Al for Si substitution is common. |
This place covers:
Alkaline earth metal silicates used as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Benitoite - BaTi(Si3O9), Phenakite - Be2SiO4
This place does not cover:
Alkaline earth metal silicates containing also aluminium oxide |
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. BeO | C04B 2235/3205 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
alkaline earth metal oxides | BeO, MgO, CaO, SrO, BaO, RaO |
This place covers:
Magnesium silicates used as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Forsterite (Mg2SiO4), Humite group (Mg,Fe)7(SiO4)3(F,OH)2, Enstatite (MgSiO3), Diopside (CaMgSi2O6), the Serpentine group
This place does not cover:
Magnesium silicates containing also aluminium oxide, e.g. mica | |
Clay starting material for making ceramics, such as talc (Mg3Si4O10(OH)2) or sepiolite (Mg4Si6O15(OH)2·6H2O) | C04B235/34H |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: magnesium silicates, e.g. talc, sepiolite | |
Mixed oxides of MgO with silica without alumina, e.g. forsterite (Mg2SiO4) | |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Catalysts comprising silica and magnesia | |
Preparation of magnesium silicate powders, sols, gels, dispersions and their after-treatments |
If the alkaline earth silicate contains both Mg and Ca, such as with diopside (CaMgSi2O6), both C04B 2235/3445 and C04B 2235/3454 are given. If the alkaline earth silicate contains both Mg and Ba, Be or Sr, both C04B 2235/3445 and C04B 2235/3436 are given.
This place covers:
Calcium silicates used as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Datolite - CaBSiO4(OH), Titanite - CaTiSiO5, Ilvaite - CaFe2+2Fe3+O(Si2O7)(OH), Pigeonite - Ca0.25(Mg,Fe)1.75Si2O6, Diopside - CaMgSi2O6, Wollastonite - CaSiO3, Tremolite - Ca2Mg5Si8O22(OH)2
This place does not cover:
Magnesium silicates containing also aluminium oxide, e.g. mica | |
Talc (Mg3Si4O10(OH)2) starting material for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: alkaline-earth metal silicates, e.g. wollastonite | |
calcium silicate based hydraulic cement | C04B 28/02 and subgroups |
Mixed oxides of CaO with silica without alumina, e.g. wollastonite (CaSiO4) | |
Calcium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. lime | |
Preparation of alkaline earth metal silicate powders, sols, gels, dispersions and their after-treatments | |
calcium silicates as compounding ingredient for polymers |
If the alkaline earth silicate contains both Mg and Ca, such as with diopside (CaMgSi2O6), both C04B 2235/3445 and C04B 2235/3454 are given. If the alkaline earth silicate contains both Ca and Ba, Be or Sr, both C04B 2235/3454 and C04B 2235/3436 are given. A silicate such as Datolite - CaBSiO4(OH) also receives the symbol C04B 2235/3409.
This place covers:
All silicates that are not clay (see C04B 2235/349 for the definition of clays) and also contain aluminium oxide, e.g. Almandine - Fe3Al2(SiO4)3, Andalusite - Al2SiO5, Kyanite - Al2SiO5, Sillimanite - Al2SiO5, Dumortierite - Al6.5-7BO3(SiO4)3(O,OH)3, Topaz - Al2SiO4(F,OH)2, Beryl/Emerald - Be3Al2(Si6O18)
This place does not cover:
Clay silicate starting materials or secondary phase of a sintered ceramic | |
Alumino-silicate fibers used in ceramic compositions |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: Aluminium silicates other than clay | |
Clay wares | C04B 33/00 and subgroups |
Alumino-silicate based ceramics | C04B 35/18 and subgroups |
Making fibres based on silica, rich in aluminium oxide | |
Aluminates other than alumino-silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. spinel (MgAl2O4) | |
Catalysts comprising silica and alumina | |
Catalysts comprising Crystalline aluminosilicate zeolites; Isomorphous compounds thereof | B01J 29/06 and subgroups |
Preparation of aluminium containing silicate powders, sols, gels, dispersions and their after-treatments | C01B 33/26 and subgroups |
Compounds having molecular sieve and base-exchange properties, e.g. crystalline zeolites; Their preparation; After-treatment, e.g. ion-exchange or de-alumination | C01B 39/00 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing aluminium silicates |
This place covers:
All alkali metal silicates that are not clay (see C04B 2235/349 for the definition of clays) and also contain aluminium oxide, e.g.:
Tourmaline - (Na,Ca)(Al,Li,Mg)3-(Al,Fe,Mn)6(Si6O18)(BO3)3(OH)4
- Sodium pyroxene series
- - Jadeite - NaAlSi2O6
- - Aegirine (Acmite) - NaFe3+Si2O6
- Spodumene - LiAlSi2O6
- Hornblende - (Ca,Na)2-3(Mg,Fe,Al)5Si6(Al,Si)2O22(OH)2
- Mica group
- - Biotite - K(Mg,Fe)3(AlSi3)O10(OH)2
- - Muscovite - KAl2(AlSi3)O10(OH)2
- - Phlogopite - KMg3(AlSi3)O10(OH)2
- - Lepidolite - K(Li,Al)2-3(AlSi3)O10(OH)2
- Alkali-feldspars
- - Potassium-feldspars
- -- Microcline - KAlSi3O8
- -- Orthoclase - KAlSi3O8
- -- Sanidine - KAlSi3O8
- - Anorthoclase - (Na,K)AlSi3O8
- - Plagioclase feldspars
- -- Albite - NaAlSi3O8
- -- Oligoclase - (Na,Ca)(Si,Al)4O8 (Na:Ca 4:1)
- -- Andesine - (Na,Ca)(Si,Al)4O8 (Na:Ca 3:2)
- -- Labradorite - (Na,Ca)(Si,Al)4O8 (Na:Ca 2:3)
- -- Bytownite - (Na,Ca)(Si,Al)4O8 (Na:Ca 1:4)
- Feldspathoid family
- - Nosean - Na8Al6Si6O24(SO4)
- - Cancrinite - Na6Ca2(CO3,Al6Si6O24).2H2O
- - Leucite - KAlSi2O6
- - Nepheline - (Na,K)AlSiO4
- - Sodalite - Na8(AlSiO4)6Cl2
- -- Hauyne - (Na,Ca)4-8Al6Si6(O,S)24(SO4,Cl)1-2
- - Lazurite - (Na,Ca)8(AlSiO4)6(SO4,S,Cl)2
- Petalite - LiAlSi4O10
- Scapolite group
- - Marialite - Na4(AlSi3O8)3(Cl2,CO3,SO4)
- Analcime - NaAlSi2O6•H2O
- Zeolite group
- - Natrolite - Na2Al2Si3O10•2H2O
- - Stilbite - NaCa2Al5Si13O36•17H2O
This place does not cover:
Illite - (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | |
Montmorillonite - (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2O |
Attention is drawn to the following places, which may be of interest for search:
Alkali metal aluminosilicates based ceramics | |
Alkali oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Na2O, K2O | C04B 2235/3201 and subgroups |
If the alumino-silicate contains both alkali and alkaline earth metals, both C04B 2235/3472 and C04B 2235/3481 are given. The presence of other oxides in the silicate is indicated with symbols from C04B 2235/32 - C04B 2235/3409.
This place covers:
All alkaline earth metal silicates that are not clay (see C04B 2235/349 for the definition of clays) and also contain aluminium oxide, e.g.:
- Garnet group
- Pyrope - Mg3Al2(SiO4)3
- Grossular - Ca3Al2(SiO4)3
- Andradite - Ca3Fe2(SiO4)3
- Uvarovite - Ca3Cr2(SiO4)3
- Hydrogrossular - Ca3Al2Si2O8(SiO4)3-m(OH)4m
- Chloritoid - (Fe,Mg,Mn)2Al4Si2O10(OH)4
- Lawsonite - CaAl2(Si2O7)(OH)2·H2O
- Epidote group (has both (SiO4)4− and (Si2O7)6− groups)
- Epidote - Ca2(Al,Fe)3O(SiO4)(Si2O7)(OH)
- Zoisite - Ca2Al3O(SiO4)(Si2O7)(OH)
- Clinozoisite - Ca2Al3O(SiO4)(Si2O7)(OH)
- Tanzanite - Ca2Al3O(SiO4)(Si2O7)(OH)
- Allanite - Ca(Ce,La,Y,Ca)Al2(Fe2+,Fe3+)O(SiO4)(Si2O7)(OH)
- Dollaseite-(Ce) - CaCeMg2AlSi3O11F(OH)
- Vesuvianite (idocrase) - Ca10(Mg,Fe)2Al4(SiO4)5(Si2O7)2(OH)4
- 4-member ring
- Axinite - (Ca,Fe,Mn)3Al2(BO3)(Si4O12)(OH)
- 6-member ring
- Beryl/Emerald - Be3Al2(Si6O18)
- Cordierite - (Mg,Fe)2Al3(Si5AlO18)
- Tourmaline - (Na,Ca)(Al,Li,Mg)3-(Al,Fe,Mn)6(Si6O18)(BO3)3(OH)4
- Mica group
- Biotite - K(Mg,Fe)3(AlSi3)O10(OH)2
- Phlogopite - KMg3(AlSi3)O10(OH)2
- Margarite - CaAl2(Al2Si2)O10(OH)2
- Glauconite - (K,Na)(Al,Mg,Fe)2(Si,Al)4O10(OH)2
- Chlorite group
- Chlorite - (Mg,Fe)3(Si,Al)4O10(OH)2•(Mg,Fe)3(OH)6
- Na-Ca feldspars
- Plagioclase feldspars
- Oligoclase - (Na,Ca)(Si,Al)4O8 (Na:Ca 4:1)
- Andesine - (Na,Ca)(Si,Al)4O8 (Na:Ca 3:2)
- Labradorite - (Na,Ca)(Si,Al)4O8 (Na:Ca 2:3)
- Bytownite - (Na,Ca)(Si,Al)4O8 (Na:Ca 1:4)
- Anorthite - CaAl2Si2O8
- Feldspathoid family
- Cancrinite - Na6Ca2(CO3,Al6Si6O24).2H2O
- Hauyne - (Na,Ca)4-8Al6Si6(O,S)24(SO4,Cl)1-2
- Lazurite - (Na,Ca)8(AlSiO4)6(SO4,S,Cl)2
- Scapolite group
- Meionite - Ca4(Al2Si2O8)3(Cl2CO3,SO4)
- Zeolite group
- Chabazite - CaAl2Si4O12•6H2O
- Heulandite - CaAl2Si7O18•6H2O
- Stilbite - NaCa2Al5Si13O36•17H2O
This place does not cover:
Illite - (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2O)] | |
Montmorillonite - (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2O | |
Vermiculite - (MgFe,Al)3(Al,Si)4O10(OH)2·4H2O |
Attention is drawn to the following places, which may be of interest for search:
Alkaline earth metal alumino-silicate based ceramics | |
Cordierite honeycombs | C04B 38/0006 and subgroups |
Alkaline earth oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. BeO | C04B 2235/3205 and subgroups |
Cordierite honeycombs containing a catalyst |
If the alumino-silicate contains both alkali and alkaline earth metals, both C04B 2235/3472 and C04B 2235/3481 are given. The presence of other oxides in the silicate is indicated with symbols from C04B 2235/32 - C04B 2235/3409.
This place covers:
Clay starting materials added to non-clay wares, thus to all ceramics that are classified in C04B 35/00 and sub-classes.
This place does not cover:
Clay starting materials used for making clay wares | C04B 33/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: clays | C04B 14/10 and subgroups |
Inorganic binders based on silicon compounds | |
Aluminates other than alumino-silicates as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. spinel (MgAl2O4) | |
Alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. mullite (3Al2O3-2SiO2) | |
Clay used as filler for polymers |
In this place, the following terms or expressions are used with the meaning indicated:
Clay | Clays are distinguished from other fine-grained soils by differences in size and mineralogy. Silts, which are fine-grained soils that do not include clay minerals, tend to have larger particle sizes than clays, but there is some overlap in both particle size and other physical properties, and there are many naturally occurring deposits which include silts and also clay . The distinction between silt and clay varies by discipline. Geologists and soil scientists usually consider the separation to occur at a particle size of 2 µm (clays being finer than silts), sedimentologists often use 4-5 μm, and colloid chemists use 1 μm. Geotechnical engineers distinguish between silts and clays based on the plasticity properties of the soil, as measured by the soils' Atterberg Limits . ISO 14688 grades clay particles as being smaller than 2 μm and silts larger. Clay minerals are hydrous aluminium phyllosilicates, sometimes with variable amounts of iron, magnesium, alkali metals, alkaline earths, and other cations. Clays have structures similar to the micas and therefore form flat hexagonal sheets Clays are commonly referred to as 1:1 or 2:1. Clays are fundamentally built of tetrahedral sheets and octahedral sheets. A 1:1 clay would consist of one tetrahedral sheet and one octahedral sheet, and examples would be kaolinite and serpentine. A 2:1 clay consists of an octahedral sheet sandwiched between two tetrahedral sheets, and examples are illite, smectite, attapulgite, and chlorite (although chlorite has an external octahedral sheet often referred to as "brucite"). Clay minerals include the following groups:Kaolin group which includes the minerals kaolinite, dickite, halloysite, and nacrite (polymorphs of Al2Si2O5(OH)4 ). Some sources include the kaolinite-serpentine group due to structural similarities. Smectite group which includes dioctahedral smectites such as montmorillonite and nontronite and trioctahedral smectites for example saponite . Illite group which includes the clay-micas. Illite is the only common mineral. Chlorite group includes a wide variety of similar minerals with considerable chemical variation. Other 2:1 clay types exist such as sepiolite or attapulgite , clays with long water channels internal to their structure. Clay mineral group Halloysite - Al2Si2O5(OH)4 Kaolinite - Al2Si2O5(OH)4 Illite - (K,H3O)(Al,Mg,Fe)2(Si,Al)4O10[(OH)2,(H2 O)] Montmorillonite - (Na,Ca)0.33(Al,Mg)2Si4O10(OH)2·nH2 O Vermiculite - (MgFe,Al)3(Al,Si)4O10(OH)2·4H2 O Talc - Mg3Si4O10(OH)2 Palygorskite - (Mg,Al)2Si4O10(OH)·4(H2 O) Pyrophyllite - Al2Si4O10(OH)2 |
This place covers:
Glass powder used as starting material for making ceramics
This place does not cover:
Glass fibers used as additive for ceramics | |
Fused silica as starting material for making ceramics | |
Amorphous silica as starting material for making ceramics, e.g. silica fume | |
Waterglass (NaSiO3) starting material for making ceramics | |
Glass phase formed in situ during sintering | C04B 2235/85 (grain boundary phase) |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: glass | C04B 14/22 and subgroups |
Waste glass used for making ceramics | |
Melting of clay material to make clay wares | |
Melting of material to make a ceramic powder | |
Inorganic binders based on silicon compounds | |
Melting of ceramic or refractory material to make a bulk ceramic | C04B 35/653 and subgroups |
Crystalline silica as starting material for making ceramics | |
Clays as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bentonites/smectites such as montmorillonite, kaolines such as halloysite, illite, talc, sepiolite and attapulgite, vermiculite | |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Glass interlayer used for joining a ceramic with another substrate |
The composition of the glass is not further classified with symbols from the C04B 2235/32 scheme. The composition of the glass should be classified in C03C.
This place covers:
All glasses that contain both silicon oxide and boron oxide and are used as starting material for making glass.
This place does not cover:
Boron oxide, borates, boric acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boric acid (HBO2) |
Attention is drawn to the following places, which may be of interest for search:
Glass compositions containing less than 40 wt% silica and also boron | C03C 3/064 and subgroups |
Glass compositions containing less than 40 wt% silica, containing lead and also boron | C03C 3/072 and subgroups |
Glass compositions containing 40-90 wt% silica and also boron | C03C 3/089 and subgroups |
Glass compositions containing 40-90 wt% silica, containing lead and also boron | |
Glass compositions containing 40-90 wt% silica, containing fluorine and also boron | C03C 3/115 and subgroups |
This place covers:
All starting materials for making ceramics containing carbide, nitride, boride or silicide phase. All sintered ceramics containing a secondary carbide, nitride, boride or silicide phase.
This place does not cover:
The use of metal fibers as reinforcement for ceramics | |
Metallic constituents or additives not added as binding phase, or present as secondary phase in a sintered ceramic | C04B 2235/40 and subgroups |
Non metallic elements added as constituents or additives, or present as secondary phase in a sintered ceramic, e.g. silicon, boron, carbon, sulphur, phosphor, selenium or tellurium | C04B 2235/42 and subgroups |
The use of phosphides as starting material for making ceramics, or their presence as secondary phase in a sintered ceramic | |
The use of halides such as fluorides as starting material for making ceramics, or their presence as secondary phase in a sintered ceramic | C04B 2235/444 and subgroups |
The use of selenides, sulfides or tellurides as starting material for making ceramics, or their presence as secondary phase in a sintered ceramic | |
Non-oxide fibers used as starting material for making ceramics | C04B 2235/524 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on non-oxide ceramics | C04B 35/515 and subgroups |
Making fibres based on non-oxide ceramic material | |
Coating or impregnating ceramic substrates with non-oxide ceramics | |
Metal oxide starting materials for making ceramics or present as secondary phase in a sintered ceramic, e.g. alumina, ferrites, titanates, cuprates | C04B 2235/32 and subgroups |
Non-metal oxide starting material or secondary phase, e.g. silica, silicates, boron oxide | C04B 2235/34 and subgroups |
Metal oxide starting material or secondary phase present in a glass phase | C04B 2235/36 and subgroups |
Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate | C04B 2235/44 and subgroups |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
Non-oxide interlayer used for joining a ceramic with another substrate | C04B 2237/08 and subgroups |
Non-oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/36 and subgroups |
If a certain starting material or secondary phase is a mixture of two or more metal non-oxides, e.g. CaMg boride, then both metal non-oxides are classified, also if one is present less than the other, e.g. Ca0.2Mg0.8B2, thus both C04B 2235/3808 (for the Mg) and C04B 2235/3804 (for the Ca) are given.
This place covers:
Starting materials for making ceramics or secondary phases of sintered ceramics containing a compound between boron and a metal or semi-metal, e.g. aluminium boride, Rare earth boride, e.g. dysprosium boride (DyB2), Lanthanum boride (LaB6), Manganese boride (Mn2B, MnB or MnB2), Iron boride (Fe2B, FeB), Cobalt boride (CoB), Nickel boride (NiB), Copper boride (Cu3B2), Gallium boride (GaB12), Scandium Iridium Boride (Sc3Ir5B2), Silver boride (AgB2), Nickel bismuth boride (Ni23-xBixB6), Silicon boride (SiBn)
This place does not cover:
Boron carbide additive or secondary phase | |
Boron nitride additive or secondary phase | |
Boron additive or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: borides | |
Ceramics based on borides | C04B 35/5805 and subgroups |
Coating or impregnating ceramic substrates with borides | |
Boron oxide or borate starting material or secondary phase | |
Borosilicate glass additive | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Preparation of metal boride powders | |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of borides | C22C 32/0073 and subgroups |
Making ferrous alloys by powder metallurgy with more than 5% preformed carbides, nitrides or borides |
This place covers:
Starting materials for making ceramics or secondary phases of sintered ceramics containing a compound between boron and magnesium, e.g. magnesium boride, MgB2
This place does not cover:
Magnesium boron carbide additive or secondary phase | |
Magnesium boron nitride additive or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on magnesium boride | |
Magnesium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Magnesium starting material or secondary phase |
This place covers:
Starting materials for making ceramics or secondary phases of sintered ceramics containing refractory metal borides or refractory metal oxy-borides, e.g. Titanium diboride (TiB2), Vanadium diboride (VB2), Chromium boride (CrB or CrB2), Zirconium of hafnium diboride (ZrB2 or HfB2), Niobium or tantalum diboride (NbB2 or TaB2), Molybdenum boride (Mo2B or Mo2B5), Tungsten boride (W2B, WB or W2B5).
This place does not cover:
Refractory boron carbide additive or secondary phase | C04B 2235/3821, C04B 2235/3839 and subgroups |
Refractory boron nitride additive or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on refractory borides | C04B 35/5805 and subgroups |
Refractory metal oxide starting material or secondary phase | C04B 2235/3231 and subgroups |
Refractory metal carbide starting material or secondary phase | C04B 2235/3839 and subgroups |
Refractory metal nitride starting material or secondary phase | |
Refractory metal silicide starting material or secondary phase | |
Refractory metal starting material or secondary phase |
This place covers:
Starting materials for making ceramics or secondary phases of sintered ceramics containing a carbide phase, a compound between carbon and a metal or semi-metal, e.g. potassium carbide, magnesium carbide, Cerium carbide (CeC2), Manganese carbide (Mn3C), Iron carbide (Fe3C), Cobalt carbide (CoC), Nickel carbide (Ni3C), Copper carbide (Cu2C), Zinc carbide (ZnC), Germanium carbide (GeC), Gold carbide (Au2C2), Silver carbide (Ag2C2), Antimony carbide (SbC)
This place does not cover:
Carbo-nitride starting material or secondary phase | |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: carbides | |
Ceramics based on carbides | C04B 35/56 and subgroups |
Carbo-nitride ceramics | C04B 35/58 and subgroups |
Making fibres based on carbides | |
Coating or impregnating ceramic substrates with carbides | |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
Carbide interlayer used for joining a ceramic with another substrate | |
Carbide catalysts | B01J 27/22, C07C 2527/22 and subgroups |
High pressure synthesis: Composition of the material to be processed: carbides | B01J 2203/063 and subgroups |
Making carbide powders | C01B 32/90 and subgroups |
Carbides used as filler for polymers | |
Making hard metals based on borides, carbides, nitrides, oxides or silicides; Preparation of the powder mixture used as the starting material | C22C 1/051 and subgroups |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of carbides | C22C 32/0052 and subgroups |
Making ferrous alloys by powder metallurgy with more than 5% preformed carbides, nitrides or borides |
Carbonitrides are seen as nitrides. If a ceramic is however a mixture of separate carbide and nitride phases, then classification occurs in the class that corresponds to the phase that is present as the largest fraction, which could be a carbide class.
Ti0.9Al0.1C and Ti0.1Al0.9C both receive both symbols C04B 2235/3817 and C04B 2235/3843.
This place covers:
Boron carbides (B4C) or boron oxy-carbides as starting materials for making ceramics or as secondary phases of sintered ceramics.
This place does not cover:
Boron carbo-nitride starting material or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: boron carbide | |
Ceramics based on boron carbide | |
Ceramics based on boron carbo-nitride | C04B 35/583 and subgroups |
Coating or impregnating ceramic substrates with boron carbide | |
Boron oxide, borates, boric acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boric acid (HBO2) | |
Borosilicate glass additive | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3804 and subgroups |
Boron nitride starting material for making ceramics or secondary phase of a sintered ceramic | |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Preparation of boron carbide powders | |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of B4C |
In the case of mixed carbides, e.g. SiBC or Si0.9B1.1C, both C04B 2235/3826 and C04B 2235/3821 are added.
This place covers:
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC
This place does not cover:
Silicon carbo-nitride starting material or secondary phase | C04B 2235/3856, C04B 2235/3873 and subgroups |
Silicon carbide fibers used as starting material for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: silicon carbide | |
Ceramics based on titanium silicon (oxy)carbide | |
Silicon carbide based ceramics | C04B 35/565 and subgroups |
Ceramics based on silicon carbo-nitride | C04B 35/584 and subgroups |
Making fibres based on silicon carbide | |
Inorganic binders based on silicon compounds | |
Coating or impregnating ceramic substrates with silicon carbide | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic | C04B 2235/3873 and subgroups |
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes | |
Silicon carbide substrate joined with another substrate or being part of a ceramic laminate | |
Silicon carbide catalyst | B01J 27/224 and subgroups, C07C 2527/224 |
High pressure synthesis: Composition of the material to be processed: silicon carbide | |
Preparation of silicon carbide powders | |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of SiC |
Ti0.9Si0.1C and Ti0.1Si0.9C both receive both symbols C04B 2235/3843 and C04B 2235/3826.
This place covers:
Alpha silicon carbide as starting material for making ceramics or as secondary phase of a sintered ceramic
This place does not cover:
The main phase of the sintered ceramic being alpha SiC |
Attention is drawn to the following places, which may be of interest for search:
Alpha silicon nitride as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Hexagonal symmetry, alpha SiC |
This place covers:
Beta silicon carbide as starting material for making ceramics or as secondary phase of a sintered ceramic
This place does not cover:
The main phase of the sintered ceramic being beta SiC |
Attention is drawn to the following places, which may be of interest for search:
Beta silicon nitride as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Cubic symmetry, e.g. beta SiC |
This place covers:
Refractory metal carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. VC, Cr3C2, ZrC, HfC, NbC, TaC, MoC or Mo2C
This place does not cover:
Refractory carbo-nitride starting material or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on refractory metal carbides | |
Refractory metal oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3231 and subgroups |
Refractory metal borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiB2, HfB2 | |
Refractory metal nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Refractory metal silicides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic | |
The preparation of tungsten or molybdenum carbide powders |
In this place, the following terms or expressions are used with the meaning indicated:
refractory carbides | titanium carbide, vanadium carbide, chromium carbide, zirconium carbide, niobium carbide, molybdenum carbide, hafnium carbide, tantalum carbide, tungsten carbide |
This place covers:
Titanium carbide as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiC or titanium oxy-carbides
This place does not cover:
Titanium carbo-nitride starting material or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on titanium (oxy)carbide | C04B 35/5611 and subgroups |
Titanium (oxy)carbonitride ceramics | |
After-treatment of mortars, concrete, artificial stone or ceramics; Treatment of natural stone: with titanium carbide | |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
Making titanium (oxy)carbide powders |
In the case of mixed refractory carbides, e.g. TiCrC, but also Ti0.9Cr1.1C both C04B 2235/3839 (for the Cr) and C04B 2235/3843 are added. Ti0.9Al0.1C and Ti0.1Al0.9C both receive both symbols C04B 2235/3817 and C04B 2235/3843.
This place covers:
Tungsten carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. WC or tungsten oxy-carbides
This place does not cover:
Tungsten carbo-nitride starting material or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on tungsten (oxy)carbide | |
Tungsten (oxy)carbonitride ceramics | |
Tungsten oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. scheelite (CaWO4) | C04B 2235/3258 and subgroups |
The preparation of tungsten or molybdenum carbide powders |
This place covers:
Starting materials for making ceramics or secondary phases of sintered ceramics containing compounds between nitrogen and a metal or semi-metal, e.g. alkali nitrides, alkaline earth metal nitrides, rare earth nitrides, carbonitrides, oxynitrides, Copper nitride (Cu3N), Mercury nitride (Hg3N2), zinc nitride (Zn3N2) , Gallium indium nitride (Ga1-xInxN), Germanium nitride (Ge3N4), Ruthenium nitride (RuN), Silver nitride (AgN3), Tin nitride (SnN), Antimony nitride (SbN), Lead nitride (Pb(N3)2), Bismuth nitride (BiN)
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: nitrides | |
Ceramics based on nitrides | C04B 35/58 and subgroups |
Making fibres based on nitrides | |
Coating or impregnating ceramic substrates with borides, nitrides or silicides | |
Gases other than oxygen used as reactant for making a ceramic phase, e.g. nitrogen used to make a nitride phase | C04B 2235/46 and subgroups |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/06 and subgroups, C01B 21/082 and s subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing nitrides | |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of nitrides | C22C 32/0068 and subgroups |
Making ferrous alloys by powder metallurgy with more than 5% preformed carbides, nitrides or borides |
Carbonitrides and oxynitrides are seen as nitrides
This place covers:
Carbonitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium carbonitride, zirconium carbonitride
This place does not cover:
Making carbonitrides per se, not preparative to the making of a ceramic |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based titanium (oxy)carbo-nitrides | |
Ceramics based zirconium (oxy)carbo-nitrides | |
Organic compounds becoming part of a ceramic after heat treatment, e.g. carbonising phenol resins | C04B 2235/48 and subgroups |
Phases that receive the C04B 2235/3856 symbol also receive other symbols from C04B 2235/3852 and the subgroups to indicate the metal, e.g. aluminum carbo-nitride receives both C04B 2235/3856 and C04B 2235/3865. If the metal nitride is classified with the main C04B 2235/3852 symbol, then only C04B 2235/3856 needs to be given. Alkali or alkaline earth carbo-nitrides receives only the C04B 2235/3856 symbol.
This place covers:
Boron nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boron oxynitrides or boron carbonitrides, cubic boron nitride, hexagonal boron nitride
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: boron nitride | |
Ceramics based on boron nitride | C04B 35/583 and subgroups |
Making fibres based on boron nitride | |
Coating or impregnating ceramic substrates with boron nitride | |
Boron oxide, borates, boric acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boric acid (HBO2) | |
Borosilicate glass additive | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3804 and subgroups |
Boron carbide starting material for making ceramics or secondary phase of a sintered ceramic | |
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl | |
Boron nitride substrate joined with another substrate or being part of a ceramic laminate | |
High pressure synthesis: Composition of the material to be processed: boronitrides | |
The preparation of boron nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/064 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
hBN | Hexagonal boron nitride |
cBN | Cubic boron nitride |
This place covers:
Aluminum nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. aluminium carbonitrides
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: aluminium nitride | |
Ceramics based on aluminum nitride | |
Coating or impregnating ceramic substrates with aluminium nitride | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Aluminium as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Aluminum nitride substrate joined with another substrate or being part of a ceramic laminate | |
The preparation of aluminium nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/072 and subgroups, C01B 21/0825 (oxy-nitrides) |
This place covers:
Starting materials or secondary phases based on oxynitrides containing at least aluminium (AlON) or also silicon (Sialon), possibly further containing rare earths
This place does not cover:
SiON starting material or secondary phase |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on AlON | |
Ceramics based on SiAlON | |
Coating or impregnating ceramic substrates with silicon oxynitrides, e.g. SIALON | |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic | C04B 2235/3873 and subgroups |
Non-oxides with a defined oxygen content as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Making aluminium oxynitrides powders per se | |
The preparation of sialon powders per se, not preparative to the making of nitride ceramics |
This place covers:
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON)
This place does not cover:
Silicon aluminium oxy-nitride (Sialon) additives or secondary phases |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: silicon nitride | |
Ceramics based on silicon (carbo)nitrides | C04B 35/484 and subgroups |
Ceramics based on silicon oxy-nitrides | |
Making fibres based on silicon nitrides | |
Inorganic binders based on silicon compounds | |
Coating or impregnating ceramic substrates with silicon nitride | |
Silica as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicon carbides as starting material for making a ceramic or as secondary phase of a sintered ceramic | C04B 2235/3826 and subgroups |
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
Si-containing organic compounds becoming part of a ceramic after heat treatment, e.g. silicone resins, (poly)silanes, (poly)siloxanes or (poly)silazanes | |
Silicon nitride substrate joined with another substrate or being part of a ceramic laminate | |
The preparation of silicon nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/068 and subgroups, C01B 21/0823 (oxy-nitrides) |
Mixed nitrides, e.g. SiBN or Si0.9B1.1N receive symbols for both nitrides, thus C04B 2235/3873 and C04B 2235/386.
This place covers:
Alpha silicon nitride as starting material for making ceramics or as secondary phase of a sintered ceramic
This place does not cover:
The main phase of the sintered ceramic being alpha Si3N4 |
Attention is drawn to the following places, which may be of interest for search:
Alpha silicon carbide as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Trigonal symmetry, alpha Si3N4 |
This place covers:
Beta silicon nitride as starting material for making ceramics or as secondary phase of a sintered ceramic
This place does not cover:
The main phase of the sintered ceramic being beta Si3N4 |
Attention is drawn to the following places, which may be of interest for search:
Beta silicon carbide as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Hexagonal symmetry, beta Si3N4 |
This place covers:
Refractory metal nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, or refractory metal oxynitrides, e.g. Titanium nitride (TiN), Vanadium nitride (VN), Chromium nitride (CrN), Zirconium of hafnium nitride (ZrN or HfN), Niobium or tantalum nitride (NbN or TaN), Molybdenum nitride (MoN), Tungsten nitride (W2N or WN2)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on refractory metal (oxy)(carbo)nitrides | C04B 35/58014 and subgroups |
Coating or impregnating ceramic substrates with titanium nitride | |
Refractory metal oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3231 and subgroups |
Refractory metal borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiB2, HfB2 | |
Refractory metal carbides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Refractory metal silicides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic | |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with vanadium, niobium or tantalum | |
The preparation of nitride powders per se, not preparative to the making of nitride ceramics, with chromium, molybdenum or tungsten | |
The preparation of titanium, zirconium or hafnium nitride powders per se, not preparative to the making of nitride ceramics | C01B 21/076 and subgroups, C01B 21/076 |
In this place, the following terms or expressions are used with the meaning indicated:
refractory nitrides | titanium nitride, vanadium nitride, chromium nitride, zirconium nitride, niobium nitride, molybdenum nitride, hafnium nitride, tantalum nitride, tungsten nitride |
This place covers:
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic, i.e. chemical compounds between silicon and a one or more metals, e.g. Titanium disilicide (TiSi2), Vanadium disilicide (VSi2), Chromium silicide (CrSi2), Zirconium of hafnium disilicide (ZrSi2 or HfSi2), Niobium or tantalum disilicide (NbSi2 or TaSi2), Molybdenum disilicide (MoSi2), Tungsten silicide (WSi2), Manganese silicide (MnSi2), Iron silicide (FeSi, FeSi2), Cobalt silicide (Co2Si, CoSi, CoSi2), Nickel silicide (Ni2Si), Copper silicide (Cu4Si), Gallium silicide (Ga3Si), germanium silicide (Si1–xGex), Osmium silicide (Os2Si3)
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on silicides | C04B 35/58085 and subgroups |
Inorganic binders based on silicon compounds | |
Coating or impregnating ceramic substrates with silicides | |
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase | C04B 2235/40 and subgroups |
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic | |
The preparation of metal silicide powders | |
Non-ferrous alloys containing at least 5% by weight but less than 50% by weight of silicides | C22C 32/0078 and subgroups |
This place covers:
Non-oxides with a defined oxygen content as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiOC, SiON, TiON. The amount of oxygen of a certain non-oxide starting material is indicated, e.g. a boride powder containing 0.1 wt% oxygen, or a silicide containing 5 wt% oxygen.
This place does not cover:
Aluminum oxynitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. AlON or sialon |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on carbides having a well-defined oxygen content, e.g. oxy-carbides | |
Products characterised by the absence or the low content of oxygen | |
The preparation of oxynitride powders per se, not preparative to the making of nitride ceramics | |
Making powders of oxycarbides, sulfocarbides or mixtures of carbides with other bodies, e.g. graphite; Carbides of other non-metals, e.g. silicocarbides, borocarbides |
A material that receive this symbol can also receive other non-oxide symbols, e.g. SiON starting powder or secondary phase receives both symbols C04B 2235/3895 and C04B 2235/3873.
This place covers:
Metal as starting material for making ceramics or as secondary phase of a sintered ceramic, not being present as a binding phase, e.g. La, Y, Mn, Re, Zn, Ga, In, Ge, Sb, Pb, Bi. The metal can be added for instance in powder form, in gaseous form or in a molten state.
This place does not cover:
Non metallic elements as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. sulphur, phosphor, carbon, boron, silicon, selenium or tellurium | C04B 2235/42 and subgroups |
Products characterised by the absence or the low content of metal phase | |
Ceramics containing a metallic binder, i.e. cermets | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: metallic additives | |
Ceramics based on non-oxide ceramics | C04B 35/515 and subgroups |
Reaction sintering of free metal or free silicon containing compositions to make a ceramic material | C04B 35/65 and subgroups |
Metal oxides, mixed metal oxides or oxide forming salts thereof, e.g. carbonates, nitrates, (oxy)hydroxides, chlorides, as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/32 and subgroups |
Metal interlayer used for joining a ceramic with another substrate | C04B 2237/12 and subgroups |
Metallic powders per se | B22F 1/09 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: metallic pigments or fillers | C09C 1/62 and subgroups |
If the end product contains a continuous metal phase, the product is regarded as a cermet and is classified in C22C 29/00 and sub subgroups. In this case neither the end product is classified in C04B 35/00 and sub-classes, nor are the starting materials classified in C04B 2235/00 and subgroups.
This place covers:
Alkaline earth metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. Mg
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on magnesium boride | |
Alkaline earth oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. BeO | C04B 2235/3205 and subgroups |
Magnesium borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. MgB2 |
This place covers:
Aluminium as starting material for making ceramics or as secondary phase of a sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Alumina based ceramics | C04B 35/10 and subgroups |
Ceramics based on aluminum nitride | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
Aluminum nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Aluminium interlayer used for joining a ceramic with another substrate |
This place covers:
Refractory metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium (Ti), chromium (Cr), tantalum (Ta)
Attention is drawn to the following places, which may be of interest for search:
Refractory metal carbide ceramics | C04B 35/5607 and subgroups |
Refractory metal nitride ceramics | C04B 35/58007 and subgroups |
Refractory metal boride ceramics | C04B 35/58064 and subgroups |
Refractory metal silicide ceramics | C04B 35/58092 and subgroups |
Titanium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. rutile or anatase | C04B 2235/3232 and subgroups |
Refractory metal borides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. TiB2, HfB2 | |
Refractory metal carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. VC, Cr3C2, ZrC, HfC, NbC, TaC, MoC or Mo2C | C04B 2235/3839 and subgroups |
Refractory metal nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. vanadium nitride (VN), tungsten nitride (WN2) | |
Refractory metal interlayer used for joining a ceramic with another substrate | |
Metallic interlayer used for joining a ceramic with another substrate, containing a refractory metal as the active component |
In this place, the following terms or expressions are used with the meaning indicated:
refractory metals | titanium, vanadium, chromium, zirconium, niobium, molybdenum, hafnium, tantalum, tungsten |
This place covers:
Iron group metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. nickel (Ni) or cobalt (Co)
Attention is drawn to the following places, which may be of interest for search:
Iron group nitride based ceramics | |
Iron group oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/327 and subgroups |
Iron group metal interlayer used for joining a ceramic with another substrate |
In this place, the following terms or expressions are used with the meaning indicated:
iron group metals | Fe, Ni, Co |
This place covers:
Copper as starting material for making ceramics or as secondary phase of a sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Copper oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. CuO or Cu2O | C04B 2235/3281 and subgroups |
Copper interlayer used for joining a ceramic with another substrate |
This place covers:
Noble metals as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silver (Ag), palladium (Pd), platinum (Pt)
This place does not cover:
Noble metal oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. IrO2, PdO, RhO2 | C04B 2235/3289 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Noble metal interlayer used for joining a ceramic with another substrate |
In this place, the following terms or expressions are used with the meaning indicated:
noble metals | Ru, Rh, Pd, Ag, Os, Ir, Pt, Au |
This place covers:
Non metallic elements as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. sulphur, phosphor, selenium or tellurium, arsenium. The elements should be in their elemental state, thus not present in any compound. The end-product is allowed to have a continuous phase of the non-metallic element, it is still regarded as a ceramic.
This place does not cover:
Metal salts chosen for the nature of the anions as starting material for making ceramics, e.g. phosphides, arsenides | |
Sulphides, tellurides or selenides as starting material for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on phosphates | |
Ceramics based on phosphides | |
Ceramics based on sulphides, selenides or tellurides | |
Phosphates or phosphites (calcium phosphates C04B 2235/3212) as starting material for making ceramics, e.g. orthophosphate (PO43-), pyrophosphate (P2O74-), hypophosphite (H2PO2-) | |
Sulphates (SO42-) or sulphites (SO3-)as starting material for making ceramics | |
Products characterised by the absence or the low content of specific components, e.g. alkali metal free alumina ceramics | C04B 2235/72 and subgroups |
This place covers:
Boron as starting material for making ceramics or as secondary phase of a sintered ceramic. The boron is in its elemental state (B).
Attention is drawn to the following places, which may be of interest for search:
Boron oxide, borates, boric acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boric acid (HBO2) | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3804 and subgroups |
Boron carbide as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. B4C | |
Boron nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Preparation of boron |
This place covers:
All starting materials for making ceramics containing an inorganic carbon phase; all secondary phases of sintered ceramics consisting out of carbon
This place does not cover:
Ceramics based on oxide ceramics, containing carbon | |
Refractory ceramics based on alumina, containing carbon | |
Adding carbonaceous materials, e.g. coal, carbon, graphite, hydrocarbons and burning-out the carbonaceous material in order to create a porous ceramic |
Attention is drawn to the following places, which may be of interest for search:
Carbon used as filler for concrete, mortar or artificial stone | C04B 14/022 and subgroups |
Ceramics based on carbon | C04B 35/52 and subgroups |
Ceramics or ceramic mixtures based on carbides | C04B 35/56 and subgroups |
Reaction sintering C to make SiC | |
Reaction sintering C to make Si3N4 | |
A carbon-based matrix containing carbon fibers | |
Coating or impregnating a ceramic substrates with carbon | C04B 41/5001 and subgroups |
Carbides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3817 and subgroups |
Carbonitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. titanium carbonitride, zirconium carbonitride | |
Organics compounds becoming part of a ceramic after heat-treatment, e.g. phenol resins | C04B 2235/48 and subgroups |
fibrous carbon additives for ceramics | |
carbon nanotube additives for ceramics | |
Ceramics or ceramic mixtures containing carbon as an impurity | |
Carbon interlayer used for joining a ceramic with another substrate | |
Carbon substrate joined with another substrate or being part of a ceramic laminate | |
Catalysts comprising carbon | B01J 21/18 and subgroups, C07C 2521/18 |
High pressure synthesis: Composition of the material to be processed: carbon | |
The preparation of carbon powders per se, not preparative to the making of carbon ceramics | C01B 32/00 and subgroups |
preparation of active carbon using carbonaceous precursors per se and binders, e.g. pitch, and producing the granules | |
Carbon used as filler for polymers | |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: carbon | C09C 1/44 and subgroups |
If the carbon additive for the oxide ceramic is plain carbon, C04B 35/013 is given and C04B 2235/422 not, but if the carbon is carbon black, graphite or diamond, C04B 35/013 is given together with the respective symbol of C04B 2235/422. Same accounts for C04B 35/103.
This place covers:
Carbon black as starting material for making ceramics or as secondary phase of a sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: carbon black | C09C 1/48 and subgroups |
This place covers:
Graphite as starting material for making ceramics or as secondary phase of a sintered ceramic
This place does not cover:
Graphite added to a ceramic to be burned away, e.g. to create porosity (pore former) |
Attention is drawn to the following places, which may be of interest for search:
Use of graphite as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of graphite specially adapted to enhance their filling properties in mortars, concrete or artificial stone | |
Ceramics based on graphite | |
Ceramics based on expanded graphite | |
High pressure synthesis: Composition of the material to be processed: graphite | |
The preparation and after-treatment of intercalated graphite powders | |
The preparation and after-treatment of graphite powders | C01B 32/20 and subgroups |
Treatment of inorganic materials, other than fibrous fillers, to enhance their pigmenting or filling properties: graphite | |
Intercalated carbon- or graphite fibres |
This place covers:
Diamond as starting material for making ceramics or as secondary phase of a sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Presses for the formation of diamonds or boronitride | B01J 3/065 and subgroups |
High pressure synthesis: Composition of the material to be processed: diamond | |
The preparation and after-treatment of diamond powders | C01B 32/25 and subgroups |
This place covers:
Silicon as starting material for making a ceramic or as secondary phase of a sintered ceramic. The end product can contain silicon up to 50% in order to be still regarded as a ceramic product.
This place does not cover:
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering Si to make SiC ceramic | |
Reaction sintering Si to make Si3N4 ceramic | |
Inorganic binders based on silicon compounds | |
Reaction sintering of free metal or free silicon containing compositions to make a ceramic material | C04B 35/65 and subgroups |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Aluminum oxynitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. AlON or sialon | |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Silicides as starting material for making a ceramic or as secondary phase of a sintered ceramic, i.e. chemical compounds between silicon and a one or more metals, e.g. chromium silicide (CrSi2), molybdenum disilicide (MoSi2), iron silicide (FeSi, FeSi2), cobalt silicide (Co2Si, CoSi, CoSi2) | |
Products characterised by the absence or the low content of silicon | |
Ceramic interlayer used for joining a ceramic with another substrate, containing silicon as the active component | |
Metallic interlayer used for joining a ceramic with another substrate, containing silicon as the active component | |
Silicon interlayer used for joining a ceramic with another substrate |
This place covers:
Metal salts chosen for the nature of the anions as starting material for making ceramics, e.g. phosphides, hydrides, acetylacetonate, hydroxides, arsenides, or present as secondary phase in the sintered ceramic. In many cases the anion cannot be present in a sintered ceramic, for instance if it is organic, since it will burn away. In other cases the anion can be present, for instance phosphate, sometimes sulphate or halides, after sintering.
This place does not cover:
Aluminium hydroxide as starting material for making ceramics |
Attention is drawn to the following places, which may be of interest for search:
Organic additives for clay mixtures | |
Ceramics based on phosphides | |
Coating or impregnating ceramic substrates with salts or salty compositions | C04B 41/5007 and subgroups |
Hydrides per se | C01B 6/00 and subgroups |
Before the introduction of the C04B 2235/00-scheme the use of metal-organic salts as additive or constituent was classified in C04B 35/6325. Normally in the case a symbol from C04B 2235/44 is given for a certain starting material, no CPC-symbol from C04B 35/63-C04B 35/638 will be given.
This place covers:
Alkoxides as starting material for making ceramics, e.g. methoxide, tert-butoxide, isopropoxide
Attention is drawn to the following places, which may be of interest for search:
Organo metallic additives for making ceramics |
If C04B 2235/441 is used, C04B 35/6325 does not need to be used.
This place covers:
Carbonates (CO32-) as starting material for making ceramics or present as secondary phase in the sintered ceramic
This place does not cover:
Dolomite, i.e. mixed calcium magnesium carbonate, or oxides derived from dolomite as starting material for making ceramics or as secondary phase of a sintered ceramic |
Attention is drawn to the following places, which may be of interest for search:
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: carbonates | C04B 14/26 and subgroups |
Coating or impregnating ceramic substrates with salts or salty compositions: containing carbon in the anion, e.g. carbonates | |
Methods for the preparation of carbonates or bicarbonates in general | |
Carbonates of sodium, potassium or alkali metals in general | C01D 7/00 and subgroups |
Lithium carbonates, bicarbonates |
This place covers:
Nitrates (NO3-) or nitrites (NO2-) as starting material for making ceramics
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with salts or salty compositions: containing nitrogen in the anion, e.g. nitrites | |
Nitrates of sodium, potassium or alkali metals in general | C01D 9/00 and subgroups |
Lithium nitrates | |
Preparation of nitrate metal compounds in general |
This place covers:
Halide containing anions as starting material for making ceramics, e.g. chlorate (ClO3-), bromide (Br-), iodate (IO3-), chlorite (ClO2-), or present as secondary phase in the sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on halogenides other than fluorides | |
Coating or impregnating ceramic substrates with salts or salty compositions: containing halogen in the anion | |
Products characterised by the absence or the low content of halogenides | |
Halogens per se | C01B 7/00, C01B 9/00, C01B 11/00 and subgroups |
Halides of sodium, potassium or alkali metals in general | C01D 3/00 and subgroups |
Lithium halides | |
Preparation of halide metal compounds in general | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing inorganic halogenide compounds | C09K 11/0827 and subgroups, C09K 11/61 and subgroups |
This place covers:
Fluoride containing anions as starting material for making ceramics, e.g. fluoride (F-), fluosilicate (SiF62-), or present as secondary phase in the sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on fluorides | |
Coating or impregnating ceramic substrates with fluorides | |
Catalysts containing fluoride | |
Fluorides per se | C01B 7/19 and subgroups, C01B 9/08, C01B 11/24 |
Fluorides of sodium, potassium or alkali metals in general |
This place covers:
Sulphides, tellurides or selenides as starting material for making ceramics or present as secondary phase in the sintered ceramic
This place does not cover:
Non metallic elements as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. sulphur, phosphor, selenium or tellurium | C04B 2235/42 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on sulfides, selenides or tellurides | |
Coating or impregnating ceramic substrates with salts or salty compositions: containing sulphur in the anion, e.g. sulfides | |
Coating or impregnating ceramic substrates with sulfides or selenides | |
Products characterised by the absence or the low content of sulphur | |
catalysts comprising sulfides | B01J 27/04 and subgroups |
Sulphide compounds per se | C01B 17/20 and subgroups |
Selenides and tellurides per se | |
Preparation of sulfides metal compounds in general | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing sulfides | C09K 11/56 and subgroups |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing two or more rare earth metals: Oxysulfides |
This place covers:
Phosphates or phosphites as starting material for making ceramics, e.g. orthophosphate (PO43-), pyrophosphate (P2O74-), hypophosphite (H2PO2-), or present as secondary phase in the sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Phosphate cements | |
Use of inorganic materials as fillers, e.g. pigments, for mortars, concrete or artificial stone; Treatment of inorganic materials specially adapted to enhance their filling properties in mortars, concrete or artificial stone: Phosphates, e.g. apatite | |
Phosphate muds used in making clay | |
Ceramics based on phosphates | |
Making fibres based on phosphates | |
Binders based on phosphoric acids or phosphates | |
Coating or impregnating ceramic substrates with salts or salty compositions: containing phosphor in the anion, e.g. phosphates | |
Coating or impregnating ceramic substrates with phosphates | |
Coating or impregnating ceramic substrates with phosphate cements | |
Coating or impregnation of artificial stone with phosphates | |
Calcium phosphates, e.g. hydroxyapatite | |
Products characterised by the absence or the low content of phosphorus | |
Phosphate catalysts | |
Catalysts comprising molecular sieves being phosphates | |
Preparation of phosphates per se, e.g. phosphates powder, not preparative to making a phosphates ceramic | |
Metallophosphates not containing aluminium having molecular sieve properties but not having base-exchange properties | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing phosphates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing phosphorus | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing arsenic, antimony or bismuth phosphates | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing rare earth phosphates |
This place covers:
Sulphates (SO42-) or sulphites (SO3-)as starting material for making ceramics or present as secondary phase in the sintered ceramic
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating ceramic substrates with salts or salty compositions: containing sulphur in the anion, e.g. sulfides | |
Products characterised by the absence or the low content of sulphur | |
Sulphates per se | C01B 17/96 and subgroups |
Sulphates or sulphites of sodium, potassium or alkali metals in general | |
Lithium sulphates, sulphites | |
Preparation of sulphate metal compounds in general | |
Luminescent, e.g. electroluminescent, chemiluminescent materials containing sulphates |
This place covers:
Organic acids as starting material for making ceramics, e.g., EDTA, citrate, acetate, oxalate
Attention is drawn to the following places, which may be of interest for search:
Organo metallic additives for making ceramics |
If C04B 2235/449 is used, C04B 35/6325 does not need to be used.
This place covers:
Nitrogen is used for making a nitride, for instance a nitride powder is made by heating a metal powder in a nitrogen atmosphere. The residual metal or silicon phase of a ceramic is reacted to a nitride phase by heating in nitrogen atmosphere.
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering N2 to make Si3N4 | |
Nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. oxynitrides, carbonitrides, oxycarbonitrides, lithium nitride (Li3N), magnesium nitride (Mg3N2) | C04B 2235/3852 and subgroups |
Products characterised by the absence or the low content of nitrogen |
This place covers:
Gases other than oxygen used as reactant for making a ceramic phase, e.g. ammonia used to make a nitride phase.
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering NH3 to make Si3N4 |
This place covers:
Organic compounds that through heat-treatment from carbon, carbide, boride, nitride, silicide and in some cases even an oxide, e.g. a (poly)siloxane is converted into a silica ceramic, or phenol resin in carbon or carbide.
This place does not cover:
Carbonaceous binders for oxide ceramics, the carbonaceous binder being formed by pyrolysis of an organic | |
Impregnating a porous carbon product with organic material that is carbonised into carbon | |
Carbon ceramic obtained from polymer precursors | |
Carbon products obtained from carbonaceous particles with a carbonisable binder | |
Pyrolysis, carbonisation or auto-combustion reactions of starting materials for making ceramics | |
Bituminous additives for ceramic materials, e.g. tar, pitch |
Attention is drawn to the following places, which may be of interest for search:
Organic additives for making clay products | |
Ceramics based on oxide ceramics, containing carbon | |
Carbon based materials obtained from polymer precursors, e.g. glass-like carbon material | |
Carbon based materials obtained from carbonaceous particles with or without other non-organic components, containing a carbonisable binder | |
Silicon carbide materials obtained from Si-containing polymer precursors or organosilicon monomers | |
Silicon nitride materials obtained from Si-containing polymer precursors or organosilicon monomers | |
Using organic waste materials that become part of the ceramic, e.g. wood that is carbonised or rice bran | C04B 35/62204 and subgroups |
Organic additives for ceramic compositions | |
Organic additives that are added to the ceramic material to create porosity after a heat treatment | C04B 38/06 and subgroups |
Non-oxides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/38 and subgroups |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Metal salt constituents or additives chosen for the nature of the anions, e.g. hydrides or acetylacetonate, the salts being both organic and inorganic | C04B 2235/44 and subgroups |
The addition of organic fibers | |
Decomposition of catalysts with carbon-containing compounds into carbon |
In the case symbols from C04B 2235/48 are given, CPC symbols from C04B 35/63404-C04B 35/6365 can be given as well. For instance, phenol resin is carbonised in making a carbon ceramic. Both C04B 2235/48 and C04B 35/63476 are given.
With certain CPC groups it is obvious that a certain type of organic additive is converted into a ceramic material, since that is required for giving the class. This applies for the groups C04B 35/524, C04B 35/571, C04B 35/589 and C04B 35/83. For C04B 35/524 and C04B 35/83 a carbonisable organic has to be used, thus C04B 2235/48 does not need to be given.
If C04B 2235/48 or one of its groups symbols is given, C04B 35/6267 does not need to be given anymore, since C04B 2235/48 means that a pyrolysis has to take place.
This place covers:
pyrolysing silicone resins, (poly)silanes, (poly)siloxanes, (poly)silazanes etc.
This place does not cover:
Silicon carbide made from silicon containing polymers or pre-polymers | |
Silicon nitride made from silicon containing polymers or pre-polymers |
Attention is drawn to the following places, which may be of interest for search:
Organic silicon containing compounds used in coating ceramic substrates | C04B 41/4905 and subgroups, C04B 41/84 |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
Silicon nitrides as starting material for making a ceramic or as secondary phase of a sintered ceramic, e.g. Si3N4, silicon carbonitride or silicon oxynitride (SiON) | C04B 2235/3873 and subgroups |
Preparation of halogenated silanes | C01B 33/107 and subgroups |
polysiloxanes | C08G 77/04, C08L 83/04 and sub-classes |
For C04B 35/571 and C04B 35/589 a Si-containing organic has to be used, therefore C04B 2235/483 does not need to be given.
This place covers:
Boron-containing organic compounds becoming part of a ceramic after heat treatment, e.g. borazine, borane or boranyl
Attention is drawn to the following places, which may be of interest for search:
Boron oxide, borates, boric acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. boric acid (HBO2) | |
Borides as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/3804 and subgroups |
Boron carbide as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. B4C | |
Boron nitrides as starting material for making ceramics or as secondary phase of a sintered ceramic | |
Carbonyl compounds derived from boron hydrides | |
Boron oxyacids | C01B 35/1045 and subgroups |
Compounds containing boron and nitrogen, phosphorus, sulphur, selenium or tellurium | C01B 35/14 and subgroups |
This place covers:
These starting materials for making ceramics all have a specific shape, e.g. fiber, whisker, sphere, or have a specific size, e.g. nanosized, microsized
Attention is drawn to the following places, which may be of interest for search:
Reinforced clay wares | |
Ceramic products containing macroscopic reinforcing agents, e.g. fibers | C04B 35/71 and subgroups |
This place covers:
These starting materials for making ceramics all have a specific shape, e.g. fiber, whisker, sphere
Attention is drawn to the following places, which may be of interest for search:
Inorganic particles per se, characterised by the particle morphology | C01P 2004/00 and subgroups |
This place covers:
Non agglomerated powders, non aggregated powders, powders in which the crystallites are present as individual particles that can disperse all individually in a liquid.
This place covers:
The addition of fibers to ceramic mixtures for making a ceramic object
This place does not cover:
Making ceramic fibers per se | C04B 35/62227 and subgroups |
Mechanical aspects of shaping ceramic objects containing fibers | |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Glass-ceramic fiber compositions | |
Making carbon fibers per se |
Attention is drawn to the following places, which may be of interest for search:
Clay wares reinforced with fibers | |
Coating ceramic and carbon fibers | C04B 35/62844 and subgroups |
Ceramic material reinforced with fibers | C04B 35/71 and subgroups, e.g. C04B 35/83, C/C composites |
Coating or impregnating ceramic substrates with fibers or whiskers | |
Fibers with a defined aspect ratio | |
Fiber or whisker reinforced substrate joined with another substrate or being part of a ceramic laminate | |
Making metallic fibers per se | |
Glass fibre or filament compositions | C03C 13/00 and subgroups |
Use of inorganic fibers as ingredient for polymers | C08K 7/02 and subgroups |
Fibers of inorganic material, not being glass or ceramic |
The method of making the fibers is usually classified in D01, e.g. spinning or electro-spinning ceramic fibers.
If the making of ceramic fibers is not described but just the use of them in a ceramic composite is mentioned, C04B 35/62227 is not used, but C04B 2235/5208 and its subgroups symbols together with C04B 35/80 and it's subgroups.
The making of ceramic fibers is normally not classified in the general oxide classes C04B 35/01-C04B 35/51 or general non-oxide classes C04B 35/515-C04B 35/597, unless the fiber composition is a new composition for that material in general or in the case the synthesis contains a new aspect that would be applicable also for making a bulk ceramic, e.g. using a new combination of starting materials that also could be used to make a bulk ceramic.
This place covers:
Organic fibers, e.g. polymeric fibers, used in making a ceramic
This place does not cover:
Organic fibers added to ceramic starting mixtures in order to be removed for creating porosity | C04B 38/06 and subgroups |
Mechanical aspects of shaping ceramic objects containing organic fibers |
Attention is drawn to the following places, which may be of interest for search:
organic or organic mineral precursor fibers as filler for concrete, cement, mortar or artificial stone | |
Polymeric fibers or whiskers added as filler to concrete, cement, mortar or artificial stone | C04B 16/06 and subgroups |
Organic additives for clay mixtures | |
Cellulose as starting material for making a ceramic |
This place covers:
Inorganic fibers or whiskers, normally ceramic fibers used as starting material for making a ceramic.
This place does not cover:
Making ceramic fibers | C04B 35/62227 and subgroups |
The use of metal fibers as reinforcement of ceramics | |
Making metallic fibers per se | |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Glass fibre or filament compositions | C03C 13/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Fibrous materials and whiskers added to cement, concrete, mortar or artificial stone | C04B 14/38 and subgroups C04B 20/0048 and subgroups |
Compositions for artificial stone, not containing binders, containing fibrous materials | |
Coating ceramic and carbon fibers | C04B 35/62844 and subgroups |
Ceramic material reinforced with fibers | C04B 35/71 and subgroups, e.g. C04B 35/83, C/C composites |
Glass compositions containing a non-glass component, e.g. compositions containing fibres, filaments, whiskers, platelets, or the like, dispersed in a glass matrix |
This place covers:
All fibers or whiskers that as a material are classified in the classes C04B 35/01-C04B 35/51, e.g. magnesia, ferrite, chromite, phosphate, titania, titanate fibers, used as starting material for making a ceramic, used as starting material for making a ceramic
This place does not cover:
Making ceramic oxide fibers | C04B 35/62231 and subgroups |
The use of asbestos, glass or fused silica fibers as reinforcement for ceramics |
Attention is drawn to the following places, which may be of interest for search:
oxidic fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/46 and subgroups |
Coating fibers with oxide ceramic | C04B 35/62847 and subgroups |
The synthesis of glass fibers | C03B 37/01 and subgroups |
Use of inorganic oxygen-containing fibers as ingredient for polymers |
This place covers:
ceramic fibers or whiskers based on aluminium oxide ceramics, e.g. spinel, alumina, YAG (yttrium aluminate garnet) fibers, used as starting material for making a ceramic
This place does not cover:
The obtaining of fibers based on aluminium oxide | |
The obtaining of fibers based on alumino-silicates |
Attention is drawn to the following places, which may be of interest for search:
alumina fibers as filler for concrete, cement, mortar or artificial stone | |
Coating fibers with alumina or aluminates | |
Aluminium oxide or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. bauxite, alpha-alumina | C04B 2235/3217 and subgroups |
This place covers:
ceramic fibers or whiskers based on alumino-silicate ceramics, e.g. mullite, cordierite, kyanite, zeolite, spodumene, vermiculite, albite, anorthite fibers, used as starting material for making a ceramic
This place does not cover:
The obtaining of fibers based on aluminium oxide | |
The obtaining of fibers based on silica | |
The obtaining of fibers based on alumino-silicates |
Attention is drawn to the following places, which may be of interest for search:
alumino-silicate fibers as filler for concrete, cement, mortar or artificial stone | |
Coating fibers with silica or silicates, e.g. alumino-silicates | |
Alumino-silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. mullite (3Al2O3-2SiO2) | C04B 2235/3463 and subgroups |
This place covers:
ceramic fibers or whiskers based on silicon oxide ceramics, e.g. silica, forsterite, wollastonite fibers, used as starting material for making a ceramic
This place does not cover:
The obtaining of fibers based on silica | |
The obtaining of fibers based on alumino-silicates | |
The synthesis of silica based glass or glass-ceramic fibers | C03B 37/01 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
silica fibers as filler for concrete, cement, mortar or artificial stone | |
silicate fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/4656 and subgroups |
Coating fibers with silica or silicates, e.g. alumino-silicates | |
Silicon oxide, silicic acids, or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. silica sol, fused silica, silica fume, cristobalite, quartz or flint (glass constituents C04B 2235/36), e.g. silicic acid H2Si2O5 | |
Silicates other than clay as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. water glass (Na2SiO3) | C04B 2235/3427 and subgroups |
Use of inorganic silicon-containing fibers as ingredient for polymers | C08K 7/08 and subgroups |
This place covers:
ceramic fibers or whiskers based on zirconium oxide ceramics, e.g. zirconia, YSZ (yttria-stabilised-zirconia), zircon, zirconate, zirconate-titanates such as PZT (lead zirconate titanate) fibers, used as starting material for making a ceramic
This place does not cover:
The obtaining of fibers based on zirconia, e.g. zirconates such as PZT |
Attention is drawn to the following places, which may be of interest for search:
zirconia or zircon fibers as filler for concrete, cement, mortar or artificial stone | |
Coating fibers with refractory metal oxides | |
Zirconium or hafnium oxides or oxide forming salts thereof as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. HfO2 | C04B 2235/3244 and subgroups |
This place covers:
ceramic fibers or whiskers based on ceramics having as the largest fraction a non-oxide material, e.g. a carbide, nitride, boride, silicide, fluoride, sulphide, selenide
This place does not cover:
The obtaining of fibers based on non-oxides | C04B 35/62272 and subgroups |
The use of carbon fiber as reinforcement for a carbon matrix | |
The synthesis of carbon nanotubes | |
The synthesis of carbon fibers |
Attention is drawn to the following places, which may be of interest for search:
non-oxide fibers as filler for concrete, cement, mortar or artificial stone | C04B 14/4687 and subgroups |
Coating fibers with non-oxide ceramics | C04B 35/62828 and subgroups |
Non-oxide glass compositions for glass fibers | C03C 13/041 and subgroups |
This place covers:
ceramic fibers based on ceramics having as the largest fraction a silicon carbide phase, e.g. alpha- or beta-silicon carbide or silicon oxy-carbide, silicon carbide whiskers, sialon fibers or whiskers
This place does not cover:
The obtaining of silicon carbide based fibers or whiskers | |
The obtaining of silicon carbo-nitride based fibers or whiskers | |
Silicon carbides as starting material for making ceramics or as secondary phase of a sintered ceramic, e.g. SiC or SiOC | C04B 2235/3826 and subgroups |
The use of silicon carbo-nitride fibers in ceramic compositions |
Attention is drawn to the following places, which may be of interest for search:
silicon carbide fibers as filler for concrete, cement, mortar or artificial stone | |
Coating fibers with silicon carbide |
This place covers:
All carbon fibers used as starting material for making a ceramic, e.g. carbon, pitch, graphite fibers or whiskers
This place does not cover:
The use of carbon fiber as reinforcement for a carbon matrix | |
The use of carbon nanotubes as starting material for making a ceramics | |
The synthesis of carbon nanotubes | |
The synthesis of carbon fibers |
Attention is drawn to the following places, which may be of interest for search:
Carbon fibers or whiskers added as filler to concrete, cement, mortar or artificial stone | |
Coating fibers with carbon | |
Carbon as starting material for making ceramics or as secondary phase of a sintered ceramic | C04B 2235/422 and subgroups |
Carbon fiber or whisker reinforced substrate joined with another substrate or being part of a ceramic laminate |
This place covers:
Fibers that have been assembled into a specific form, for instance a 3D form
This place does not cover:
Fiber pre-forms used in metallic alloys | C22C 47/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures as ceramic preforms for the fabrication of metal matrix comp, e.g. cermets | C04B 2111/00913 and subgroups |
This place covers:
Fibers that have been assembled into a specific two-dimensional form, for instance by weaving or pressing
This place covers:
The length of the individual fibers is specified, e.g. 100 nm or 1 mm. Is also used for other elongated particles that are classified with C04B 2235/5276, e.g. whiskers, needles, pins, spindles.
Attention is drawn to the following places, which may be of interest for search:
Fibers with a defined aspect ratio | |
Particle size related information of particles used for making ceramics | C04B 2235/54 and subgroups |
This place covers:
The diameter of the individual fibers is specified, e.g. 100 nm or 1 mm. Is also used for other elongated particles that are classified with C04B 2235/5276, e.g. whiskers, needles, pins, spindles.
Attention is drawn to the following places, which may be of interest for search:
Fibers with a defined aspect ratio | |
Particle size related information of particles used for making ceramics | C04B 2235/54 and subgroups |
This place covers:
The fibers are for instance intentionally randomly oriented, or are oriented parallel, or are aligned to a certain degree
This place does not cover:
Fibers oriented parallel through weaving | |
Oriented fibers used in metallic alloys |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures containing oriented fibers |
This place covers:
The ceramic mixture contains different fibers of the same material, e.g. alumina fibers of 1 mm length and of 5 mm length, or carbon fibers of 100 nm thickness and of 200 nm thickness.
Attention is drawn to the following places, which may be of interest for search:
Fibrous materials and whiskers added to cement, concrete, mortar or artificial stone: mixtures of fibres of different physical characteristics, e.g. different lengths | |
Fibers with a defined aspect ratio |
This place covers:
Starting materials added to ceramics where they have an aspect ratio of greater than 1, but are not that long as to be considered a fiber, e.g. whiskers, spindles, needles, pins.
Attention is drawn to the following places, which may be of interest for search:
Whiskers added as filler to concrete, cement, mortar or artificial stone | |
Whiskers, spindles, needles, pins with a defined aspect ratio | |
Inorganic particles per se, extending in one dimension, e.g. needle-like | C01P 2004/10 and subgroups |
This place covers:
Particles of which it is mentioned that they are spherical, or that have an aspect ratio as close to 1 as possible.
This place does not cover:
addition of hollow spheres for creating porosity |
Attention is drawn to the following places, which may be of interest for search:
Spheres with a defined aspect ratio | |
Spherical metallic particles | |
Inorganic spherical particles per se | C01P 2004/32 and subgroups |
This place covers:
Fibers that are characterised by the fact that they are hollow. Most are nanotubes.
Attention is drawn to the following places, which may be of interest for search:
nanotubes used as filler in mortars, concrete or artificial stone | |
Nanotubes with a defined aspect ratio | |
metallic nanofibers or nanotubes | |
Inorganic nanotubes per se | C01P 2004/13 and subgroups |
This place covers:
All carbon nanotubes used as starting material for making ceramics, e.g. single wall nanotubes (SWNT, SWCNT), multi wall nanotubes (MWNT, MWCNT). Nanofibers often are in fact nanotubes.
Attention is drawn to the following places, which may be of interest for search:
Catalysts comprising carbon nanotubes | |
The preparation and after-treatment of carbon nanotubes | C01B 32/158 and subgroups |
Structure or properties of carbon nanotubes | C01B 2202/00 and s subgroups |
This place covers:
All particles used for making ceramics that are flat, e.g. graphite flakes
Attention is drawn to the following places, which may be of interest for search:
Minute sintered alumina entities, e.g. sintered abrasive grains or shaped particles such as platelets | |
Flakes, platelets or plates with a defined aspect ratio | |
Flake metallic powder per se | |
Inorganic particles per se, extending in two dimension, e.g. plate-like | C01P 2004/20 and subgroups |
This place covers:
All solid starting materials for making ceramics, e.g. fibers, whiskers, spindles, pins, nanotubes, spheres, flakes, platelets, that have a defined aspect ratio (or a so called D/H ratio)
Attention is drawn to the following places, which may be of interest for search:
Spherical particles used for making ceramics | |
Spherical metallic powder per se | |
Inorganic particles per se characterised by their aspect ratio, i.e. the ratio of sizes in the longest to the shortest dimension |
This place covers:
Information that relates to the size of the particles that are used for making a ceramic. Can apply both to particles with a defined shape, e.g. whiskers, platelets, pins, flakes, spheres, as to particles of which the shape is not defined.
This place does not cover:
Aspect ratio of starting material, e.g. particles, spheres, whiskers, flakes, platelets, used for making a ceramic |
Attention is drawn to the following places, which may be of interest for search:
Decreasing the particle size of starting material for making a ceramic through milling | C04B 35/6262 and subgroups |
Increasing the particle size of starting material for making a ceramic through granulation or pelletising |
This place covers:
The specific surface, for instance expressed by the BET-surface, of the particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic.
Attention is drawn to the following places, which may be of interest for search:
Surface area of inorganic powders per se | C01P 2006/12 and subgroups |
This place covers:
The particle size of particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic is specified. The code C04B 2235/5418 in principle has little use, since all possible particle size can be indicated by one of the sub-codes.
This place does not cover:
Length of fibers and other elongated particles such as whiskers and pins, used as starting material for making a ceramic | |
Width of fibers and other elongated particles such as whiskers and pins, used as starting material for making a ceramic |
Attention is drawn to the following places, which may be of interest for search:
Inorganic particles per se characterised by their size | C01P 2004/60 and subgroups |
This place covers:
The particle size of particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic is specified, where these particles can be larger than 100 microns.
This place covers:
The particle size of particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic is specified, where these particles can be in the size range of 1-100 microns.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on magnesia, the ceramic having a grain size below 100 microns (fine ceramic) | |
Ceramics based on alumina, the ceramic having a grain size below 100 microns (fine ceramic) | C04B 35/111 and subgroups |
Ceramics based on zirconia, the ceramic having a grain size below 100 microns (fine ceramic) | C04B 35/486 and subgroups |
Ceramics based on silicon nitride, the ceramic having a grain size below 100 microns (fine ceramic) | |
Inorganic particles per se characterised by their size: micrometer sized, i.e. from 1-100 micrometer |
This place covers:
The particle size of particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic is specified, where these particles can be in the size range of 100-1000 nanometers.
Attention is drawn to the following places, which may be of interest for search:
Inorganic particles per se characterised by their size: submicrometer sized, i.e. from 0.1-1 micrometer |
This place covers:
The particle size of particles, spheres, whiskers, platelets, flakes that are used as starting material for making a ceramic is specified, where these particles can be in the size range of below 100 nanometers
Attention is drawn to the following places, which may be of interest for search:
Powder used for coating or impregnating a ceramic substrate characterised by the grain distribution: nanometer sized particles | |
Nanometer sized metallic particles | |
Inorganic particles per se characterised by their size: nanometer sized, i.e. from 1-100 nanometer |
This place covers:
Information is give on how the particle size is distributed. This relates to particles of the same type only. It is mentioned how many particles of different size ranges for the same type of particle are present.
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: characterised by the grain distribution | |
Powder used for coating or impregnating a ceramic substrate characterised by the grain distribution | C04B 41/4547 and subgroups |
Size distribution of metallic particles | |
Inorganic particles per se with a specific particle size distribution | C01P 2004/51 and subgroups |
This place covers:
The document mentions that the inorganic starting materials of the same type e.g. all alumina, deliberately have different mesh sizes, such as a fraction of < 400 mesh, a fraction of 200-400 mesh and a fraction > 200 mesh, or the document mentions different particle sizes, e.g. two fractions, one with size below and one with size above 0,1 mm. A certain constituent is added with two different particle sizes, by adding for instance SiC with a size of 1 micron and SiC with a size of 10 micron. A powder is added that contains one fraction, but this fraction has a bimodal particle size distribution.
This place does not cover:
Clay powders consisting of a mixture of materials with different sizes, e.g. multi-fraction powder | |
Ceramic mixtures in which the organic additives have different size fractions | C04B 35/632 and subgroups |
Mixtures of particles having different sizes, where the different sizes result from different types of particles, e.g. a mixture of alumina of 1 micron with silica of 0,1 micron |
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: characterised by the grain distribution: fillers with bimodal grain size distribution | |
Separation of particles of different sizes through sedimentation | B01D 21/00 and subgroups |
Inorganic particles per se with a bimodal particle size distribution |
This place covers:
A certain powder consisting of one type of material, e.g. only alumina particles, only zirconia spheres, only silica sol particles, only silicon carbide whiskers, is used as starting material for making a ceramic and has a narrow distribution of the particle size.
Attention is drawn to the following places, which may be of interest for search:
Fillers added to cement, concrete, mortar or artificial stone: characterised by the grain distribution: fillers with mono- or narrow grain size distribution | |
Inorganic particles per se with a highly monodisperse size distribution |
This place covers:
It is specified that the mentioned particle size is not of the agglomerates/aggregates, as is usual, but of the individual crystals/crystallites, that possibly are assembled together into larger particles (agglomerates/aggregates).
Attention is drawn to the following places, which may be of interest for search:
Monocrystalline powder used as starting material for making a ceramic | |
Inorganic powders characterised by their crystallite size |
This place covers:
The shaping of the starting materials for making a ceramic into a certain shape, the so called green body, which is not yet heat treated into a sintered ceramic.
Mechanical aspects of the shaping of ceramics B28B
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating green ceramic bodies | C04B 41/4578 and subgroups |
This place covers:
Using a mould for shaping the starting mixture for making a ceramic into a green ceramic
This place does not cover:
The mechanical aspects of the ceramic moulding techniques |
Attention is drawn to the following places, which may be of interest for search:
Compositions of refractory mould or core materials; Grain structures thereof; Chemical or physical features in the formation or manufacture of moulds | B22C 1/00 and subgroups |
Moulds and cores and moulding processes for making metallic articles | B22C 9/00 and subgroups |
Moulds for plastic materials | B29C 33/00 and subgroups |
This place covers:
Extrusion moulding is a manufacturing process used to make pipes, hoses, drinking straws, curtain tracks, rods, and fibres. The machine used to extrude materials is very similar to an injection moulding machine. A motor turns a screw which feeds granules of plastic through a heater. The granules melt into a liquid which is forced through a die, forming a long 'tube like' shape. The shape of the die determines the shape of the tube. The extrusion is then cooled and forms a solid shape. The tube may be printed upon, and cut at equal intervals. The pieces may be rolled for storage or packed together. Shapes that can result from extrusion include T-sections, U-sections, square sections, I-sections, L-sections and circular sections.
This place does not cover:
Mechanical aspects of extruding ceramic mixtures | B28B 3/20 and subgroups, B28B 21/52 and subgroups (tubes) |
Attention is drawn to the following places, which may be of interest for search:
Extrusion of cement, concrete, mortar or artificial stone | |
Extrusion of metallic objects | B22F 3/20 and subgroups, B22F 3/227 |
Extrusion of plastics | B29C 48/00 and subgroups s |
This place covers:
a manufacturing process for producing parts using both thermoplastic and thermosetting plastic materials for the plasticity of the ceramic mixture. Material is fed into a heated barrel, mixed, and forced into a mould cavity where it cools and hardens to the configuration of the cavity. After a product is designed, usually by an industrial designer or an engineer, moulds are made by a moldmaker (or toolmaker) from metal, usually either steel or aluminum, and precision-machined to form the features of the desired part.
This place does not cover:
Mechanical aspects of injection moulding ceramic materials | B28B 1/24 (general), B28B 1/265 (ceramic slips) , B28B 21/38 (tubes) |
Attention is drawn to the following places, which may be of interest for search:
Injection moulding of cement, concrete, mortar or artificial stone | |
Making metallic articles by injection moulding | |
Making plastic articles by injection moulding | B29C 45/00 and subgroups |
This place covers:
Gel casting is a method in which a ceramic powder is mixed with a liquid in order to make a slurry. The slurry also contains organic monomers. The monomers are subsequently polymerised. Due to the formation of the polymeric network the slurry is solidified. After solidification the gel cast object is dried and sintered, thereby burning away the polymer.
This place does not cover:
sol-gel processing |
This place covers:
Tape casting is a casting process used in the manufacture of thin ceramic tapes from ceramic slurry. The feed stock for the tape casting process is a slip made from a suspension of ceramic, metal or polymer particles in an organic solvent or water, mixed together with strengthening plasticizers and/or binders. The actual tape is formed when the slip is cast onto a flat surface by doctor blade to a carrier film or steel belt.
Attention is drawn to the following places, which may be of interest for search:
Obtaining ceramics films, e.g. by using temporary support | |
Inert electrodes with catalytic activity, e.g. for fuel cells, obtained by casting, e.g. tape casting, vacuum slip casting |
If C04B 2235/6025 is given, C04B 35/62218 does not need to be given anymore.
This place covers:
All methods that use a robot system for 3D shaping, such as rapid prototyping. The use of additive manufacturing for rapid prototyping takes virtual designs from computer aided design (CAD) or animation modelling software, transforms them into thin, virtual, horizontal cross-sections and then creates successive layers until the model is complete. Some solid freeform fabrication techniques use two materials in the course of constructing parts. The methods that can be used are 3D printing, stereolithography.
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures specially adapted for three-dimensional printing (3DP), stereo-lithography or prototyping | |
using stereolithographic techniques for making dental prostheses | |
using 3D printing for making dental prostheses | |
Implantable joints made by stereolithography | |
Implantable joints made by 3D printing | |
Rapid manufacturing and prototyping of 3D objects by additive depositing, agglomerating or laminating of plastics material, e.g. by stereolithography or selective laser sintering | |
Devices or arrangements of selective printing mechanisms, e.g. ink-jet printers, thermal printers, for supporting or handling copy material in sheet or web form: for treating before, during or after printing or for uniform coating or laminating the copy material before or after printing | B41J 11/0015 and subgroups |
Photomechanical, e.g. photolithographic, production of textured or patterned surfaces, e.g. printing surfaces; Materials therefore, e.g. comprising photoresists; Apparatus specially adapted therefore | G03F 7/00 and subgroups |
This place covers:
This is where slip, liquid clay, is poured into a plaster mould. The water in the slip is drawn out of the slip, leaving an inside layer of solid clay. When this is thick enough, the excess slip can be removed from the mould. When dry, the solid clay can then also be removed. The slip used in slip casting is often liquefied with a substance that reduces the need for additional water to soften the slip; this prevents excessive shrinkage which occurs when a piece containing a lot of water dries
This place does not cover:
The slip casting of clay/porcelain mixtures | |
Mechanical features of slip-casting ceramic materials | B28B 1/26 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Making a clay slurry | |
semi-permeable membranes for separation processes made by slurry techniques, e.g. die or slip-casting | |
Slip casting metallic articles | |
Making clay or ceramic tubular articles by slip casting and moulds therefore | |
Slip casting plastics | |
Inert electrodes with catalytic activity, e.g. for fuel cells, obtained by casting, e.g. tape casting, vacuum slip casting |
This place covers:
A ceramic is shaped (partially) around for instance a polymeric object or a wax object with a certain form, possibly in a mould. The polymer or wax is removed after shaping by melting or burning, leaving the ceramic with a certain, hollow shape. The core could also be made of metal or glass, which can be melted away.
This place does not cover:
Creating porous ceramics by dissolving-out added substances | |
Creating porous ceramics by burning-out added substances by burning natural expanding materials or by sublimating or melting out added substances | C04B 38/06 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Creating a porous ceramic by dissolving-out added substances, the dissolved-out substance being a monolithic element having approximately the same dimensions as the final article, e.g. a prepreg obtained by bonding together dissolvable particles | |
Creating porous ceramics by burning-out added substances: the burned-out substance being a monolithic element having approximately the same dimensions as the final article, e.g. a porous polyurethane sheet or a prepreg obtained by bonding together resin particles | C04B 38/0615 and subgroups |
Forming ceramic laminates or joined ceramic articles comprising holes, channels or other types of openings |
This place covers:
Dry pressing of ceramic powder mixtures, possibly with heating but below the sintering temperature, either through uniaxial pressing (pressing from one side) or isostatic pressing (pressing from all sides).
Presses in general B30B
This place does not cover:
Pressing of clay mixtures | |
Pressing a starting mixture for making a ceramic in an injection moulding machine | |
Mechanical aspects of pressing ceramic materials | B28B 3/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Pressing of clay mixtures at sintering temperature | |
Granulation or pelletising of starting material for making a ceramic | |
Pressing of ceramic mixtures at sintering temperature | C04B 35/645 and sub-class |
Making metallic articles by compacting | B22F 3/02 and subgroups |
Press moulds and press-ram assemblies for shaping clay or other ceramic compositions |
This place covers:
Pressing, moulding, casting or using any other shaping technique while at the same time a magnetic field is applied to the mixture that is being shaped, in order to influence the material that is being shaped.
This place does not cover:
Manufacturing of magnetic circuits by moulding or by pressing powder | |
manufacturing permanent magnets by Moulding; Pressing |
This place covers:
Drying of shaped ceramic bodies, e.g. green ceramics, moulded bodies, cast ceramic bodies.
Drying solid materials or objects by removing liquid therefrom F26B
This place does not cover:
Drying clay or porcelain powder mixtures or clay green bodies | |
Drying, e.g. freeze-drying, spray-drying, microwave or supercritical drying of powder mixtures, slurries | |
Curing of starting mixtures for making ceramics or of green bodies | |
Mechanical aspects of drying a green ceramic body |
Attention is drawn to the following places, which may be of interest for search:
involving the removal of at least part of the materials of the treated article, e.g. etching, drying of hardened concrete | C04B 41/53 and subgroups |
processing clay- or ceramic containing substances in non-fluid condition by heating, drying | |
Surface treatment of glass not in the form of fibres or filaments: drying; dehydroxylation |
This place covers:
The density of the green body (the green density) is specified, or the density of a pre-form is mentioned.
This place does not cover:
Intentionally porous ceramics | C04B 38/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Density of sintered ceramics | C04B 2235/77 and subgroups |
This place covers:
At least one (or more) mechanical property of the green ceramic body, or ceramic pre-form, such as a fiber form, is measured and mentioned. This can be the strength, e.g. bending or compressive strength, the toughness, hardness, the stiffness determined by the modulus of elasticity, etc.
Attention is drawn to the following places, which may be of interest for search:
Mechanical properties of sintered ceramics |
This place covers:
All methods that lead to the removal of at least a part of the green body or preform, while leaving the green body or perform at a smaller size, e.g. grinding or polishing to smoothen the surface, cutting or grinding the green body into different parts.
This place does not cover:
Destroying a green body or pres-sintered ceramic by milling | |
Cutting ceramic objects | B28B 11/12 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramic products characterised by their shape | C04B 2235/94 and subgroups |
Ceramic products characterised by their size |
This place covers:
Gas phase techniques, such as CVD (chemical vapour deposition), PVD (physical vapour deposition) are used to infiltrate a porous green body, a preform, a fiber body, a partially sintered ceramic, in principle any ceramic body that still has open porosity, and leave a deposit inside the open pores.
Attention is drawn to the following places, which may be of interest for search:
Coating of inorganic particles or of fibers applied by a gas phase technique | |
Porous ceramics in general | C04B 38/00 and subgroups |
Coating or impregnating a ceramic substrate applied from the gas phase | C04B 41/4529 and subgroups |
Non-superficial impregnation or infiltration of the substrate | |
Gases other than oxygen used as reactants, e.g. nitrogen | C04B 2235/46 and subgroups |
Inorganic membrane formation by deposition from the gaseous phase, e.g. sputtering, CVD, PVD | |
PVD, CVD methods or coating in a gas-phase using a fluidized bed of preparing the interference pigments | |
Coating metallic substrates by chemical coating by decomposition of gaseous compounds, without leaving reaction products of the surface material in the coating, e.g. chemical vapour deposition (CVD) processes | C23C 16/00 and subgroups |
This place covers:
Infiltrating a porous green body, a preform, a fiber body, a partially sintered ceramic, in principle any ceramic body that still has open porosity with for instance liquid silicon, or with a solution of organic carbonisable material, or with carbonisable liquid polymer and leave a deposit inside the open pores.
Making ceramic powders by gas phase techniques C01
This place does not cover:
Ceramics based on carbon, made by impregnation of a carbon product with carbonisable material | |
Joining two substrates of which at least one is porous by infiltrating the porous substrate with a liquid, such as a molten metal, causing bonding of the two substrates, e.g. joining two porous carbon substrates by infiltrating with molten silicon |
Attention is drawn to the following places, which may be of interest for search:
Coating of inorganic particles or of fibers applied by wet chemical techniques | |
Porous ceramics in general | C04B 38/00 and subgroups |
Coating or impregnating a ceramic substrate applied as a solution, emulsion, dispersion or suspension | C04B 41/4535 and subgroups |
Non-superficial impregnation or infiltration of the substrate | |
Chemically coating metallic substrates by decomposition of either liquid compounds or solutions | C23C 18/00 and s subgroups |
Chemically coating metallic substrates by decomposition of either solid compounds or suspensions | C23C 20/00 and subgroups |
This place covers:
All heat treatments of ceramic green bodies, already sintered ceramics, joining treatment of a ceramic body that is joined with another body
Furnaces, kilns, ovens, or retorts F27
This place does not cover:
Drying of ceramic powders | |
Curing of starting mixtures for making ceramics or of green bodies | |
Superficial sintering of ceramic objects with the goal of creating a porous object | C04B 38/0038 and subgroups |
Mechanical aspects of the heat treatments |
Attention is drawn to the following places, which may be of interest for search:
Heat treatment, e.g. precalcining, burning, melting; Cooling of hydraulic cements | C04B 7/43 and subgroups |
Burning methods for clay wares | C04B 33/32 and subgroups |
Heat treatments of ceramic powders | C04B 35/62645 and subgroups |
Removing organic binders from a shaped green ceramic by burning them out | |
Sintering methods for shaped ceramic materials | C04B 35/64 and subgroups |
Making ceramic materials by melting | C04B 35/653 and subgroups |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: making use of a rise in temperature, e.g. caused by an exothermic reaction | |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: heating up to sintering temperatures | |
After-treatment of mortars, concrete, artificial stone or ceramics: Heat treatment | |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
Sintering glass | C03B 19/06 and subgroups |
Shaft or like vertical or substantially vertical furnaces wherein no smelting of the charge occurs, e.g. calcining or sintering furnaces |
C04B 35/64 generally does not need to be given, since the subgroups of C04B 2235/65 can give more specific information, while C04B 35/64 is very general.
The symbols from the range C04B 2235/65-C04B 2235/668 are used for binder burnout, pre-sintering, sintering, melting, annealing.
This place covers:
The shaped ceramic material is reduced, for instance by using carbon as reducer, or by heating in a reducing atmosphere (e.g. hydrogen, argon, nitrogen, carbon monoxide), e.g. reducing an oxide to a carbide, or reducing a titanate by heating in hydrogen atmosphere.
This place does not cover:
Reduction or oxidation treatment of starting mixture or components of the starting mixture used for making ceramics | |
Reductive annealing of shaped ceramics |
Attention is drawn to the following places, which may be of interest for search:
coating or impregnating with a product reacting with the substrate, e.g. generating a metal coating by surface reduction of a ceramic substrate | |
Hydrogen containing atmosphere during thermal treatment of green, sintered or melted ceramic |
This place covers:
The sintering into a shaped ceramic at a certain specific temperature. The temperature at which the heat treatment is performed is of particular importance and is claimed in the claims, or the heating temperature is varied in the examples.
This place does not cover:
Heat treatments of starting mixtures for making ceramics, characterised by the treatment temperature | |
Curing of starting mixtures for making ceramics or of green bodies |
Attention is drawn to the following places, which may be of interest for search:
Burning methods for clay-wares | C04B 33/32 and subgroups |
Binder burnout | |
Burning or sintering processes for ceramics | C04B 35/64 and subgroups |
Multi step sintering | C04B 2235/661 and subgroups |
Mechanical aspects of sintering clay or ceramic objects |
This place covers:
The heating rate of the binder burnout, pre-sinter step, sinter step, melting step, annealing step, is of particular importance, e.g. heating slowly with 1°C/hour or heating fast with 100°C/min.
This place covers:
The cooling rate of the binder burnout, pre-sinter step, sinter step, melting step, annealing step, is of particular importance, e.g. cooling slowly with 1°C/hour or cooling fast by direct quenching in water.
Attention is drawn to the following places, which may be of interest for search:
Cooling of a ceramic, e.g. freezing: In this group the term "cooling" is used in the sense of an additional cooling treatment, different from the traditional cooling step in the fabrication of materials involving a heating step, such as sintering of ceramics | |
After treatment of ceramics: heat treatment characterised by the subsequent cooling step | |
Cooling rate after sintering metallic objects |
This place covers:
The treatment time of the binder burnout, pre-sinter step, sinter step, melting step, annealing step, is of particular importance, e.g. heating shortly during only 5 minutes or heating for a long time during for instance 1 day.
This place covers:
Using a pressurised atmosphere during binder burnout, pre-sinter step, sinter step, melting step or annealing step, e.g. an atmosphere of 2 bar nitrogen, or if a specific gas is used, such as hydrogen, water, carbon monoxide, carbon dioxide, or if an unexpected atmosphere is used, e.g. heat treating oxide material in nitrogen or argon or heat treating non-oxide material in air.
This place does not cover:
Heat treatments of starting mixtures for making ceramics, characterised by the applied pressure of type of atmosphere | |
Sintering using pressure | C04B 35/645 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
application of coatings on ceramics under inert, e.g. non-oxidising, atmosphere | |
application of coatings on ceramics under another specific atmosphere | |
Atmosphere during sintering of metallic articles |
This place covers:
The atmospheric pressure during binder burnout, pre-sinter step, sinter step, melting step or annealing step is reduced to below 1 atmosphere, e.g. to a vacuum, for instance with vacuum sintering, or to facilitate the burning away of the organic binder during binder burnout.
Attention is drawn to the following places, which may be of interest for search:
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: hardening under vacuum or reduced pressure | |
Processes in general utilising sub-atmospheric pressure; Apparatus therefore |
This place covers:
The atmosphere during binder burnout, pre-sinter step, sinter step, melting step or annealing step contains hydrogen (H2) gas, for instance in order to create a reducing atmosphere.
Attention is drawn to the following places, which may be of interest for search:
Reduction treatment of the ceramic body during binder burnout, pre-sinter step, sinter step or melting step | |
Reductive annealing of shaped ceramics |
This place covers:
The oxygen partial pressure is of particular importance, for instance because it is varied during the heat treatment in order to create a more reducing or a more oxidising atmosphere.
This place covers:
The oxygen percentage is below 20%, i.e. below a partial pressure of 0.2 atmosphere, for instance to create a reducing atmosphere.
This place covers:
The oxygen percentage is above 20%, i.e. above a partial pressure of 0.2 atmosphere, for instance to create an oxidising atmosphere.
This place covers:
The atmosphere during binder burnout, pre-sintering, sintering, melting or annealing is not static. A gas (e.g. nitrogen oxygen, argon) is passed through the heating furnace.
This place covers:
If a ceramic is heated that contains an oxide that can vaporise (e.g. lead or bismuth oxide), the ceramic to be heat treated is surrounded by a powder or other solid of the fugitive oxide, e.g. a lead oxide containing powder. The vaporising of the lead oxide from the sacrificial powder reduces or even prevents the vaporising of the lead oxide of the to-be-sintered ceramic.
This place covers:
The atmosphere during binder burnout, pre-sinter step, sinter step, melting step or annealing step contains water vapour.
This place covers:
Specific sintering methods not covered by any of the subgroups, such as using enhanced gravity during sintering
Furnaces, kilns, ovens, or retorts F27
This place does not cover:
Heat treatments of non-shaped powders that are used for making ceramics | C04B 35/62645 and subgroups |
Curing of starting mixtures for making ceramics or of green bodies | |
Mechanical aspects of sintering clay or ceramic objects | |
chamber type furnaces | |
Travelling or movable supports or containers for the charge of furnaces, kilns, ovens, retorts in so far as they are of kinds occurring in more than one kind of furnace |
Attention is drawn to the following places, which may be of interest for search:
Burning or sintering processes of clay products | |
Removing organic binders from a shaped green ceramic by burning them out | |
Burning or sintering processes of ceramic or refractory products | |
Making ceramic materials by melting | C04B 35/653 and subgroups |
Heat treatments such as] Calcining; Fusing Pyrolysis in general | B01J 6/00 and subgroups |
Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; Apparatus specially adapted therefore; Presses and furnaces | B22F 3/00 and subgroups |
Manufacture of composite layers, workpieces, or articles, comprising metallic powder, by sintering the powder, with or without compacting wherein at least one part is obtained by sintering or compression | B22F 7/00 and subgroups |
Sintering glass | C03B 19/06 and subgroups |
This place covers:
The sintering (or melting process) consists out of more than one step, after a possible binder burnout step. The process encompasses for instance a pre sintering step and a subsequent pressure sintering step, e.g. a hot isostatic pressing step, or a sintering step and a subsequent annealing step. The process can also be first a melting step and then a sintering step.
This place does not cover:
First a binder burnout step and then a sintering step |
Attention is drawn to the following places, which may be of interest for search:
Multiple heating or additional steps during the sintering of metallic articles |
This place covers:
After the main sintering step at the highest temperature of the heating process the ceramic is heated at a lower temperature for a prolonged period of time, for instance to reduce the stress in the material.
Attention is drawn to the following places, which may be of interest for search:
After treatment of ceramics: heat treatment | |
Annealing glass products | C03B 25/00 and subgroups |
This place covers:
After the main sintering step at the highest temperature of the heating process the ceramic is heated at a lower temperature for a prolonged period of time, with the effect of oxidising the sintered ceramic.
This place does not cover:
Reduction or oxidation treatment of starting mixture or components of the starting mixture used for making ceramics | |
oxidative annealing for making a coating layer | C04B 41/45 and subgroups |
Oxidising the surface of a substrate that is joined with a ceramic substrate before joining |
Attention is drawn to the following places, which may be of interest for search:
Atmosphere during the heat treatment enriched in oxygen content to above the level normal in air |
This place covers:
After the main sintering step at the highest temperature of the heating process the ceramic is heated at a lower temperature for a prolonged period of time, with the effect of reducing the sintered ceramic.
This place does not cover:
Reduction or oxidation treatment of starting mixture or components of the starting mixture used for making ceramics | |
reductive annealing for making a coating layer | C04B 41/45 and subgroups |
Reduction treatment of the ceramic body during binder burnout, pre-sinter step, sinter step or melting step |
Attention is drawn to the following places, which may be of interest for search:
Hydrogen containing atmosphere during thermal treatment of green, sintered or melted ceramic | |
Atmosphere during the heat treatment reduced in oxygen content to below the level normal in air |
This place covers:
A ceramic is not sintered completely at the same moment, first one part is sintered, then possibly another and another and so on, until either the whole ceramic is sintered, or the process is stopped and a part is left unsintered. This can for instance be done with a laser that scans the surface of the sintered, and sinters part by part.
This place does not cover:
After treatment of ceramics by laser beam |
Attention is drawn to the following places, which may be of interest for search:
Selective sintering of metallic powders, i.e. stereolithography | |
working by laser beam | B23K 26/00 and subgroups |
using layers of powder being selectively joined, e.g. by selective laser sintering or melting |
This place covers:
Spark plasma sintering (SPS), also known as field assisted sintering technique (FAST) or pulsed electric current sintering (PECS), is a sintering technique. The main characteristic of SPS is that the pulsed DC current directly passes through the graphite die, as well as the powder compact, in case of conductive samples. Therefore, the heat is generated internally, in contrast to the conventional hot pressing, where the heat is provided by external heating elements. This facilitates a very high heating or cooling rate (up to 1000 K/min), hence the sintering process generally is very fast (within a few minutes). The general speed of the process ensures it has the potential of densifying powders with nanosize or nanostructure while avoiding coarsening which accompanies standard densification routes. Whether plasma is generated has not been confirmed yet, especially when non-conductive ceramic powders are compacted. It has, however, been experimentally verified that densification is enhanced by the use of a current or field.
Electrical Resistance Heating (ERH) is a method that uses the flow of alternating current electricity to the ceramic. Electric current is passed through a targeted ceramic. The resistance to electrical flow that exists in the ceramic causes the formation of heat, resulting in an increase in temperature up to the desired sintering temperature.
This place does not cover:
After treatment of ceramics with plasma |
Attention is drawn to the following places, which may be of interest for search:
Pressing clay at sintering temperatures | |
Pressure sintering to make silicon carbide based ceramics | |
Pressure sintering to make silicon nitride based ceramics | |
Flame, melting or plasma treatment of powders used for making ceramics | |
Pressing at sintering temperatures of ceramic or refractory mixtures | C04B 35/645 and subgroups |
Sintering metallic powder by using electric current other than for infrared radiant energy, laser radiation or plasma | |
Heating by electric, magnetic, or electromagnetic fields | H05B 6/00 and subgroups |
SPS is normally performed using pressure, which means it should in theory also be classified in C04B 35/645. This is only done, however, if it is specified which pressure has been used. If the sintering pressure is not specified, only C04B 2235/666 is used.
This place covers:
Heating making use of for instance IR, UV or microwaves.
Dielectric heating, also known as electronic heating, RF heating, high-frequency heating and diathermy, is the process in which a high-frequency alternating electric field, or radio wave or microwave electromagnetic radiation heats a dielectric material. At higher frequencies, this heating is caused by molecular dipole rotation within the dielectric. At lower frequencies in conductive fluids, other mechanisms such as ion-drag are more important in generating thermal energy.
Microwaves are used for heating of various materials in cooking and various industrial processes. The rate of heating of the material depends on the energy absorption, which depends on the dielectric constant of the material. The dependence of dielectric constant on temperature varies for different materials; some materials display significant increase with increasing temperature. This behaviour, when the material gets exposed to microwaves, leads to selective local overheating, as the warmer areas are better able to accept further energy than the colder areas—potentially dangerous especially for thermal insulators, where the heat exchange between the hot spots and the rest of the material is slow. These materials are called thermal runaway materials. This phenomenon occurs in some ceramics.
Electrobeam heating.
Attention is drawn to the following places, which may be of interest for search:
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone composition: making use of electric or wave energy or particle radiation | C04B 40/0003 with subgroups |
Processes, in general, for influencing or modifying the properties of mortars, concrete or artificial stone compositions: Selection of the hardening environment: making use of electric or wave energy or particle radiation | C04B 40/0204 and subgroups |
Melting in electric furnaces by microwave heating | |
Heating by electric, magnetic, or electromagnetic fields: microwave heating | H05B 6/64 and subgroups |
This place covers:
If a certain material that normally is sintered using pressure, since otherwise it is difficult to sinter, is sintered without pressure, this code is give. This applies specially to non-oxide materials, such as silicon carbide or silicon nitride. If a document stresses that a material is sintered without pressure, this code can be given.
Attention is drawn to the following places, which may be of interest for search:
Pressing clay at sintering temperatures | |
Pressure sintering to make silicon carbide based ceramics | |
Pressure sintering to make silicon nitride based ceramics | |
Pressure sintering of ceramics in general | C04B 35/645 and sub-class |
This place covers:
Compositional aspects of the produced ceramic, e.g. the presence of impurities, the crystal structure of the sintered product, the density, the microstructure, the presence of secondary phases.
Shape and size of the ceramic end product and the properties of the ceramic end product.
This place covers:
Materials that have a specified amount of certain impurities or certain undesired phases, materials that are specifically free of certain metal oxides, e.g. titanates or niobates that are lead-free. This code is used for all metal ions that are bound either to oxygen (oxides), carbon (carbides), boron (borides), nitrogen (nitrides) or silicon (silicides). It is also for bound silicon, e.g. low silica, silicon carbide, silicon nitride.
This place does not cover:
Eliminating lime or iron from clay mixtures |
Attention is drawn to the following places, which may be of interest for search:
Unintentionally added compounds, such as impurities in raw materials, e.g. alkali sulphates in construction grade cement | |
Cement, concrete, mortar or artificial stone mixtures characterised by the absence or the very low content of a specific material | C04B 2111/10 and subgroups |
Pure silica glass, e.g. pure fused quartz: impurity concentration specified | |
Doped silica-based glasses: impurity concentration specified |
This place covers:
A ceramic product being free of free carbon (C) or having a low content in free carbon.
This place does not cover:
A ceramic product being free of bound carbon or having a low content in bound carbon, e.g. a low content in carbides or carbon nitride. |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by the absence or the very low content of a specific material: Carbon free or very low carbon content fly ashes; Fly ashes treated to reduce their carbon content or the effect thereof | C04B 2111/1087 and subgroups |
This place covers:
A ceramic product being free of undesired nitrogen compounds or having a low content in undesired nitrogen compounds, e.g. a low content in nitrides.
This place covers:
A ceramic product being free of undesired oxygen compounds or having a low content in undesired oxygen compounds, while possibly containing a desired oxygen compound, for instance a silicon carbide, containing yttria (Y2O3) sintering aid, being characterised by the fact that it does not contain any further oxygen than the oxygen present in the yttria.
This place covers:
A ceramic product characterised by the fact it contains a low amount of undesired halogenide compounds, while possibly containing also one or more desired halogenide compounds, e.g. an alumina ceramic containing less than 1% fluoride.
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by the absence or the very low content of a specific material: halogen free or very low halogen-content materials | C04B 2111/1062 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
halogenide | fluoride, chloride, bromide, iodide |
This place covers:
A ceramic being characterised by the fact that it contains a low amount or even no metal phase, e.g. a sintered diamond compact that is free of cobalt metal, or a sintered tungsten carbide compact being free of nickel binder.
This place does not cover:
A ceramic being characterised by the fact that it contains a low amount of metal oxide, metal carbide, metal nitride, metal boride or metal silicide. |
This place covers:
A ceramic containing a low amount of sulphur compounds or low amount of elemental sulphur, e.g. less than 1% sulphate (SO42-), sulphite (SO32-), sulphide (S2-)
This place covers:
A ceramic containing a low amount of phosphor compounds or low amount of elemental phosphor, e.g. less than 1% phosphate, phosphide, phosphor
This place covers:
Ceramics having a low content in unbound silicon, e.g. a silicon carbide made by reaction sintering of carbon and silicon, containing less than 1% residual unreacted silicon.
This place does not cover:
A ceramic having a low silica (SiO2) content |
This place covers:
Characteristics such as the crystal structure, density, microstructure of the sintered product.
This place covers:
Ceramics products that have a gradient in the composition of the product of at least one of the components, for instance an alumina-zirconia ceramic that on one side has 20% alumina and 80% zirconia, and on the other side of the product 80% alumina and 20% zirconia, while in between there is a gradient from one side to the other.
This place does not cover:
Ceramics products with a gradient in the density | |
Ceramic products with a gradient in the composition, where this gradient results from joining ceramic layers that have different compositions | C04B 2237/58 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures with a gradually increasing or decreasing concentration of ingredients or property from one layer to another |
This place covers:
The ceramic product having a specific lattice system, e.g. triclinic, monoclinic, orthorombic or rhomohedral, or a specific crystal system such as trigonal (see below for the relation between lattice system and crystal system), or a specific crystal structure, e.g. perovskite, garnet, spinel
Attention is drawn to the following places, which may be of interest for search:
Three-dimensional structures of inorganic powders |
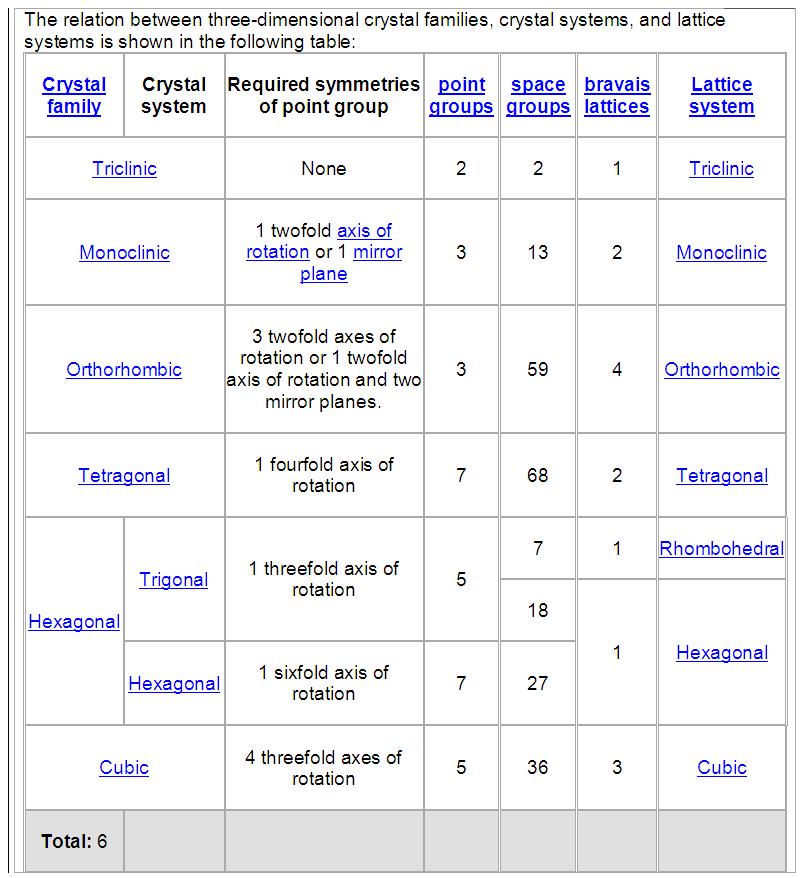
This place covers:
At least one of the lattice constants of the ceramic material is defined. The lattice constant [or lattice parameter] refers to the constant distance between unit cells in a crystal lattice. Lattices in three dimensions generally have three lattice constants, referred to as a, b, and c. However, in the special case of cubic crystal structures, all of the constants are equal and we only refer to a. Similarly, in hexagonal crystal structures, the a and b constants are equal, and we only refer to the a and c constants. A group of lattice constants could be referred to as lattice parameters. However, the full set of lattice parameters consist of the three lattice constants and the three angles between them.
For example the lattice constant for a common carbon diamond is a = 3.57Å at 300 K. The structure is equilateral although its actual shape cannot be determined from only the lattice constant. Furthermore, in real applications, typically the average lattice constant is given. As lattice constants have the dimension of length, their SI unit is the meter. Lattice constants are typically on the order of several angstroms (i.e. tenths of a nanometre). Lattice constants can be determined using techniques such as X-ray diffraction or with an atomic force microscope.
Attention is drawn to the following places, which may be of interest for search:
Inorganic powders defined by unit-cell parameters, atom positions or structure diagrams |
This place covers:
Ceramics having a cubic lattice or crystal system. In crystallography, the cubic (or isometric) crystal system is a crystal system where the unit cell is in the shape of a cube. This is one of the most common and simplest shapes found in crystals and minerals.
There are three main varieties of these crystals, called simple cubic (sc), body-centered cubic (bcc), and face-centered cubic (fcc, also known as cubic close-packed or ccp), plus a number of other variants listed below. Note that although the unit cell in these crystals is conventionally taken to be a cube, the primitive unit cell often is not. This is related to the fact that in most cubic crystal systems, there is more than one atom per cubic unit cell.
This place does not cover:
Cubic boron nitride ceramics |
The symbol only needs to be given if it is not standard that the material is cubic, e.g. perovskites can be either cubic, tetragonal or orthorombic, therefore it is not standard that the perovskite is cubic.
If a ceramic material has beta-SiC as the main phase, C04B 2235/762 is given to indicate that the silicon carbide is in the beta form.
This place covers:
The spinels are any of a class of minerals of general formulation A2+B23+O42- which crystallise in the cubic (isometric) crystal system, with the oxide anions arranged in a cubic close-packed lattice and the cations A and B occupying some or all of the octahedral and tetrahedral sites in the lattice. A and B can be divalent, trivalent, or quadrivalent cations, including magnesium, zinc, iron, manganese, aluminium, chromium, titanium, and silicon. Although the anion is normally oxide, the analogous thiospinel structure includes the rest of the chalcogenides. A and B can also be the same metal under different charges, such as the case in Fe3O4 (as Fe2+Fe23+O42-). The main groups of spinels are aluminate spinels, ferrite spinels and chromite spinels.
This place does not cover:
Magnesium aluminate spinel ceramics |
Attention is drawn to the following places, which may be of interest for search:
Magnesium aluminate spinel ceramics | |
Coating or impregnating ceramic substrates with spinels | |
Spinel catalysts | |
Oxide powders with spinel symmetry | |
Single crystals of complex oxides with formula BMe2O4, wherein B is Mg, Ni, Co, Al, Zn, or Cd and Me is Fe, Ga, Sc, Cr, Co, or Al |
If the class C04B 35/443 is given, C04B 2235/763 does not need to be given.
This place covers:
The crystallographic structure of garnets has been expanded from the silicon containing prototype to include chemicals with the general formula A3B2(CO4)3. Besides silicon, a large number of elements have been put on the C site, including Ge, Ga, Al, V and Fe. Yttrium aluminium garnet (YAG), Y3Al2(AlO4)3, is used for synthetic gemstones. When doped with neodymium (Nd3+), these YAl-garnets may be used as the lasing medium in lasers. In yttrium iron garnet (YIG), Y3Fe2(FeO4)3, the five iron(III) ions occupy two octahedral and three tetrahedral sites, with the yttrium(III) ions coordinated by eight oxygen ions in an irregular cube. The iron ions in the two coordination sites exhibit different spins, resulting in magnetic behaviour. YIG is a ferrimagnetic material having a Curie temperature of 550 K. All garnets have cubic symmetry.
Attention is drawn to the following places, which may be of interest for search:
Other ferrites containing rare earth metals, e.g. rare earth ferrite garnets | |
Single crystals of complex oxides with formula A3Me5O12 wherein A is a rare earth metal and Me is Fe, Ga, Sc, Cr, Co or Al, e.g. garnets | |
Soft magnetic material, e.g. ferrite garnets | |
Thin magnetic films, e.g. of one-domain structure made of garnet ferrites | H01F 10/24 and subgroups |
This place covers:
Ceramics having a cubic lattice or crystal system. In crystallography, the tetragonal crystal system is one of the 7 lattice point groups. Tetragonal crystal lattices result from stretching a cubic lattice along one of its lattice vectors, so that the cube becomes a rectangular prism with a square base (a by a) and height (c, which is different from a).
There are two tetragonal Bravais lattices: the simple tetragonal (from stretching the simple-cubic lattice) and the centered tetragonal (from stretching either the face-centered or the body-centered cubic lattice).
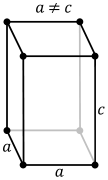
The code only needs to be given if it is not standard that the material is tetragonal, e.g. perovskites can be either cubic, tetragonal or orthorombic, therefore it is not standard that the perovskite is tetragonal.
This place covers:
Ceramics having a trigonal crystal system. In crystallography, the trigonal crystal system is one of the seven crystal systems, and the rhombohedral lattice system is one of the seven lattice systems. They are often confused with each other: crystals in the rhombohedral lattice system are always in the trigonal crystal system, but some crystals such as quartz are in the trigonal crystal system but not in the rhombohedral lattice system. The rhombohedral lattice system consists of the rhombohedral lattice, while the trigonal crystal system consists of the five point groups of the seven space groups with a rhombohedral lattice. There are 25 space groups whose point groups are one of the five in the trigonal crystal system, consisting of the seven space groups associated with the rhombohedral lattice system together with 18 of the 45 space groups associated with the hexagonal lattice system.
The trigonal crystal system is the only crystal system whose point groups have more than one lattice system associated with their space groups: the hexagonal and rhombohedral lattices both appear.
Unit cells for trigonal crystal system:
Rhombohedral
Hexagonal

If a ceramic material has alpha silicon nitride or alpha sialon as the main phase, C04B 2235/766 is given to indicate that the silicon nitride or sialon is in the alpha form.
This place covers:
Ceramics having a tetragonal lattice or crystal system. In crystallography, the hexagonal crystal system is one of the 7 crystal systems, the hexagonal lattice system is one of the 7 lattice systems, and the hexagonal crystal family is one of the 6 crystal families. They are closely related and often confused with each other, but they are not the same. The hexagonal lattice system consists of just one Bravais lattice type: the hexagonal one. The hexagonal crystal system consists of the 7 point groups such that all their space groups have the hexagonal lattice as underlying lattice. The hexagonal crystal family consists of the 12 point groups such that at least one of their space groups has the hexagonal lattice as underlying lattice, and is the union of the hexagonal crystal system and the trigonal crystal system. Graphite is an example of a crystal that crystallizes in the hexagonal crystal system.
Attention is drawn to the following places, which may be of interest for search:
Soft magnetic material, e.g. Hexaferrites with decreased hardness or anisotropy, i.e. with increased permeability in the microwave (GHz) range | |
Thin magnetic films, e.g. of one-domain structure made of hexagonal ferrites | |
LaMgAl11O19 (LNA, Lanthanum Magnesium Hexaluminate) used for lasers |
If a ceramic material has alpha SiC, beta silicon nitride or beta sialon as the main phase, C04B 2235/767 is given to indicate that the silicon nitride or sialon is in the beta form, or the silicon carbide is in the alpha form.
This place covers:
A perovskite structure is any material with the same type of crystal structure as calcium titanium oxide (CaTiO3), known as the perovskite structure, or XIIA2+VIB4+X2−3 with the oxygen in the face centers. The general chemical formula for perovskite compounds is ABX3, where 'A' and 'B' are two cations of very different sizes, and X is an anion that bonds to both. The 'A' atoms are larger than the 'B' atoms. The ideal cubic-symmetry structure has the B cation in 6-fold coordination, surrounded by an octahedron of anions, and the A cation in 12-fold cuboctahedral coordination. The perovskite structure is adopted by many oxides that have the chemical formula ABO3. The perovskite can be either cubic, orthorombic or tetragonal.
This place does not cover:
Ceramics based on barium titanate perovskite | C04B 35/4682 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Catalysts comprising metals or metal oxides or hydroxides: Mixed oxides other than spinels, e.g. perovskite | |
Oxide powders with perovskite symmetry | |
Single crystals of complex oxides with formula AMeO3, wherein A is a rare earth metal and Me is Fe, Ga, Sc, Cr, Co or Al, e.g. orthoferrites |
If the presence of a perovskite structure is inherent due to the class that is given, this symbol does not need to be given. This is the case with C04B 35/4682 and C04B 35/4684, barium titanate perovskites.
If the perovskite is cubic or tetragonal, C04B 2235/762 or C04B 2235/765 are given, respectively. If the perovskite is orthorombic, C04B 2235/76 is given.
This place covers:
The density of the pre-sintered, sintered or melted ceramic is indicated, either as the theoretical density, e.g. 99% dense, or as the absolute density, e.g. 5.0 g/cm3.
This place does not cover:
Ceramics characterised by the porosity | |
Density of green ceramic |
Attention is drawn to the following places, which may be of interest for search:
Density of cement, concrete, mortar or artificial stone | |
Solid density of inorganic powders per se |
This place covers:
The ceramic product has a gradient in the theoretical density, one side has a higher density than the other, e.g. one side is 95% dense, the other side 90%.
This place does not cover:
Ceramics products with a gradient in the composition | |
Forming a gradient in composition or in properties across the laminate or the joined articles by joining layers or articles of the same composition but having different densities | C04B 2237/58 and subgroups |
If a ceramic has a gradient in the composition, it automatically will have a gradient in the absolute density as well, unless the two different phases have the same specific density, which will be rarely the case. The gradient therefore has to be in the theoretical density, since the theoretical density does not depend on the composition.
This place covers:
The sintered (or melted) ceramic has a specific microstructure, a certain grain, certain grain shapes, etc.
Attention is drawn to the following places, which may be of interest for search:
Constituents or additives for ceramic mixtures characterised by their shapes | C04B 2235/52 and subgroups |
The symbol C04B 2235/78 is given if the grain size is higher than 100 microns and if the microstructure is being shown, e.g. in SEM pictures or TEM pictures.
This place covers:
The average grain size of the sintered (or melted) ceramic is below 100 nanometers.
Attention is drawn to the following places, which may be of interest for search:
Particles or aggregates of a component of the ceramic starting mixture have an average particle size of below 100 nanometers |
This place covers:
Information is give on how the grain size is distributed. This relates to particles of the same type only. It is mentioned how many grains of different size ranges for the same type of grain are present.
Attention is drawn to the following places, which may be of interest for search:
Particle size distribution of an individual component (of the same material) of the starting mixture for making a ceramic |
This place covers:
The grains of the sintered (or melted) ceramic do not have a uniform grain size distribution, there is at least one type of grains with a smaller size and one type of grains with a larger size.
Attention is drawn to the following places, which may be of interest for search:
Clay powders consisting of a mixture of materials with different sizes, e.g. multi-fraction powder | |
Particle size distribution of an individual component (of the same material) of the starting mixture for making a ceramic being bimodal, multi-modal or multi-fraction |
This place covers:
The grains of the sintered (or melted) ceramic have a uniform grain size distribution, at least as so far the grains belong to the same phase.
Attention is drawn to the following places, which may be of interest for search:
Particle size distribution of an individual component (of the same material) of the starting mixture for making a ceramic being monomodal. |
This place covers:
The average grain size of the sintered (or melted) ceramic is in the range of 0.1-1 microns.
Attention is drawn to the following places, which may be of interest for search:
Particles or aggregates of a component of the ceramic starting mixture have an average particle size of 0.1-1 micron |
This place covers:
The average grain size of the sintered (or melted) ceramic is in the range of 1-100 microns.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on magnesia, the ceramic having a grain size below 100 microns (fine ceramic) | |
Ceramics based on alumina, the ceramic having a grain size below 100 microns (fine ceramic) | C04B 35/111 and subgroups |
Ceramics based on zirconia, the ceramic having a grain size below 100 microns (fine ceramic) | C04B 35/486 and subgroups |
Ceramics based on silicon nitride, the ceramic having a grain size below 100 microns (fine ceramic) | |
Particles or aggregates of a component of the ceramic starting mixture have an average particle size of 1-100 micron |
This place covers:
The grains of the sintered (or melted) ceramic are to a certain extent aligned along a certain axis, e.g. it has elongated grains that are aligned in a certain direction, or it has grains whose magnetic moment has been aligned. This is often the case for dielectric or piezoelectric films.
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures containing oriented fillers or elements |
This place covers:
The grains are elongated and the average width divided by the average length has been measured. The aspect ratio of spherical grains can also be mentioned.
This place covers:
Materials having a stoichiometry that deviates from what is normal for that specific material, e.g. bismuth sodium titanate normally is (Bi0.5Na0.5)TiO3, (Bi0.48Na0.52)TiO3 therefore has a deviating stoichiometry.
This place does not cover:
The use of sub-stoichiometric titanium oxides for making ceramics | |
The use of sub-stoichiometric niobium oxides for making ceramics | |
Sintered ferrites that contain ferrous iron, iron with an oxidation state of +2. |
This place covers:
All sintered ceramics that contain at least one secondary phase, where this secondary phase is not a grain boundary phase, e.g. a titanate ceramic containing a secondary niobate phase, or a silicate ceramic containing a main silicate phase and a secondary silicate phase.
This place does not cover:
Reinforced clay wares | |
Magnesia based refractories from grain sized mixtures containing refractory metal compounds other than chromium oxide or chrome ore | |
Magnesia based refractories from grain sized mixtures containing chromium oxide or chrome ore | C04B 35/047 and subgroups |
Magnesia based refractories obtained by fusion casting containing chromium oxide or chrome ore | |
Alumina based refractories from grain sized mixtures containing refractory metal compounds other than those covered by C04B 35/103 - C04B 35/106 | |
Alumina based refractories from grain sized mixtures containing non-oxide refractory materials, e.g. carbon | |
Alumina based refractories from grain sized mixtures containing chromium oxide or chrome ore | C04B 35/105 and subgroups |
Alumina based refractories containing zirconia | |
Alumina refractories containing zirconia, made by melt-casting | |
fine alumina ceramics containing one or more secondary phases | C04B 35/117 and subgroups |
Zirconia-based ceramics containing one or more secondary phases | C04B 35/488 and subgroups |
Ceramic products containing macroscopic reinforcing agents, e.g. fibers | C04B 35/71 and subgroups |
Ceramics where the secondary phase is a continuous glass phase | C03C 10/00 and subgroups |
Ceramics where the secondary phase is a continuous metallic phase | C22C 29/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramics containing a grain boundary phase |
The symbol C04B 2235/80 is not used for the above-mentioned classes, where by default a different secondary phase has to be present.
All secondary phases are indicated with symbols from the scheme C04B 2235/32-C04B 2235/428.
This place covers:
Sintered ceramics that, with the exception of a grain boundary phase, contain only one phase, e.g. single phase barium titanate or single phase translucent alumina.
This place covers:
Sintered ferrites that contain ferrous iron, iron with an oxidation state of +2.
This place covers:
The sintered ceramic contains an intergranular or grain boundary phase, e.g. glassy grain pockets or an amorphous phase along the grain boundaries. The brain boundary phase can also be crystalline.
This place covers:
It is specifically mentioned or it is clear from SEM- or TEM-pictures that the sintered ceramic does not contain grain boundary phases. The ceramic can contain secondary phases, though, by no means it has to be single phase.
This place covers:
The sintered ceramic has a specific shape, e.g. is a disc, tube, hollow core, radome, etc.
working by grinding or polishing B24
shaping of ceramics B28B
Working stone or stone-like materials, e.g. brick, concrete or glass , not provided for elsewhere; machines, devices, tools therefore B28D
This place does not cover:
Free standing ceramic films or tapes | |
Ceramic coating | C04B 35/62222, C04B 41/00 and subgroups |
Ceramic fibers | C04B 35/62227 and subgroups, C04B 2235/5216 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Slip casting of clay wares | |
making the green bodies or pre-forms by moulding | C04B 2235/602 and subgroups |
pressing at non-sintering temperatures to make green bodies or pre-forms | C04B 2235/604 and subgroups |
Forming ceramic laminates or joined ceramic articles comprising at least one member in the form other than a sheet or disc, e.g. two tubes or a tube and a sheet or disc | C04B 2237/76 and subgroups |
This place covers:
The shaped sintered ceramic containing macro-sized unevenness at the surface
Attention is drawn to the following places, which may be of interest for search:
Forming ceramic laminates or joined ceramic articles comprising grooves or cuts | |
Apparatus or processes for reshaping the surface of ceramic objects, e.g. smoothing, roughening, corrugating, making screw-threads | B28B 11/08 and subgroups |
Methods or apparatus for grooving or corrugating ceramic tubes |
This place covers:
ceramics of which the size is specified, the ceramic being either unusually large or unusually small, or just one dimension having a specific size, e.g. the ceramic being very long
This place covers:
Ceramic products having specific mechanical properties, such as hardness, toughness, strength, wear resistance, elasticity
Attention is drawn to the following places, which may be of interest for search:
Strengthening clay wares through the addition of reinforcing additives | |
Strengthening ceramics through the addition of reinforcing additives | C04B 35/71 and subgroups |
Properties and uses of cement, concrete, mortar or artificial stone | C04B 2111/00 and subgroups |
Cement, concrete, mortar or artificial stone characterised by specific physical values for the mechanical strength | C04B 2201/50 and subgroups |
Mechanical properties of the green compact | |
Mechanical properties of membranes, e.g. strength | |
Mechanical properties of the layers of laminates | B32B 2307/50 and subgroups |
Mechanical properties of carbon nanotubes |
The symbol C04B 2235/96 is used only to indicate the mechanical properties of ceramics, the symbols of the subgroups of C04B 2235/96 are for other properties. Electrical properties are not coded in C04B 2235/96 and subgroups, as these are normally indicated by the relevant CPC symbols from the H-part of the ECLA-scheme, e.g. the piezoelectric properties are indicated by the classification in H10N 30/00 and subgroups, dielectric properties are indicated for instance by a class from H01G 4/12 and subgroups, semiconducting properties are indicated by a class from H10W 99/00 and subgroups, etc.
This place covers:
Properties such as the thermal expansion coefficient, thermal conductivity, melting point, heat capacity, thermal shock resistance
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone characterised by specific physical values for heat transfer properties such as thermal insulation values, e.g. R-values | C04B 2201/30 and subgroups |
This place covers:
The shrinkage of the green body or pre-sintered body during sintering, normally indicated in percentage, e.g. a linear firing shrinkage of -20%, meaning that the ceramic shrinks 20% during sintering
Furnaces, kilns, ovens, or retorts F27
This place does not cover:
Shrinkage during curing | |
Shrinkage during drying of green compact |
Attention is drawn to the following places, which may be of interest for search:
Burning methods for clay-wares | C04B 33/32 and subgroups |
Sintering of ceramics | C04B 35/64 and subgroups |
Aspects relating to heat treatment of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes | C04B 2235/65 and subgroups |
Mechanical aspects of sintering clay or ceramic objects |
This place covers:
The use of substrates, supports, jigs during heating steps, mainly the sintering step, that have the function of giving mechanical support to the ceramic that is being sintered.
Furnaces, kilns, ovens, or retorts F27
This place does not cover:
Using sacrificial powder or objects to influence the atmosphere during a heating step | |
Using constraining layers before or during sintering of ceramic laminates or ceramic substrates that are joined with other substrates | C04B 2237/56 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Burning methods for clay-wares | C04B 33/32 and subgroups |
Sintering of ceramics | C04B 35/64 and subgroups |
Aspects relating to heat treatment of ceramic bodies such as green ceramics or pre-sintered ceramics, e.g. burning, sintering or melting processes | C04B 2235/65 and subgroups |
Mechanical aspects of sintering clay or ceramic objects |
This place covers:
Properties that are surface related, e.g. the surface roughness
This place does not cover:
Applying of coatings to the surface of a ceramic | C04B 41/00 and subgroups |
Oxidising the surface of a ceramic as preparation for joining the ceramic |
Attention is drawn to the following places, which may be of interest for search:
Pre-treatment of the joining surfaces of a substrate that is joined with a ceramic substrate, e.g. cleaning, machining | C04B 2237/52 and subgroups |
Apparatus or processes for smoothing the surface of ceramic objects | |
Methods or apparatus for smoothing, roughening, corrugating or for removing burr from ceramic tubes |
This place covers:
An individual ceramic that is defined by having very accurate dimensions, or a series of ceramic objects that have all the same dimensions within a certain narrow range (the tolerance).
This place does not cover:
Forming ceramic laminates or joined ceramic articles showing high dimensional accuracy, e.g. indicated by the warpage |
This place covers:
Properties such as IR or UV absorption, light scattering or reflection
This place does not cover:
Luminescent, e.g. electroluminescent, chemiluminescent materials | C09K 11/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by the optical properties, e.g. transparency or reflexibility | C04B 2111/80 and subgroups |
Optical properties of inorganic powders per se | C01P 2006/60 and subgroups |
This place covers:
Transparent or translucent ceramics, such as aluminate (YAG or spinel), AlON, zirconia, yttria
This place does not cover:
Transparent or translucent alumina ceramics |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by the transparency |
This place covers:
All ceramic materials that are defined by their colour, including black and white, or whose colour is influenced by the addition of colouring additives
This place does not cover:
Coloured clay ceramics |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by a white colour | |
Cement, concrete, mortar or artificial stone mixtures characterised by the colour |
This place covers:
The chemical resistance of ceramics against oxidation, reduction, reaction.
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures characterised by the resistance against chemical, physical or biological attack | C04B 2111/20 and subgroups |
This place covers:
The resistance of ceramic materials, e.g. refractory linings used in converters, ladles, tundishes, etc., against molten metals such as steel, aluminium
Attention is drawn to the following places, which may be of interest for search:
Hot tops from refractory material for ingot moulds | |
Linings for casting melt-holding vessels, e.g. ladles, tundishes, cups or the like | B22D 41/02 and subgroups |
Blast furnaces with special refractories, e.g. linings | C21B 7/04 and subgroups |
Refractory linings for carbon-steel converters | C21C 5/44 and subgroups |
Refractory coated lances; Immersion lances for carbon-steel converters |
This place covers:
The resistance against oxidation, e.g. when heating non-oxides such as silicon carbide in an oxygen containing atmosphere
Attention is drawn to the following places, which may be of interest for search:
Directional oxidation or solidification, e.g. Lanxide process | |
oxidative annealing for making a coating layer | C04B 41/45 and subgroups |
Atmosphere during the heat treatment enriched in oxygen content to above the level normal in air | |
Oxidative annealing of shaped ceramics | |
Oxidising the surface of a substrate that is joined with a ceramic substrate before joining |
This place covers:
The resistance against alkalis such as cryolite, molten glass
Attention is drawn to the following places, which may be of interest for search:
Resistance against alkali-aggregate reaction of mortars, concrete or artificial stone | |
Acid resistance, e.g. against acid air or rain of mortars, concrete or artificial stone |
This place covers:
Secondary aspects of making ceramic laminates (B32B 18/00) and of joining ceramic articles with other articles through heating (C04B 37/00 and sub-classes), e.g. the composition of the layers or articles that are laminated or joined, the composition of the interlayers that are used for joining, processing aspects such as surface treatments to the layers-to-be-joined and also the geometrical configuration of the articles that are joined, e.g. joining both layers on their small side or one layer on the largest surface with one layer on the shortest surface.
Soldering or unsoldering; welding; cladding or plating by soldering or welding; cutting by applying heat locally e.g. flame cutting; working by laser beam B23K
Coatings applied to the outside of the metallic substrate, thus the side of the substrate that is not bonded with another substrate C23C
joining constructional elements in general F16B
This place does not cover:
Layered products essentially comprising metal | B32B 15/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Clay-wares | C04B 33/00 and subgroups |
Ceramic materials | C04B 35/00 and subgroups |
joining of a ceramic layer to another layer | C04B 37/00 and subgroups |
porous ceramic products | |
Honeycomb structures assembled from subunits | |
Aspects relating to ceramic starting mixtures or sintered ceramic products | C04B 2235/00 and s subgroups |
Application of procedures in order to connect objects or parts, e.g. coating with sheet metal otherwise than by plating | B21D 39/00 and subgroups |
Friction heat forging | |
Riveting | B21J 15/00 and subgroups |
Uniting components to form integral members, e.g. turbine wheels and shafts, caulks with inserts, with or without shaping of the components | B21K 25/00 and subgroups |
Connecting metal parts or objects by metal-working techniques, not covered wholly by either B21J or B23K | B23P 11/00 and subgroups |
Joining or sealing of preformed parts, e.g. welding of plastics materials; Apparatus therefore | B29C 65/00 and subgroups |
Layered products essentially comprising ceramics , e.g. refractory products | |
Uniting glass pieces by fusing without substantial reshaping | |
Joining pieces of glass to pieces of other inorganic material; Joining glass to glass other than by fusing | C03C 27/00 and subgroups |
Connecting constructional elements or machine parts by sticking or pressing them together, e.g. cold pressure welding | F16B 11/00 and subgroups |
Seals between parts of vessels of electric discharge tubes or discharge lamps |
This place covers:
An interlayer is a layer that is applied in-situ on a substrate, e.g. by a coating a substrate, or by laying a sheet or foil upon a substrate, or by chemically treating the surface of a substrate to such an extent that a separate layer is formed at the surface, e.g. by oxidising a metal or non-oxide substrate. The function of the interlayer has to be to bond two layers or two objects to each other. Any coating that is applied on a substrate at the side of the substrate that is bonded is regarded as interlayer and is indicated with a symbol from the range C04B 2237/02-C04B 2237/16. Any material that is not classified in any of the sub-classes is classified in this class, such as boron interlayers.
This place does not cover:
Coatings applied to the outside of the ceramic substrate, thus the side of the substrate that is not bonded with another substrate | C04B 41/00 and subgroups |
Interlayers between two metallic substrates, two glass substrates or between a glass and metallic substrate | C03C 27/04 (Joining glass to metal by means of an interlayer ) |
Electrodes or electrode layers that are inside multilayer ceramics, e.g. multilayer ceramic capacitors (unless it is mentioned that these electrodes are used specifically for joining) | H01G 4/30 (stacked capacitors) |
Attention is drawn to the following places, which may be of interest for search:
Honeycomb structures assembled from subunits characterised by the material used for joining separate subunits | |
Cement, concrete, mortar or artificial stone mixtures used as glue or binder for uniting building or structural materials | |
Thickness of the interlayer | |
Chemical nature of materials in mouldable or extrudable form for sealing or packing joints or covers: Oxides, hydroxides, carbonates | |
Chemical nature of materials in mouldable or extrudable form for sealing or packing joints or covers: Silica-rich compounds, e.g. silicates, cement, glass | C09K 2200/0243 and subgroups |
Chemical nature of materials in mouldable or extrudable form for sealing or packing joints or covers: ceramics | |
Seals between parts of vessels of electric discharge tubes or discharge lamps |
Documents classified in C04B 38/0019 should normally also be classified in C04B 37/003, as most honeycombs are made from ceramic material. The interlayer used for joining the honeycomb parts receives a symbol from C04B 2237/04-C04B 2237/16.
This place covers:
All interlayers consisting mainly out of a ceramic material, the ceramic materials being the materials that are classified in C04B 33/00 (clay materials), C04B 35/00-C04B 35/597 (ceramic materials), C04B 35/62204 (ceramic materials made out of waste material), C04B 35/62227( ceramic fibers), C04B 35/628 (coated ceramic powders or coated ceramic fibers), C04B 35/6303 (inorganic additives), C04B 35/66 (refractories and refractory mortars) and C04B 35/71-C04B 35/83 (ceramic materials containing macroscopic reinforcing agents), where consisting mainly means that the ceramic materials at least have to form the largest fraction.
This place does not cover:
Interlayers that are glass-ceramic material | |
Joining glass to metal by means of an interlayer consisting of glass, glass-ceramic or ceramic material only |
If the interlayer is a mixture of ceramic and metallic material, e.g. a cermet, then contrary to what is done with the substrates (cermets are coded with the metals normally), the cermet interlayer receives a symbol from C04B 2237/04-C04B 2237/083 to indicate the largest ceramic fraction and also the symbol C04B 2237/126 (or a symbol form subgroups of C04B 2237/126, if appropriate) to indicate that the active fraction is a metal.
This place covers:
All oxide materials that are normally, as a ceramic material, are classified in the groups C04B 35/01-C04B 35/51, thus also phosphate materials.
This place covers:
All materials mainly comprising silica and silicates, e.g. lanthanum silicate (LaSiO3), all alumino-silicates, such as clays
This place does not cover:
zircon (ZrSiO4) |
This place covers:
all aluminates, e.g. spinel (MgAl2O4), lanthanum aluminate (LaAlO3), all alumina materials such gamma-alumina, boehmite, corundum, gibbsite, etc.
This place does not cover:
alumino-silicates |
This place covers:
all rare earth oxides not present in a chemical compound with oxides other than rare earth oxides, e.g. Y2O3, Ce2O3, CeO2, La2O3, LaCeO3, YLaO3
This place does not cover:
rare earth ferrite interlayer | |
rare earth cuprate interlayer | |
rare earth phosphate interlayer | |
rare earth silicate interlayer | |
rare earth aluminate interlayer | |
rare earth titanate interlayer | |
rare earth zirconate interlayer | |
rare earth niobate interlayer | |
rare earth chromite interlayer |
This place covers:
materials based on the oxides of the nine refractory oxides, e.g. all titanates, such as barium titanate (BaTiO3), aluminium titanate (Al2TiO5), bismuth titanate (Bi4Ti3O12) and all titania based material; all materials consisting mainly of zirconates and hafnates, such as zircon (ZrSiO4), calcium zirconate (Ca2ZrO4), lead hafnate (PbHfO3), zirconate-titanates such as PZT (lead zirconate-titanate), for all values for the relation Ti/Zr; all zirconia based materials such as yttria-stabilised-zirconia (YSZ), unstabilised zirconia, cubic zirconia, etc.; all niobates such as alkaline earth niobates (Na0.5K0.5NbO3)
This place does not cover:
other refractory oxide materials such as alumina,or magnesia | |
refractory non-oxide materials such as silicon carbide |
In this place, the following terms or expressions are used with the meaning indicated:
refractory metal oxides | titanium oxide, vanadium oxide, chromium oxide, zirconium oxide, niobium oxide, molybdenum oxide, hafnium oxide, tantalum oxide, tungsten oxide |
This place covers:
Materials based on all non-oxide materials that are classified in the groups C04B 35/515-C04B 35/597, e.g. nitrides such as silicon nitride (Si3N4), aluminium nitride (AlN) or boron nitride (BN), carbonitrides, borides such as magnesium boride (MgB2) or titanium boride (TiB2), silicides such as molybdenum silicide (MoSi2), fluorides such as aluminium fluoride (AlF3), sulfides, selenides. It also covers non-oxide layers that are formed in-situ during bonding, e.g. a silicon nitride layer that is formed during bonding, due to the reaction of the Si interlayer with a nitrogen-containing substrate
This place covers:
all carbide interlayers, whether they are present before bonding or whether they are formed in-situ during bonding, e.g. a Ti-layer reacts during bonding to form a TiC-interlayer
This place does not cover:
carbonitrides, e.g. SiCN, or oxycarbonitrides, e.g. AlCON |
This place covers:
All interlayers based on inorganic carbon, e.g. graphite, diamond, carbon nanotubes, fullerenes, carbon black, glassy carbon
This place does not cover:
interlayers based on organic carbon, e.g. pitch, tar, polymers |
If the joining composition is a mixture of carbon and polymer, both C04B 37/008 and C04B 37/005 are given.
This place covers:
An interlayer that for the largest part contains ceramic components, where the component responsible for the bonding with the substrate is not the component present as the largest fraction.
This place does not cover:
Glass is the minority and ceramic phases the majority, but the glass forms a continuous phase as a binding phase | |
cermet interlayers, interlayers containing a majority of ceramic material with a metallic binder | C04B 2237/126 and s subgroups |
This symbol will always be given in combination with a symbol from C04B 2237/04 - C04B 2237/083, since the largest component has to be a ceramic component
This place covers:
An interlayer that for the largest part contains ceramic components, where the component responsible for the bonding with the substrate is not the component present as the largest fraction, but is Si
This place does not cover:
cermet interlayers, interlayers containing a majority of ceramic material with a metallic binder |
This symbol will always be given in combination with a symbol from C04B 2237/04 - C04B 2237/083, since the largest component has to be a ceramic component
This place covers:
Glass and glass-ceramic interlayers. Also when glass is the minority and ceramic phases the majority, but the glass forms a continuous phase as a binding phase, this symbol is given. When a frit or flux is melted to form an interlayer, this symbol is used as well.
This place does not cover:
Joining glass to metal by means of an interlayer consisting of glass, glass-ceramic or ceramic material only | |
Joining metals with the aid of glass |
In this place, the following terms or expressions are used with the meaning indicated:
glass-ceramic | a crystallised glass or a mixture of glass particles and ceramic particles, in which the glass forms a continuous matrix phase |
This place covers:
metallic interlayers, cermet interlayer in which the metal is the bonding material, e.g. zinc or a mixture , also metallic layers that react during bonding to form a ceramic layer, e.g. Ti-layer that reacts to form TiC
This place does not cover:
Joining glass to metal by means of an interlayer consisting of metals, metal oxides or metal salts only | |
Joining glass to glass by means of an interlayer with the aid of intervening metal |
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces | B23K 35/001 and subgroups |
Selection of soldering or welding materials proper | B23K 35/24 and subgroups |
If it is mentioned that methods such as brazing or soldering, in which the joining material is always metallic, are used, but the metal used for brazing or soldering is not specified, then C04B 2237/12 can be allocated.
Electrode and electrodes layers that are inserted between ceramic substrate layers are normally not seen as interlayer, since they normally do not have the function of joining the two ceramic substrates. They therefore are not classified with a C04B 2237/12 symbol. Only if it is clear that the electrode does have a joining effect, it is regarded as interlayer, and C04B 2237/12 or a symbol of its subgroups is given.
In this place, the following terms or expressions are used with the meaning indicated:
cermet | a mixture of a ceramic phase and a metal phase, in which the metal phase forms a continuous matrix |
This place covers:
alloys in which aluminium has the largest weight fraction and all aluminides or aluminide alloys, e.g. titanium aluminide (TiAl), nickel aluminide (Ni3Al)
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces | B23K 35/001 and subgroups |
Selection of soldering or welding materials proper with the principal constituent melting at less than 950 degrees C: Al as the principal constituent |
This place covers:
Interlayers having as the largest fraction alloys in which the nine refractory metals together have the largest weight fraction. For instance, if the interlayer has the composition Cu50Ti25Zr25, both C04B 2237/122 and C04B 2237/124 are given, since copper and refractory metals are present in equal amount. If the interlayer has the composition Fe40Ti30V15Ag15, only C04B 2237/122 is given.
Attention is drawn to the following places, which may be of interest for search:
Selection of soldering or welding materials proper with the principal constituent melting at more than 1550°C | |
Selection of soldering or welding materials proper with the principal constituent melting at more than 1550 degrees C; Ti as the principal constituent |
In this place, the following terms or expressions are used with the meaning indicated:
refractory metal | titanium , vanadium , chromium , zirconium , niobium , molybdenum , hafnium , tantalum , tungsten |
This place covers:
Alloys in which the three iron group metals together have the largest weight fraction. If the interlayer has the composition Ti40Fe30Ni12Ag18, only C04B 2237/123 is given, since Fe and Ni together are the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Ni as the principal constituent | B23K 35/3033 and subgroups |
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Co as the principal constituent | |
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Fe as the principal constituent | B23K 35/3053 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
iron group metals | Fe, Co, Ni |
This place covers:
Alloys in which copper has the largest weight fraction.
Attention is drawn to the following places, which may be of interest for search:
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Cu as the principal constituent |
This place covers:
Alloys in which the eight noble metals together have the largest weight fraction. If the interlayer has the composition Fe45Pd20Pt20Ag10Ti5, only C04B 2237/125 is given, since Pd, Pt and Ag together are the largest fraction.
Attention is drawn to the following places, which may be of interest for search:
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Ag as the principal constituent | |
Selection of soldering or welding materials proper with the principal constituent melting at less than 1550 degrees C: Au as the principal constituent | |
Selection of soldering or welding materials proper with the principal constituent melting at more than 1550 degrees C; a Pt-group metal as principal constituent |
Often interlayer materials based on noble metals contain minorities of other metals. These other metals usually form the active component, since noble metals are not very reactive. The C04B 2237/126 symbol and symbols of its subgroups can be used to indicate the presence of these minority metals, even if it is not mentioned that these minority metals are the active component, since it can be assumed these minority metals act as active component.
In this place, the following terms or expressions are used with the meaning indicated:
noble metals | ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), Osmium (Os), iridium (Ir), Platinum (Pt), gold (Au) |
This place covers:
An interlayer that has a metallic nature, where the component responsible for the bonding with the substrate is not the component present as the largest fraction. The interlayer can either be a cermet, where ceramic material form the majority but the bonding component is a metal, or the interlayer can be a metallic alloy, having at least two different metals. You can have more than one active component per joining composition. The amount of active component can be very low, lower even than 1 wt% or 1 mole%.
This symbol will normally be given in combination with a symbol from C04B 2237/12 - C04B 2237/125, since the largest component normally is a metallic component. In the case of a cermet mixture C04B 2237/12 is given as well.
In patent documents, the following expressions/words are often used as synonyms.
Active component | wetting component, wetting agent, joining component, joining agent |
This place covers:
An interlayer that has a metallic nature, where the component responsible for the bonding with the substrate is not the component present as the largest fraction, this active component being a refractory metal. The interlayer can either be a cermet, where ceramic material form the majority but the bonding component is a refractory metal, or the interlayer can be a metallic alloy, containing the refractory metal as a minor component.
This symbol will normally be given in combination with a symbol from C04B 2237/12 - C04B 2237/125, since the largest component normally is a metallic component. In the case of a cermet mixture C04B 2237/12 is given as well.
This place covers:
An interlayer that has a metallic nature, where the component responsible for the bonding with the substrate is not the component present as the largest fraction, this active component being silicon. The interlayer can either be a cermet, where ceramic material form the majority but the bonding component is a metal, where silicon is also present, or the interlayer can be a metallic alloy, containing silicon as a minor component.
This symbol will normally be given in combination with a symbol from C04B 2237/12 - C04B 2237/125, since the largest component normally is a metallic component. In the case of a cermet mixture C04B 2237/12 is given as well.
This place covers:
Alloys in which Si has the largest weight fraction, but has not reacted to form a silicide compound. If the starting material of the bonding layer is Si and the Si reacts during bonding to something else, e.g. SiC, then both the Si starting layer and SiC final layer are coded.
This place does not cover:
silica interlayers | |
silicate interlayers | |
silicon nitride (Si3N4) interlayers | |
silicide interlayers, e.g. MoSi2 | |
silicon carbide interlayers |
This place covers:
all individual layers of a ceramic laminate classified in B32B 18/00; all objects that are joined, either directly or by use of an interlayer, and are classified in C04B 37/00 and subgroups, this can be a layer but also tubes, fiber forms, etc. Substrates that are neither ceramic nor metallic will be classified also with the symbol C04B 2237/30. These are half-metals such as Si polymers, single crystals
This place does not cover:
the composition of interlayers, the layers that are used for the joining |
In this place, the following terms or expressions are used with the meaning indicated:
substrate | the object that is joined or is part of the laminate |
This place covers:
All layers/objects consisting mainly out of a ceramic material, the ceramic materials being the materials that are classified in C04B 33/00 and subgroups (clay materials), C04B 35/00-C04B 35/597 (ceramic materials), C04B 35/62204 (ceramic materials made out of waste material), C04B 35/66 (refractories and refractory mortars) and C04B 35/71-C04B 35/83 (ceramic materials containing macroscopic reinforcing agents), where consisting mainly means that the ceramic materials at least have to form the largest fraction.
This place does not cover:
layers/objects that are glass-ceramic material | C04B 37/04 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Ceramic substrate/layer characterised by its composition | C04B 35/00 and subgroups |
Thickness of the ceramic substrate | |
Laminates containing only one ceramic layer | B32B 9/005 and subgroups |
This place covers:
All oxide materials that are normally, as a ceramic material, are classified in the groups C04B 35/01-C04B 35/51, thus also phosphate materials.
Attention is drawn to the following places, which may be of interest for search:
Ceramic oxide based substrate/layer characterised by its composition | C04B 35/01 and subgroups |
This place covers:
All materials mainly comprising silica and silicates, e.g. lanthanum silicate (LaSiO3), all alumino-silicates, such as clays, silicates such as mullite, cordierite, spodumene, forsterite, wollastonite
This place does not cover:
zircon (ZrSiO4) |
Attention is drawn to the following places, which may be of interest for search:
Clay based substrate/layer characterised by its composition | C04B 33/00 and subgroups |
Silica based substrate/layer characterised by its composition | |
Silicate based substrate/layer characterised by its composition | C04B 35/16 and s subgroups |
This place covers:
all aluminates, e.g. spinel (MgAl2O4), lanthanum aluminate (LaAlO3), all alumina materials such gamma-alumina, boehmite, corundum, gibbsite, etc.
This place does not cover:
alumino-silicates |
Attention is drawn to the following places, which may be of interest for search:
Alumina based substrate/layer characterised by its composition | C04B 35/10 and s subgroups |
Aluminate based substrate/layer characterised by its composition | C04B 35/44 and subgroups |
This place covers:
materials based mainly on the oxides of the nine refractory oxides, e.g. all niobates such as alkaline earth niobates (Na0.5K0.5NbO3)
This place does not cover:
other refractory oxide materials such as alumina,or magnesia | |
refractory non-oxide materials such as silicon carbide | C04B 2237/365 (SiC) |
Attention is drawn to the following places, which may be of interest for search:
Chromium oxide based substrate/layer characterised by its composition | |
Chromite based substrate/layer characterised by its composition | |
Vanadium, niobium, tantalum, molybdenum or tungsten oxide, or vanadate, niobate, tantalate, molybdate or tungstate based substrate/layer characterised by its composition | C04B 35/495 and subgroups |
In this place, the following terms or expressions are used with the meaning indicated:
refractory oxides | titanium oxide, vanadium oxide, chromium oxide, zirconium oxide, niobium oxide, molybdenum oxide, hafnium oxide, tantalum oxide, tungsten oxide |
This place covers:
All titanates, e.g. barium titanate (BaTiO3), aluminium titanate (Al2TiO5), bismuth titanate (Bi4Ti3O12) and all titania based material
This place does not cover:
niobate-titanate containing more niobium than titanium | |
zirconate-titanates such as PZT (lead zirconate-titanate), for all values for the relation Ti/Zr |
Attention is drawn to the following places, which may be of interest for search:
Titania or titanate based substrate/layer characterised by its composition | C04B 35/46 and subgroups |
This place covers:
all materials consisting mainly of zirconates and hafnates, such as zircon (ZrSiO4), calcium zirconate (Ca2ZrO4), lead hafnate (PbHfO3), zirconate-titanates such as PZT (lead zirconate-titanate), for all values for the relation Ti/Zr; all zirconia based materials such as yttria-stabilised-zirconia (YSZ), unstabilised zirconia, cubic zirconia, etc.
Attention is drawn to the following places, which may be of interest for search:
Zirconia, hafnia, hafnate or zirconate based substrate/layer characterised by its composition | C04B 35/48 and subgroups |
Fuel cells with solid electrolyte, where the electrolyte contains zirconia |
This place covers:
Materials based on all non-oxide materials that are classified in the groups C04B 35/515-C04B 35/597, e.g. carbides such as boron carbide (B4C), nitrides such as titanium nitride (TiN), carbonitrides such as silicon carbonitride (SiCN), borides such as magnesium boride (MgB2) or titanium boride (TiB2), silicides such as molybdenum silicide (MoSi2), fluorides such as aluminium fluoride (AlF3), sulfides, selenides.
Attention is drawn to the following places, which may be of interest for search:
Non-oxide based substrate/layer characterised by its composition | C04B 35/515 and subgroups |
This place covers:
layers/objects based on boron nitride, carbo boron nitride, boron oxynitride, materials that would be classified in the groups C04B 35/583 and C04B 35/5831
Attention is drawn to the following places, which may be of interest for search:
Boron nitride based substrate/layer characterised by its composition | C04B 35/583 and subgroups |
This place covers:
Layers/objects made of material that consists for the largest fraction of carbon or carbon-like materials, materials that would be classified in the groups C04B 35/52 - C04B 35/536, thus graphite, diamond, glassy carbon, expanded graphite, etc.
This place does not cover:
carbide based layers/objects | C04B 2237/365 (SiC) or C04B 2237/36 (other carbides) |
Attention is drawn to the following places, which may be of interest for search:
Carbon based substrate/layer characterised by its composition | C04B 35/52 and subgroups |
This place covers:
layers/objects based on silicon carbide (SiC), silicon boron carbide (SiBC), silicon oxy-carbide (SiOC), silicon carbide reinforced with (any kind of) fibers, materials that would be classified in the groups C04B 35/565 - C04B 35/5755
This place does not cover:
silicon carbonitride (SiCN) | C04B 2237/368 (silicon nitride) |
Attention is drawn to the following places, which may be of interest for search:
Silicon carbide based substrate/layer characterised by its composition | C04B 35/565 and subgroups |
This place covers:
layers/objects based on aluminium nitride (AlN), aluminium oxynitride (AlON), aluminium carbonitride (AlCN), aluminium boronitride (AlBN), materials that would be classified in the group C04B 35/581
This place does not cover:
layers or objects based on sialon | C04B 2237/368 (silicon nitride) |
Attention is drawn to the following places, which may be of interest for search:
Aluminum nitride based substrate/layer characterised by its composition |
This place covers:
layers/objects based on silicon nitride (Si3N4), silicon oxynitride (SiON), silicon aluminium oxynitride (Sialon), silicon carbonitride (SiCN), silicon boronitride (SiBN), silicon nitride reinforced with (any kind of) fibers, materials that would be classified in the groups C04B 35/584 - C04B 35/597
Attention is drawn to the following places, which may be of interest for search:
Silicon nitride based substrate/layer characterised by its composition | C04B 35/584 and subgroups |
This place covers:
all ceramic layers/objects containing fibers, whiskers, nanotubes, nanowires and similar elongated reinforcements
Attention is drawn to the following places, which may be of interest for search:
Clay ware based substrate/layer containing fibers or whiskers characterised by its composition | |
Ceramic substrate/layer containing metallic fibers or whiskers characterised by its composition | |
Ceramic substrate/layer containing non-metallic fibers or whiskers characterised by its composition | C04B 35/80 and subgroups |
The matrix of the reinforced ceramic layer/object should be indicated with a symbol from C04B 2237/32 - C04B 2237/368. The symbol C04B 2237/38 therefore is always given together with another symbol from the range C04B 2237/32-C04B 2237/368.
This place covers:
Materials based on with carbon fibers reinforced carbon materials, which would be classified in C04B 35/83
This place does not cover:
Materials based on a carbide matrix reinforced with carbon fibers | C04B 2237/38 and C04B 2237/365 (silicon carbide matrix) or C04B 2237/38 (matrix made of other carbide material) |
Attention is drawn to the following places, which may be of interest for search:
Carbon substrate/layer containing carbon fibers or whiskers characterised by its composition |
In the case C04B 2237/385 is given, it is not necessary to also give the class C04B 2237/363, since C04B 2237/385 already indicates the matrix of the layer/object is mainly carbon
This place covers:
All layers/objects based on metallic phases as well as ceramic layers/objects having a metallic binder (cermets). If the layer/object has a continuous metallic phase, it is regarded as metallic, even if the amount of metal is as low as for instance 5 wt%.
This place does not cover:
Silicon layers/articles joined with a ceramic layer/article | |
A second metal layer/object that is joined to a first metal layer/object, which itself is joined to a ceramic layer/object. | B32B 15/00 and subgroups (Layered products essentially comprising metal) |
Attention is drawn to the following places, which may be of interest for search:
Thickness of the metallic substrate | |
Manufacture of workpieces or articles from metallic powder characterised by the manner of compacting or sintering; Apparatus specially adapted therefore; Presses and furnaces | B22F 3/00 and subgroups |
Manufacture of workpieces or articles from metallic powder characterised by the special shape of the product | B22F 5/00 and subgroups |
This place covers:
Layers/objects containing a mixture of at least one ceramic material and one metallic material)
Attention is drawn to the following places, which may be of interest for search:
Cermet substrate/layer containing carbon fibers or whiskers characterised by its composition | C22C 29/00 and subgroups |
If the largest metallic fraction is one from the list of C04B 2237/402-C04B 2237/408, this symbol is given as well. The largest ceramic fraction is not classified with a ceramic layer/object symbol, since cermets are regarded intrinsically as metals.
This place covers:
Layers/objects containing as the largest fraction alloys in which aluminium has the largest weight fraction, as well as all aluminides or aluminide alloys, e.g. titanium aluminide (TiAl), nickel aluminide (Ni3Al).
This place covers:
Layers/objects containing as the largest fraction alloys in which the nine refractory metals together have the largest weight fraction, e.g. Mn40Ti25Nb25Ag10 will be classified in C04B 2237/403, not C04B 2237/404 for the 40Mn, since Ti and Nb together have 50. Also a mixture of 95 wt% ceramic and 5 wt% Mn40Ti25Nb25Ag10 binder will be classified in C04B 2237/403 (together with C04B 2237/401).
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces at least one of the workpieces being of a refractory metal |
In this place, the following terms or expressions are used with the meaning indicated:
refractory metal | titanium , vanadium , chromium , zirconium , niobium , molybdenum , hafnium , tantalum , tungsten |
This place covers:
Layers/objects containing as the largest fraction alloys in which manganese and rhenium refractory metals together have the largest weight fraction, e.g. Mn40Ti25Nb25Ag10 will be classified in C04B 2237/403, not C04B 2237/404 for the 40Mn, since Ti and Nb together have 50. Also a mixture of 95 wt% ceramic and 5 wt% Mn40Ti25Nb25Ag10 binder will be classified in C04B 2237/403 (together with C04B 2237/401).
This place covers:
Layers/objects containing as the largest fraction alloys in which the iron group metals together have the largest weight fraction, e.g. Cr49Fe20Co20Ni10Ag1 will get C04B 2237/405, not C04B 2237/403 for the 49 Cr, since Fe, Co and Ni together have 50.
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces at least one of the workpieces being of a metal of the iron group |
This place covers:
Layers/objects containing as the largest fraction alloys in which iron has the largest weight fraction, e.g. Cr49Fe50Ag1 will be classified in C04B 2237/406, while Cr49Fe48Ni2Ag1 will be classified in C04B 2237/405
This place covers:
Layers/objects containing as the largest fraction alloys in which copper has the largest weight fraction
This place does not cover:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces at least one of the workpieces being of copper or another noble metal |
This place covers:
Layers/objects containing as the largest fraction alloys in which the eight noble metals together have the largest weight fraction, e.g. Mn20Re20Pd10Pt10Rh10Ru11Ni19 will be classified in C04B 2237/408, not C04B 2237/404 for the 40 Mn and Rh, since the noble metals together have 41.
Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: Interlayers, transition pieces for metallurgical bonding of workpieces at least one of the workpieces being of copper or another noble metal |
In this place, the following terms or expressions are used with the meaning indicated:
noble metals | ruthenium (Ru), rhodium (Rh), palladium (Pd), silver (Ag), Osmium (Os), iridium (Ir), Platinum (Pt), gold (Au) |
This place covers:
The processes used in joining ceramic articles with other articles or making ceramic laminates
This place does not cover:
Details of heat treatments used in the joining or laminating process |
The details of the heat treatments used in the joining or laminating process are classified as well with symbols from the range C04B 2235/65-C04B 2235/668. The heating rate, atmosphere used during the heat treatment, e.g. vacuum or hydrogen-containing, the use of multi-step heating treatments or use of wave energy or a laser for heating can all be classified with these symbols.
This place covers:
Cleaning of the surfaces to-be-joined with solvents or with acids that etch the surface, vacuum cleaning, wiping, scraping, machining the surface-to-be-treated etc.
This place does not cover:
treatment of a ceramic surface that is not to be joined involving the removal of at least part of the materials of the treated article | C04B 41/53 and subgroups |
cleaning of ceramic objects in general | |
cutting of ceramic | B28D 1/22 , B28B 11/14, B23K 26/55 (with laser beam) |
Attention is drawn to the following places, which may be of interest for search:
Surface roughness of a ceramic substrate | |
Adhesive processes involving pre-treatment of the surfaces to be joined | |
etching, surface-brightening or pickling compositions | C09K 13/00 and subgroups |
If the surface of the ceramic substrate is machined to obtain a certain surface roughness, C04B 2235/963 (surface properties of ceramics) is allocated and C04B 2237/52 does not need to be given anymore. If the surface of the metal, glass or other non-ceramic substrate is machined, C04B 2237/52 is allocated.
This place covers:
Heat treatment of the surface which does not lead to bonding or to the creation of a bonding layer, but is directed at removing things from the surface that prevent bonding
This place does not cover:
Oxidising a surface before joining | |
Pre-heat treatment of a substrate other than oxidation treatment in order to form an active joining layer |
Attention is drawn to the following places, which may be of interest for search:
Cleaning the surface of a ceramic substrate by burning | |
Details of heat treatments used in the joining or laminating process |
This place covers:
Any oxidation treatment before bonding of a surface that is later joined to another surface
This place does not cover:
Oxidation of pore formers | C04B 38/00 and subgroups |
Heat treatment of the surface which does not lead to bonding or to the creation of a bonding layer, but is directed at removing things from the surface that prevent bonding |
Attention is drawn to the following places, which may be of interest for search:
Coating or impregnating involving the chemical conversion of an already applied layer, e.g. obtaining an oxide layer by oxidising an applied metal layer | C04B 41/4558 and s subgroups |
Oxidative annealing of shaped ceramics | |
Pre-heat treatment of a substrate other than oxidation treatment in order to form an active joining layer |
This place covers:
For instance heating a substrate already coated with a joining layer to activate/pre-react the joining layer, before joining with the other substrate
This place does not cover:
Heat treatments done while coating a substrate with a joining interlayer | |
Heat treatment of the surface which does not lead to bonding or to the creation of a bonding layer, but is directed at removing things from the surface that prevent bonding |
Attention is drawn to the following places, which may be of interest for search:
Oxidising a surface before joining |
During the application of a bonding layer to a to-be-joined substrate usually heating is used for the application of the coating that will form the bonding layer. In this case C04B 2237/55 is not used. Only if after the step of coating the substrate a non-oxidising heating treatment is performed in order to prepare the coating for the bonding step, C04B 2237/55 is used.
This place covers:
For instance a reduction treatment to form a reduced surface layer, e.g. heating a Si3N4 substrate in a reducing atmosphere to form a Si-layer at the surface
This place does not cover:
Heat treatments done while coating a substrate with a joining interlayer | |
Heat treatment of the surface which does not lead to bonding or to the creation of a bonding layer, but is directed at removing things from the surface that prevent bonding |
Attention is drawn to the following places, which may be of interest for search:
Reduction treatment for making a ceramic | |
Reductive annealing of shaped ceramics | |
Oxidising a surface before joining |
This place covers:
Layers or objects that are temporarily attached or put next to other layers/objects with the aim of hindering any movement of the other layers/objects, e.g. hindering shrinkage during the heat treatment due to the fact that the constraining layer has a higher sintering temperature
Furnaces, kilns, ovens, or retorts F27
This place does not cover:
Pressure sintering of clay ceramics | |
Pressure sintering of ceramics | C04B 35/645 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Using setters during sintering | |
Thickness of the constraining layer |
Weights that are put on the substrates or clamps that are used to restrain the substrate also are regarded as constraining layers.
This place covers:
A constraining layer that for instance is shorter than the layer it is covering
Attention is drawn to the following places, which may be of interest for search:
Forming laminates or joined articles comprising holes, channels or other types of openings | |
Thickness of the constraining layer | |
Joining the largest surface of one substrate with a smaller surface of the other substrate, e.g. butt joining or forming a T-joint | |
Both substrates not completely covering the other substrate, e.g. two plates in a staggered position | |
Joining of two substrates at their largest surfaces, one surface being complete joined and covered, the other surface not, e.g. a small plate joined at its largest surface on top of a larger plate |
This place covers:
constraining layers made of aluminates, e.g. spinel (MgAl2O4), lanthanum aluminate (LaAlO3), or alumina materials such gamma-alumina, boehmite, corundum, gibbsite, etc.
This place does not cover:
alumino-silicate constraining layers |
Attention is drawn to the following places, which may be of interest for search:
Alumina or aluminate substrate joined with another substrate or being part of a ceramic laminate |
This place covers:
glass and glass-ceramic constraining layers
Attention is drawn to the following places, which may be of interest for search:
Glass substrate joined with a ceramic substrate | C04B 37/04 and sub-classes |
This place covers:
constraining layers made of materials based on the oxides of the nine refractory oxides, e.g. all titanates, such as barium titanate (BaTiO3), aluminium titanate (Al2TiO5), bismuth titanate (Bi4Ti3O12) and all titania based material; all materials consisting mainly of zirconates and hafnates, such as zircon (ZrSiO4), calcium zirconate (Ca2ZrO4), lead hafnate (PbHfO3), zirconate-titanates such as PZT (lead zirconate-titanate), for all values for the relation Ti/Zr; all zirconia based materials such as yttria-stabilised-zirconia (YSZ), unstabilised zirconia, cubic zirconia, etc.; all niobates such as alkaline earth niobates (Na0.5K0.5NbO3).
This place does not cover:
other refractory oxide materials such as alumina, or magnesia | |
refractory non-oxide materials such as silicon carbide |
Attention is drawn to the following places, which may be of interest for search:
Refractory metal oxide substrate joined with another substrate or being part of a ceramic laminate |
In this place, the following terms or expressions are used with the meaning indicated:
refractory oxides | titanium oxide, vanadium oxide, chromium oxide, zirconium oxide, niobium oxide, molybdenum oxide, hafnium oxide, tantalum oxide, tungsten oxide |
This place covers:
constraining layers/objects based on metallic phases as well as ceramic layers/objects having a metallic binder (cermets). If the layer/object has a continuous metallic phase, it is regarded as metallic, even if the amount of metal is as low as for instance 5 wt%.
Attention is drawn to the following places, which may be of interest for search:
Metal substrate joined with a ceramic substrate | C04B 37/02 and subgroups, C04B 2237/40 and subgroups |
This place covers:
constraining layers based on all non-oxide materials that are classified in the groups C04B 35/515-C04B 35/597, e.g. nitrides such as silicon nitride (Si3N4), aluminium nitride (AlN) or boron nitride (BN), carbonitrides, borides such as magnesium boride (MgB2) or titanium boride (TiB2), silicides such as molybdenum silicide (MoSi2), carbides such as silicon carbide (SiC) or boron carbide (B4C), fluorides such as aluminium fluoride (AlF3), sulfides, selenides, carbon or carbon-like materials such as graphite, diamond, glassy carbon, expanded graphite, etc.
Attention is drawn to the following places, which may be of interest for search:
Non-oxide substrate joined with another substrate or being part of a ceramic laminate | C04B 2237/36 and subgroups |
Carbon fiber reinforced carbon substrate joined with another substrate or being part of a ceramic laminate |
This place covers:
At least two adjacent layers/objects are similar but have a small difference in composition or properties, e.g. one ZTA-layer (zirconia-toughened alumina, zirconia with a minority of alumina) next to an ATZ-layer (alumina-toughened zirconia, alumina with a minority of zirconia)
This place does not cover:
Ceramic products with a gradient within one layer or monolithic object | |
Two adjacent layers that have a completely different composition, e.g. one alumina layer next to a zirconia layer | B32B 18/00, C04B 37/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Cement, concrete, mortar or artificial stone mixtures with a gradually increasing or decreasing concentration of ingredients or property from one layer to another |
This place covers:
At least two adjacent layers/articles have the same main component, being the component present in the largest amount, but one or more different minor components
This place does not cover:
Adjacent layers/articles that have differing main components | B32B 18/00, C04B 37/00 and s subgroups |
This place covers:
At least two adjacent layers/objects contain fibers or whiskers, but the fibers or whiskers have a different composition, length, width or spatial orientation
This place does not cover:
A ceramic layer/article containing fibers of the same material but with different dimensions |
This place covers:
Two adjacent layers/objects have a similar composition but a different relative density/porosity, e.g. one alumina layer with 50% porosity adjacent to an alumina layer with 70% porosity
This place does not cover:
One ceramic layer or article having a gradient in the density within that layer or article | |
Two adjacent layers/articles having different absolute densities due to differing compositions | B32B 18/00, C04B 37/00 and subgroups |
This place covers:
For instance one alumina layer has an average grain of 1 micron, while an adjacent alumina layer has an average grain size of 2 microns, due for instance to a small difference in the composition or to different pre-treatments
This place does not cover:
Two adjacent layers/articles having different grain sizes due to the fact that the compositions are very different | B32B 18/00, C04B 37/00 and subgroups |
Attention is drawn to the following places, which may be of interest for search:
Grain sizes and shapes of sintered or melt-casted ceramics | C04B 2235/78 and subgroups |
If the difference in grain size is due to a small difference in composition, both C04B 2237/588 and C04B 2237/582 are given.
This place covers:
Aspects not relating to the composition of the interlayer, but the continuity and porosity of the interlayer
This place does not cover:
the composition of the interlayer | C04B 2237/02 and s subgroups |
reaction phases at the interlayer-substrate joining area |
Attention is drawn to the following places, which may be of interest for search:
thickness of the interlayer | |
Two interlayers next to each other |
This place covers:
The interlayer is interrupted, while the substrates or other interlayers on both sides continue, e.g. a printed or patterned interlayer is normally not continuous
This place does not cover:
An interruption of the interlayer due to openings/holes in at least one of the two substrates, e.g. an interruption of the interlayer due to the presence of an opening in the substrate as in C04B 2237/62 | |
Printed or patterned non-bonding electrodes not covering the whole ceramic substrate | B32B 18/00 or C04B 37/00 and subgroups |
Electrodes that do not seem to have the function of bonding two substrates are not regarded as interlayer. If these electrodes are discontinuous, not covering the whole substrate they are coated on, as is usually the case, they therefore do not receive the symbol M04B237/62B.
This place covers:
The interlayer is not interrupted, while the substrates or other interlayers on both sides continue. In a horizontal interlayer the different materials are encountered in a lateral direction.

This place does not cover:
An interlayer containing different materials that are homogeneously mixed, e.g. oxide powder mixed with fibers | C04B 2237/06 and subgroups |
Interlayers being on a macro-scale heterogeneous, where one of the macro-parts is void, where the void continuous in the substrate | |
Interlayers being on a macro-scale heterogeneous, where one of the macro-parts is void, where the void is restricted to the interlayer | M04B237/62B |
Electrodes that do not seem to have the function of bonding two substrates are not regarded as interlayer. If these electrodes are discontinuous, not covering the whole substrate they are coated on, as is usually the case, they therefore do not receive the symbol M04B237/62B.
This place covers:
The interlayer is continuous, while the substrates on both sides continue
This place does not cover:
An interruption of the interlayer due to openings/holes in at least one of the two substrates, e.g. an interruption of the interlayer due to the presence of an opening in the substrate as in C04B 2237/62 |
Attention is drawn to the following places, which may be of interest for search:
Hollow or porous granular material used as fillers for mortars, concrete or artificial stone | C04B 20/002 and sub-classes |
Porous or hollow ceramic granular materials, e.g. microballoons |
This place covers:
For instance a reaction between Si from the interlayer with C from the substrate in order to form SiC at the interphase between interlayer and substrate, or diffusion of Ti from a titanium alloy interlayer into a metallic substrate
This place does not cover:
reaction between two adjacent substrates, possibly resulting in the formation of an interlayer | C04B 37/001 (direct ceramic-ceramic junction) or C04B 37/021 (direct ceramic-metal junction) and C04B 2237/02- C04B 2237/16 for the in-situ formed interlayer |
Attention is drawn to the following places, which may be of interest for search:
Reaction sintering of free metal or free silicon containing compositions | C04B 35/65 and sub-classes |
This place covers:
Infiltrating a porous ceramic with metal or silicon to join the resulting cermet or ceramic with a metal or ceramic substrate.
Attention is drawn to the following places, which may be of interest for search:
Ceramics based on carbon, made by impregnation of a carbon product with carbonisable material | |
Making silicon carbide ceramic by reaction sintering, e.g. infiltrating a porous carbon body with Si and let them react to form SiC | |
Reaction sintering of free metal or free silicon containing compositions | C04B 35/65 and subgroups |
Porous ceramics in general | C04B 38/00 and subgroups |
Non-superficial impregnation or infiltration of a ceramic substrate | |
Liquid infiltration of green bodies or pre-forms |
Normally a joint between a cermet and a metal substrate is not classified in C04B 37/00, only in the above-mentioned case that a porous ceramic is joined to a metal through an infiltrated metal. The infiltration can lead to a bonding layer in between the two bodies, but it is also possible that there is no bonding layer after bonding, which means the bonding is a direct bonding
This place covers:
Laminates or joined articles having openings, for instance for electrodes and/or conductors. The openings normally should pass fully through at least one substrate layer. The openings normally are filled in the end-product with electrodes/conductors, but at least an intermediate product contains the hole.
This place does not cover:
Porous mortars, concrete, artificial stone or ceramic ware, containing continuous channels, e.g. of the "dead-end" type or obtained by pushing bars in the green ceramic product | |
Porosity in honeycomb structures | C04B 38/0006 and subgroups |
Laminates or joined articles having superficial holes, not penetrating the whole substrate layer | |
Joined articles of which at least one article is a tube, at least one article being ceramic |
Attention is drawn to the following places, which may be of interest for search:
Making a ceramic by shaping around a core that is later removed |
This place covers:
Openings/holes are at the surface of at least one of the substrates, but do not penetrate the whole substrate. The grooves can have the function of providing a mechanical bonding force at the joining surface, e.g. as saw-tooth on both substrate surfaces that are joined
This place does not cover:
Openings/holes that penetrate a whole substrate |
Attention is drawn to the following places, which may be of interest for search:
Ceramic articles per se containing grooves or cuts | |
A ceramic surface having a certain surface roughness |
If the surface of a ceramic substrate has grooves or cuts on micro- or nanolevel , C04B 2235/963 is attributed. If the surface roughness of the ceramic substrate is specified, C04B 2235/963 is used as well.
This place covers:
Laminates/joined articles that should have very specific dimensions
This place does not cover:
Laminates/joined articles of which the dimensions are mentioned but no indication is given on the desirability of having those dimensions | C04B 37/00 and subgroups or B32B 18/00 |
Attention is drawn to the following places, which may be of interest for search:
Ceramics in general characterised by having a high dimensional accuracy, indicated e.g. by the tolerance |
This place covers:
The two different parts can be of the same material and of different material. It can be for instance a layer with a checkerboard pattern, containing blocks of two kinds of different material.


This place does not cover:
Substrates that contain two different materials mixed on a small scale, e.g. smaller than 1 mm, for instance a substrate containing a homogeneous mixture of ceramic and metallic material | C04B 2237/30 and subgroups |
This place covers:
The whole laminate/joined article having a certain specific thickness, and also the glass layer of a glass-ceramic joint having a certain specific thickness
Attention is drawn to the following places, which may be of interest for search:
Ceramic objects in general characterised by their dimensions, e.g. having a specific size |
This place covers:
The constraining layer having a certain specific thickness
This place covers:
The ceramic substrate having a certain specific thickness
This place does not cover:
ceramic interlayers having a specific thickness |
This place covers:
The metallic substrate having a certain specific thickness
This place does not cover:
metallic interlayers having a specific thickness |
This place covers:
all interlayers, whether ceramic, metallic, glass, silicon, adhesive resin, having a specific thickness
This place covers:
Two substrates are joined by at least two interlayers. Non-bonding electrode layers do not count as interlayer.

Attention is drawn to the following places, which may be of interest for search:
Rods, electrodes, materials, or media, for use in soldering, welding, or cutting: layered sheets or foils for use in soldering or brazing | |
Adhesive processes involving separate application of adhesive ingredients to the different surfaces to be joined |
If there are two interlayers of which one is a non-bonding electrode layer, C04B 2237/72 is not attributed.
This place covers:
A sandwich that has at least 3 substrates, substrate 1, substrate 2 and substrate 3. The interlayer 1 between substrate 1 and 2 is different from the second interlayer, interlayer 2, between substrate 2 and substrate 3.

Non-bonding electrode layers do not count as interlayer. If two substrates contain only a non-bonding electrode in between, these substrates are regarded to be directly bonded.
This place covers:
Joining irregular shapes. Plates and discs are considered as regular shapes. A shaft or cylinder is considered as a regular shape as well.
This place does not cover:
joining of the blocks of a honeycomb | |
Sheets that are not joined at their longest side, but at one of the short sides |
This place covers:
Joining two substrates of which at least one is a tube, either ceramic or metallic
If C04B 2237/765 is given, C04B 2237/62 does not need to be given, since it is obvious a tube contains a hole.
This place covers:
Connecting substrates both at the sides that do not have the largest surface, e.g. two cylinders at the curved side, not at the end
This place does not cover:
Connecting one plate with its long side at another plate with its short side |
This place covers:
Joining the side surface of a plate with the largest surface of another plate
This place does not cover:
Joining the end of a cylinder with the largest surface of a plate | |
Sheets that are joined at both their shortest side |
This place covers:
This place covers:
The joining surface is for instance the inside of the outer tube and the outside of the inner tube. Or joining something to the inside of a vessel or to the inside of a box.
C04B 2237/62 and C04B 2237/64 do not need to be attributed for the hole/opening/groove that is used for joining. C04B 2237/62 and/or C04B 2237/64 might still need to be given for another hole/opening/groove.
This place covers:
This place does not cover:
Two plates that are joined at their largest surfaces with parts of both sheet remaining uncovered |
This place covers:
See for instance document US2010231129.
This scheme is associated mainly with groups C04B 2/00 - C04B 12/04 but also C04B 26/00 - C04B 32/00, C04B 38/00 and C04B 41/00