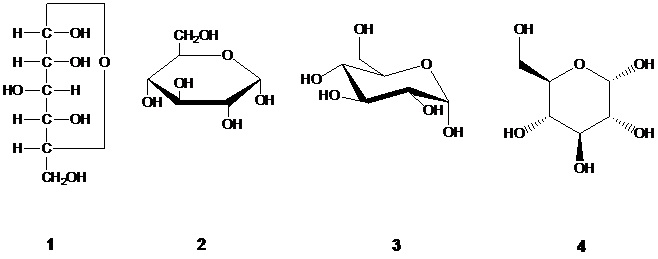
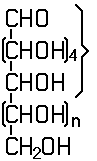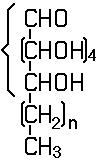CPC Definition - Subclass C07H
This place covers:
Compounds containing saccharide radicals, sugars and their derivatives, e.g.:
- Saccharides, deoxysugars, anhydrosugars and 1,2-ketoaldoses;
- Aminosugars, aza-, thio-, seleno- and telluro analogues;
- Sugar esters, sugar ethers, glucosides and cyclic acetals;
- Sugar derivatives containing acylic, carbocyclic or heterocyclic radicals;
- Sugar derivatives containing boron, silicon or a metal;
Nucleosides, nucleotides
Nucleic acids produced by chemical preparation
Processes for the preparation of the above compounds.
In class C07, the last place priority rule is used, i.e. in the absence of an indication to the contrary, a compound is classified in the last appropriate subclass. Hence, while individual heterocycle-containing amino acids are classified in C07D, peptides are generally classified in C07K. Similarly, compounds containing saccharide radicals, with the exception of polysaccharides, are classified in this subclass, and heterocyclic steroids are classified under C07J. Heterocycles incorporating elements other than C, H, halogen, N, O, S, Se, Te are classified in C07F.
This subclass is a function oriented entry for the compounds themselves and does not cover the application or use of the compounds under the subclass definition. For classifying such information other entries in IPC exist, for example:
- Compounds or compositions for preservation of bodies of humans, animals, plants, or parts thereof, as biocides, e.g. disinfectants, pesticides, herbicides, as pest repellents or attractants, and as plant growth regulators are classified in A01N.
- Preparations for medical, dental, or toilet purposes are classified in A61K.
MULTIPLE CLASSIFICATION
Compounds classified in C07H are given additional classification according to the table below:
Field | Further classified in subclass |
Preservation of bodies of humans or animals or plants or parts thereof; biocides, e.g. as disinfectants, as pesticides or as herbicides; pest repellants or attractants; plant growth regulators | |
Biocidal, pest repellent, pest attractant, or plant growth regulatory activity of chemical compounds or preparations | |
Foods or foodstuffs; Their preparation or treatment | |
Preparations for medical, dental, or toilet purposes | A61K ( IPC) |
Therapeutic activity of chemical compounds | |
Uses of cosmetics or similar toilet preparations | |
Chemical or physical processes, e.g. catalysis, colloid chemistry; their relevant apparatus | |
Nanotechnology | |
General methods of organic chemistry; apparatus therefor | |
Macromolecular compounds obtained by reactions only involving carbon-to-carbon unsaturated bonds | |
Macromolecular compounds obtained otherwise than by reactions only involving carbon-to-carbon unsaturated bonds | |
Organic dyes or closely-related compounds for producing dyes; mordants; lakes | |
Measuring or testing processes involving enzymes or microorganisms; compositions therefor; processes of preparing such compositions | |
Combinatorial chemistry; libraries, e.g. chemical libraries, in silico libraries |
This place does not cover:
Derivatives of aldonic or saccharic acids | |
Sugar alcohols that are hydrogenated forms of carbohydrates, whose carbonyl group (aldehyde or ketone) has been reduced to a primary or secondary hydroxyl group, when they do not have an anomeric acetal or ketal (such as xylitol, mannitol, sorbitol).Maltitol ( (4-O-α-D-Glucopyranosyl-D-glucitol) contains a glycosidic linkage (i.e. anomeric acetal group) and is classified in C07H. | |
Alcohols (polyols) of cyclohexane, such as inositol | |
Aldonic acids, saccharic acids | |
Cyanohydrins | |
Glycals | |
Compounds of unknown constitution, glycosides | |
Steroid glycosides | |
Peptides | |
Polysaccharides, derivatives thereofwhich for the purpose of this subclass are defined as having more than five saccharide radicals attached to each other by glycosidic linkages | |
DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification | |
Using enzymes or microorganisms for the preparation of compounds containing saccharide radicals | |
Production of sucrose. Saccharides, other than sucrose, obtained from natural sources or by hydrolysis of naturally occurring di-, oligo- or polysaccharides |
Examples of places where the subject matter of this place is covered when specially adapted, used for a particular purpose, or incorporated in a larger system:
Sugar industry |
Attention is drawn to the following places, which may be of interest for search:
Brewing of beer | |
Preparation of wine or other alcoholic beverages | |
Measuring or testing processes involving nucleic acids | |
Electrolytic or electrophoretic processes for the production of compounds. | |
Chemical features in the manufacture of artificial filaments, threads, fibres, bristles, or ribbons; apparatus specially adapted for the manufacture of carbon filaments | |
Investigating or analysing materials by determining their chemical or physical properties |
Only explicitly disclosed examples that are covered by the claims ("claimed examples") are classified.
The examiner first look at the claims and only classify the single examples of the description/claims falling within the scope of the claims.
Claimed embodiments are thus classified only if they represent an enabling disclosure, which could be used for a novelty/inventive step reasoning against another document.
Further compounds/processes of the description, which have not been claimed, are not classified.
Broad claims are hence disregarded for the purporse of classification as they would not be useful for novelty/inventive step.
If the patent (application) is of interest for other fields, further classification(s) must be given ("circulation of documents"), in order to ensure that the document can be found when searching in the relevant field.
Should the compound/process be classifed in C07H, the two following additional classifications are given only in the IPC (not in CPC).
Preparations for medical, dental, or toilet purposes | A61K ( IPC) |
Therapeutic activity of chemical compounds |
In this subclass, in the absence of an indication to the contrary, a compound is classified in the last appropriate place.
In general, and in the absence of any indication to the contrary, the terms "acyclic" and "aliphatic" are used to describe compounds in which there is no ring; and, if a ring were present, the compound would be taken by the "last place" rule to a later group for cycloaliphatic or aromatic compounds, if such a group exists.
Where a compound or an entire group of compounds exists in tautomeric forms, it is classified as though existing in the form which is classified last in the system, unless the other form is specifically mentioned earlier in the system.
Chemical compounds and their preparation are classified in the groups for the type of compound prepared. The processes of preparation are also classified in the groups for the types of reaction employed, if of interest.
General processes for the preparation of a class of compounds falling into more than one main group are classified in the groups for the processes employed, when such groups exist.
The compounds prepared are also always classified in the groups for the types of compound prepared.
That means that a process will be always be given at least a class for the products obtained by that process, regardless of whether the product is known or not.
For instance, a process for preparing glucose will always be assigned the classification for glucose (i.e. C07H 3/02) and additionally main group C07H 1/00 or one of its subgroups, if the process is of interest.
Salts of a compound, unless specifically provided for, are classified as that compound.
Metal chelates are classified in C07H 23/00.
Salts, adducts or complexes formed between two or more organic compounds are classified according to all compounds forming the salts, adducts or complexes.
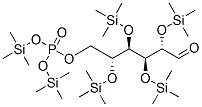
In this subclass only compounds derived from acyclic polyhydroxy-aldehydes or acyclic polyhydroxy-ketones, or from their cyclic tautomers, by removing hydrogen atoms or by replacing hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium are classified.
The compounds of this subclass must have at least one of the following functional groups, wherein the two hetero bonds to oxygen can be replaced by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium:
- aldehyde
- ketone
- hemiacetal
- hemiketal
- acetal
- ketal
Examples:
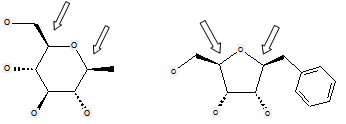
These compounds are not classified in C07H but in C07D, as no acetal group is present (the carbon atoms not forming a cyclic acetal are indicated by the arrows in the formulas below):
(classified only in C07D)
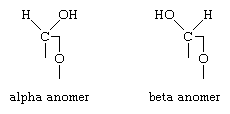
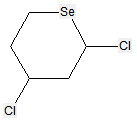
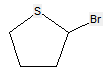
According to definition (a)(iii) and (b) below for a saccharide radical, some compounds comprising hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium should be classified in C07H and not in C07D (following the last place rule). Nonetheless, both classifications C07H and C07D have sometimes been given, for example, the following compounds should be classified in C07H but might also have additional classification in C07D:
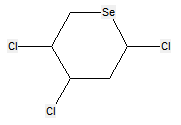
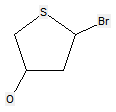
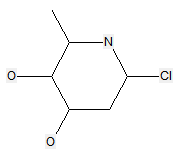
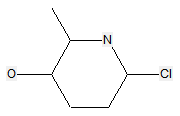
The following compounds should only be classified in C07D (see definition (a)(iii) and (b) below for a saccharide radical), as they do not have at least three carbon atoms - at least two in the case of a skeleton having only four carbon atoms - having one single bond to oxygen (or to halogen, nitrogen, sulfur, selenium, or tellurium atom) as the only hetero bond:
(classified only in C07D)
Sugar alcohols are not classified in C07H, if they have neither a carbonyl group (aldehyde or ketone) nor an anomeric acetal or ketal. For example sorbitol, is classified only in C07C
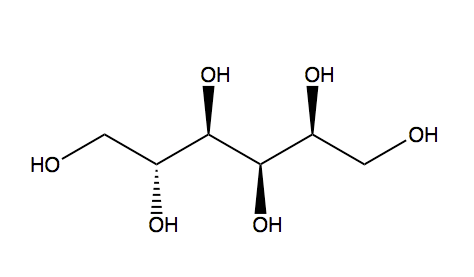 sorbitol (classified in C07C)
sorbitol (classified in C07C)
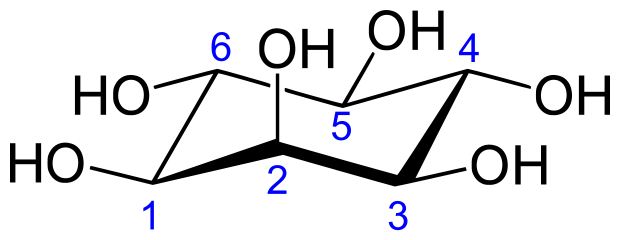
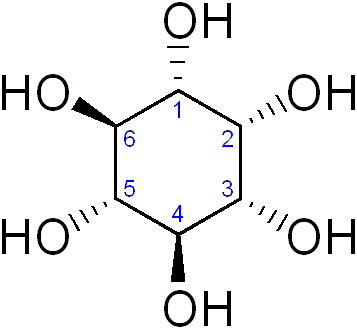
Inositol or cyclohexane-1,2,3,4,5,6-hexol is a sixfold alcohol (polyol) of cyclohexane, does not contain, in the cyclic sequence, at least one other carbon atom having two single bonds to oxygen atoms as the only hetero bonds
myo-inositol (classified in C07C 35/16)
 or
or
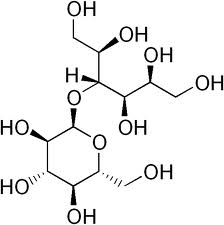
Maltitol ( (4-O-α-D-Glucopyranosyl-D-glucitol) contains a glycosidic linkage (i.e. anomeric acetal group) and is classified in C07H.
maltitol (classified in C07H)
In this place, the following terms or expressions are used with the meaning indicated:
Acyclic, aliphatic | Describe compounds in which there is no ring. Acyclic chains may be linear or branched. |
Carbocyclic | Where all ring members in a ring are carbon atoms. |
Chelate | Intracomplex compound i.e. compound containing intramolecular donor-acceptor bonds. |
Heteroatom | Any atom that is not carbon or hydrogen. |
Heterocyclic radical or hetero ring | Wherein at least one ring member in a molecule containing a ring of atoms is not a carbon atom.These are considered to exclude saccharide radicals as defined below. |
Inorganic compound | A compound devoid of a carbon atom and containing a non-metallic element, or a compound containing a carbon atom, and satisfying one of the following criteria: the compound cannot have a carbon atom having direct bonding to another carbon atom, or the compound cannot have direct bonding between a carbon atom and a halogen or hydrogen atom, or the compound cannot have direct bonding between a carbon and a nitrogen atom by a single or double bond.The following are exceptions to the above and are to be considered as inorganic compounds: compounds consisting of only carbon atoms, (e.g. fullerenes), cyanogen, cyanogen halides, cyanamide, phosgene, thiophosgene, hydrocyanic acid, isocyanic acid, isothiocyanic acid, fulminic acid, unsubstituted carbamic acid, and salts of the previously mentioned acids and which contain the same limitations as to a carbon atom. |
Non-metal | The elements of hydrogen, carbon, halogen (fluorine, chlorine, bromine, iodine and astatine), oxygen, sulfur, phosphorus, silicon, nitrogen, boron, selenium, tellurium and noble gases (helium, neon, argon, krypton, xenon and radon). |
Metal | Any element other than a non-metal. |
Organic compound | Compound satisfying one of the following criteria: At least two carbon atoms bonded to each other, or One carbon atom bonded to at least one hydrogen atom or halogen atom, or One carbon atom bonded to at least one nitrogen atom by a single or double bond.Exceptions to the above criteria are: compounds consisting of only carbon atoms (e.g., fullerenes, etc.), cyanogen, cyanogen halides, cyanamide, metal carbides, phosgene, thiophosgene, hydrocyanic acid, isocyanic acid, isothiocyanic acid, fulminic acid, unsubstituted carbamic acid, and salts of the previously mentioned acids; these exceptions are considered to be inorganic compounds for classification purposes. |
Phosphonic acid | R-P(=O)(OH)2 (wherein R-P is a P-C bond) |
Phosphinic acid | (R)2P(=O)OH (wherein R-P is a P-C bond)) |
Polysaccharide | A compound having more than five saccharide radicals attached to each other by glycosidic linkages. |
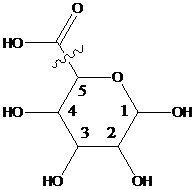
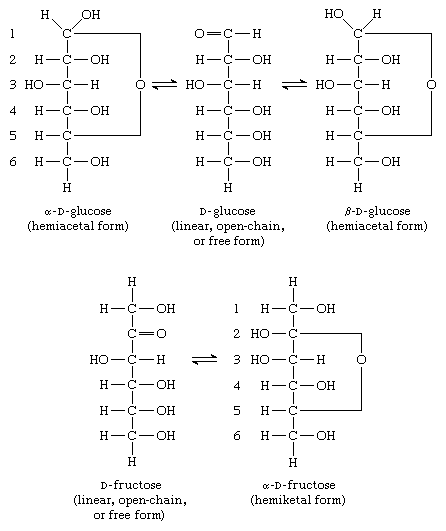
Saccharide radical | Radical derived from acyclic polyhydroxy-aldehydes or acyclic polyhydroxy-ketones, or from their cyclic tautomers, by removing hydrogen atoms or by replacing hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium, in accordance with either of the following definitions: Consists of an uninterrupted carbon skeleton and oxygen atoms directly attached thereto, andIs considered to be terminated by every bond to a carbon atom of a cyclic structure and by every bond to a carbon atom having three bonds to hetero atoms, e.g. ester or nitrile radicals, and example:the saccharide radical consists of an uninterrupted carbon skeleton of 5 carbon atoms and oxygen atoms directly attached thereto, and is considered to be terminated by the bond to the carbon atom having three bonds to oygen (i.e. hetero atom ) |
Salt | Compound consisting of at least one anionic part and at least one cationic part. Carboxylate salts – products where the hydrogen in a carboxyl group is replaced by an ion of metal or other cation. |
Chemical compounds and their preparation are classified in the groups for the type of compound prepared. The processes of preparation are also classified in the groups for the types of reaction employed, if of interest.
General processes for the preparation of a class of compounds falling into more than one main group are classified in the groups for the processes employed, when such groups exist.
The compounds prepared are also always classified in the groups for the types of compound prepared.
That means that a process will be always be given at least a class for the products obtained by that process, regardless of whether the product is known or not.
This place covers:
Phosphorylation is the addition of a phosphate radical (such as PO43- or esters thereof) or polyphosphoric acid radicals (having general formula -O(PO2OH)x-, where x = number of phosphoric units in the molecule). Polyphosphoric acid racidals can be linear or cyclic or be esterified.
This place covers:
Examples:
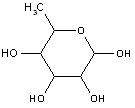
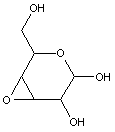
Attention is drawn to the following places, which may be of interest for search:
Production of sucrose; apparatus specially adapted therefor (chemically synthesised sugars or sugar derivatives C07H ; fermentation or enzyme-using processes for preparing compounds containing saccharide radicals C12P 19/00 ) | |
Saccharides, other than sucrose, obtained from natural sources or by hydrolysis of naturally occuring di-, oligo- or polysaccharides (chemically synthesised sugars or sugar derivatives C07H ; polysaccharides, e.g. starch, derivatives thereof C08B ; malt C12C ; fermentation or enzyme-using processes for preparing compounds containing saccharide radicals C12P 19/00 ) |
This place covers:
Examples:
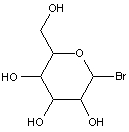
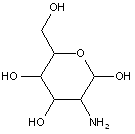
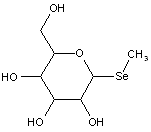
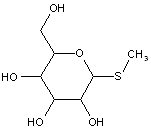
This place covers:
Examples:

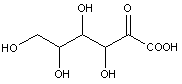
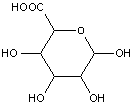
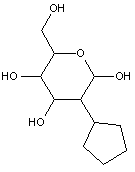
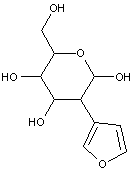
Compounds wherein the anomeric carbon is connected to non-saccharide radicals through a carbon-to-carbon bond are not classified in C07H (but in C07D).
The anomeric carbon cannot be connected to non-saccharide radicals through a carbon-to-carbon bond (i.e. the anomeric carbon must have two bonds to oxygen, or the two hetero bonds to oxygen can be replaced by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium)
This place covers:
Examples:
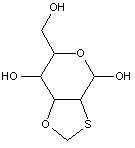
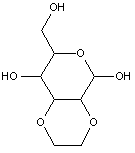
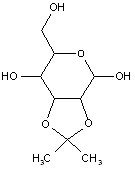
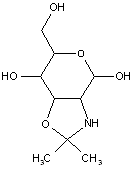
This place covers:
Examples:
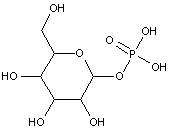
Attention is drawn to the following places, which may be of interest for search:
Phosphonates i.e. esters of R-P(=O)(OH)2 (wherein R-P is a P-C bond) |
This place covers:
Examples:
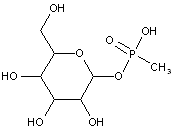
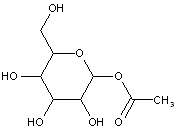
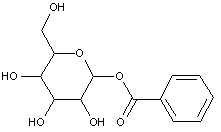
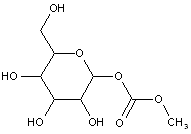
This place covers:
Examples:
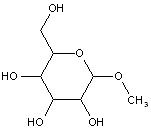
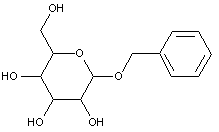
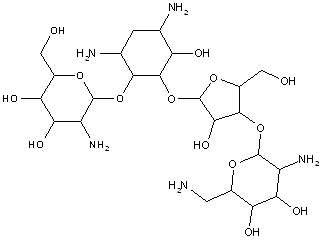
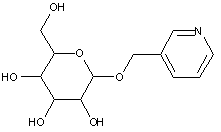
This place covers:
Examples:
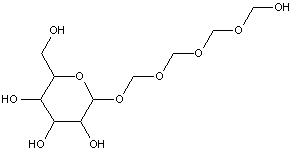
Attention is drawn to the following places, which may be of interest for search:
Polyoxyalkylene derivatives of polyols in general |
This place covers:
Examples:
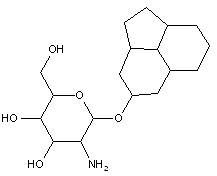
Attention is drawn to the following places, which may be of interest for search:
Steroid glycosides |
This place covers:
Examples:
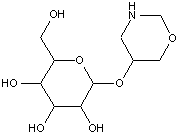
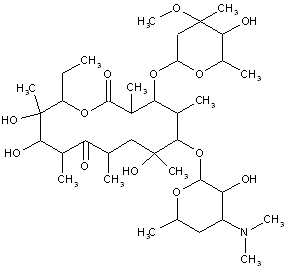
This place covers:
Examples:
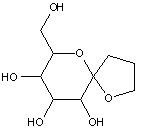
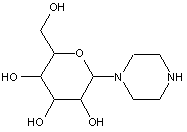
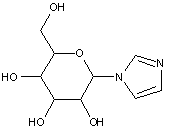
This place covers:
Examples:
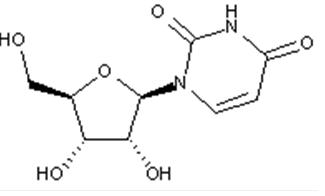
Compound falling within main group C07H 19/00 with ribosyl as the saccharide radical:
This sub-group contains only saccharide radicals wherein the saccharide radical is ribosyl or its derivatives obtained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the ribosyl), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
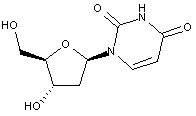
Compound falling within main group C07H 19/00 with 2-deoxyribosyl as the saccharide radical:
This sub-group contains only saccharide radicals wherein the saccharide radical is 2-deoxyribosyl or its derivatives obatained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the 2-deoxyribosyl), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
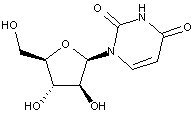
Compound falling within main group C07H 19/00 with arabinosyl as the saccharide radical:
This sub-groups contains only saccharide radicals wherein the saccharide radical is arabinosyl, or its derivatives obatained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the arabinosyl radical), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
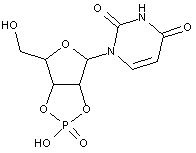
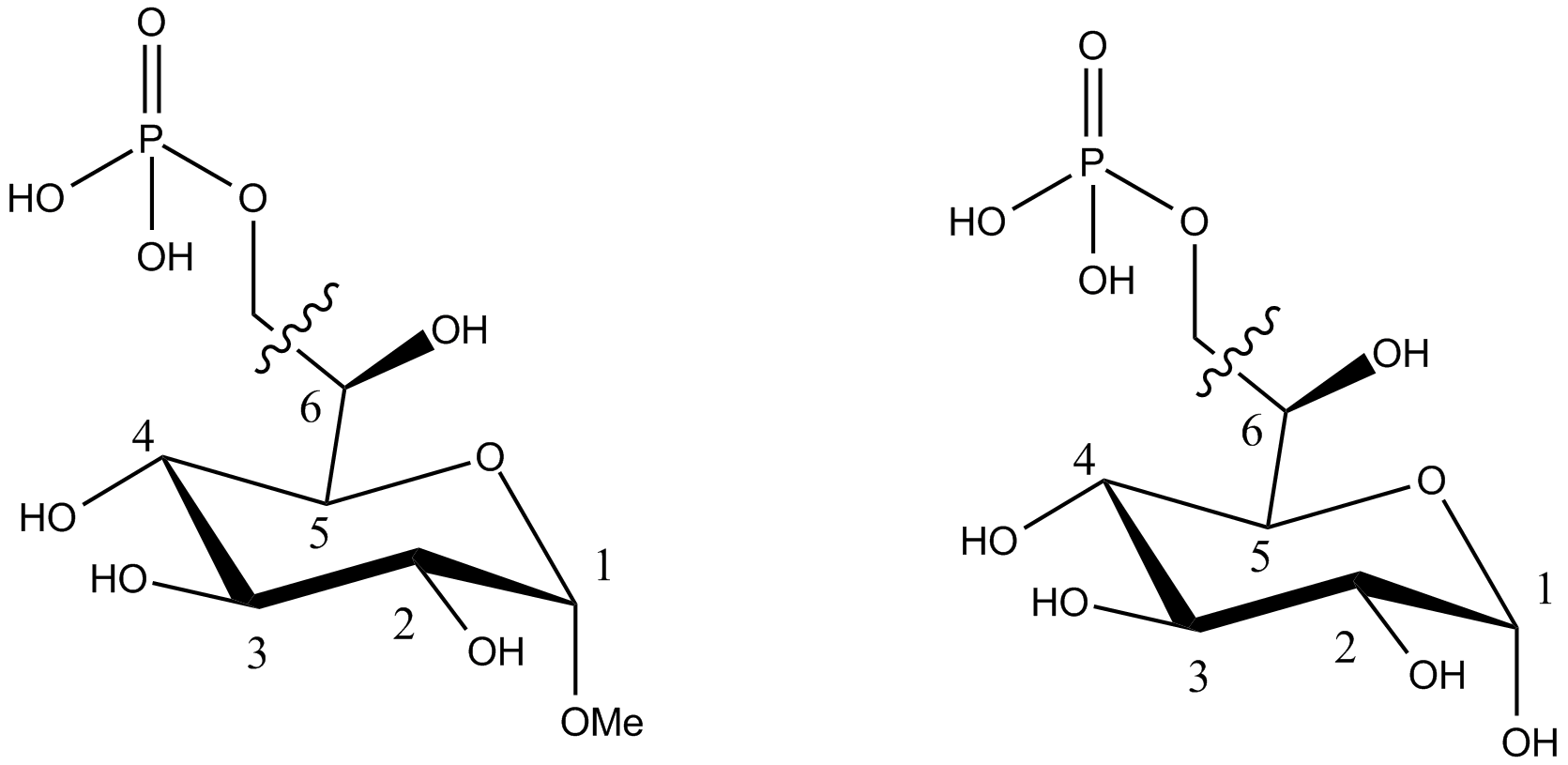
Compound falling within main group C07H 19/00 with the saccharide radical being esterified by phosphoric or polyphosphoric acids, or cyclic phosphates thereof:
this sub-group contains only saccharide radicals wherein the phosphor has valence (V), i.e. phosphoric acid derivatives.
Compounds wherein the phosphor atom has valence (III) are not classfied in those sub-groups but are classified in a previous sub-group.
The molecule above is classified in the lower sub-group C07H 19/11.
This place covers:
Examples:
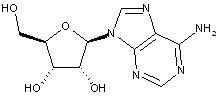
Compound falling within main group C07H 19/00 with ribosyl as the saccharide radical:
This sub-group contains only saccharide radicals wherein the saccharide radical is ribosyl or its derivatives obatained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the ribosyl radical), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
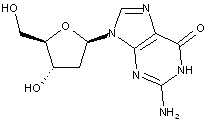
Compound falling within main group C07H 19/00 with 2-deoxyribosyl as the saccharide radical:
This sub-group contains only saccharide radicals wherein the saccharide radical is 2-deoxyribosyl or its derivatives obatained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the 2-deoxyribosyl radical), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
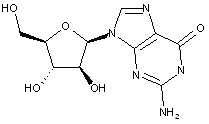
Compound falling within main group C07H 19/00 with arabinosyl as the saccharide radical:
This sub-group contains only saccharide radicals wherein the saccharide radical is arabinosyl, or its derivatives obatained by replacing at the most four of the specified hetero bonds to oxygen by the same number of hetero bonds to halogen, nitrogen, sulfur, selenium, or tellurium (without changing the original stereochemistry of the arabinosyl radical), in accordance with the definition b) above for a saccharide radical.
Compounds not falling exactly within the definition above are classified in a previous sub-group.
This place covers:
Examples:
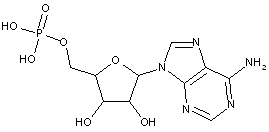
Compound falling within main group C07H 19/00 with the saccharide radical being esterified by phosphoric or polyphosphoric acids, or cyclic phosphates thereof:
this sub-group contains only saccharide radicals wherein the phosphor has valence (V), i.e. phosphoric acid derivatives.
Compounds wherein the phosphor atom has valence (III) are not classfied in those sub-groups but are classified in a previous sub-group.
This place covers:
Examples:
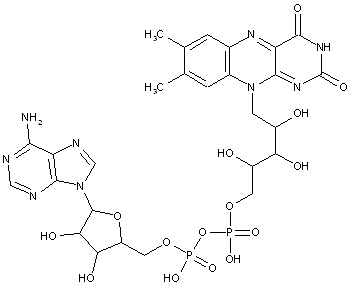
Compound falling within main group C07H 19/00 with the saccharide radical being esterified by phosphoric or polyphosphoric acids, or cyclic phosphates thereof:
this sub-group contains only saccharide radicals wherein the phosphor has valence (V), i.e. phosphoric acid derivatives.
Compounds wherein the phosphor atom has valence (III) are not classfied in those sub-groups but are classified in a previous sub-group.
This place covers:
Examples:
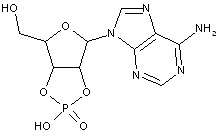
Compound falling within main group C07H 19/00 with the saccharide radical being esterified by phosphoric or polyphosphoric acids, or cyclic phosphates thereof:
this sub-group contains only saccharide radicals wherein the phosphor has valence (V), i.e. phosphoric acid derivatives.
Compounds wherein the phosphor atom has valence (III) are not classfied in those sub-groups but are classified in a previous sub-group.
This place covers:
Examples:
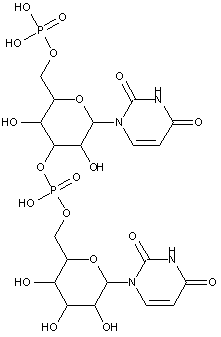
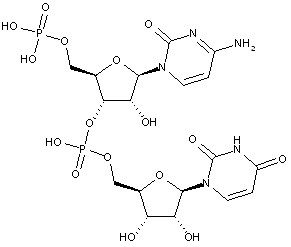

Compounds are classified in this main group only if they are obtained by chemical synthesis using nucleoside/nucleotide building blocks.
Compounds containing two or more mononucleotide units that are not obtained by chemical coupling of nucleoside/nucleotide building blocks together are classified in the two groups below. No classification in C07H 21/00 or sub-groups is given in this case.
Attention is drawn to the following places, which may be of interest for search:
DNA or RNA prepared by recombinant technology, concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification | |
Fermentation or enzyme-using processes for the preparation of compounds containing saccharide radicals |
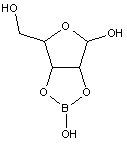
This place covers:
Illustrative examples of subject matter classified in this place:
1.
2.
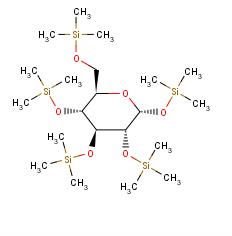
This place does not cover:
Esters with inorganic acids |
Saccharide radicals containing silicon-protecting groups are to be classified in this main group.