CPC Definition - Subclass A61K
This place covers:
Compositions which are:
Used as preparations for dentistry, e.g. for artificial teeth, for filling or for capping teeth, or for taking dental impressions.
Used for cosmetic purposes for treating the skin, hair, nails, teeth or oral cavity with a view to cleaning them, changing their appearance, correcting body odours, protecting them or keeping them in good condition.
Used for medicinal purposes, e.g. drugs, biological compositions, when they are capable of:
- preventing, alleviating, treating or curing abnormal or pathological conditions of the living body by such means as destroying a parasitic organism or limiting the effect of the disease or abnormality by chemically altering the physiology of the host or parasite;
- maintaining, increasing, decreasing, limiting or destroying a physiological body function, e.g. vitamin compositions, sex sterilants, fertility inhibitors, growth promotors or the like;
- diagnosing a physiological condition or state by an in vivo test, e.g. X-ray contrast or skin patch test compositions.
Processes:
- of preparing these compositions;
- of using these compositions or single compounds for medical, dental or toiletry purposes.
Subclass A01N covers the preservation of bodies of humans or animals or plants or parts thereof; biocides, e.g. as disinfectants, as pesticides or as herbicides. The distinction between classification in subclass A61K and subclass A01N is probably best envisaged in terms of the purpose. Subject-matter classified in subclass A61K imparts a direct benefit to the organism to which it is administered, e.g. curative. Subject-matter classified in subclass A01N on the other hand has no benefit to the target organism even though there may well be a secondary benefit to another organism, e.g. pesticides in crop protection.
Subclass C08K covers the use of substances as compounding ingredients.
Compositions per se containing polymers are classified in subclass C08L.
Microorganisms per se are classified in subclass C12N.
Multiple classification
The therapeutic activity of medicinal preparations is further classified in subclass A61P.
The use of cosmetics or similar toiletry preparations is further classified in subclass A61Q.
This place does not cover:
Devices or methods specially adapted for bringing pharmaceutical products into particular physical or administering forms | |
Disinfection, sterilisation or deodorisation of air; Chemical aspects or use of materials for bandages, dressings, absorbent pads or surgical articles | |
Detergent compositions |
Attention is drawn to the following places, which may be of interest for search:
Sex sterilants for invertebrates per se | |
Biocides, pest repellents or attractants per se | |
Wigs | |
Hair pieces, inserts, rolls, pads or the like | |
Processes of waving, straightening or curling hair | |
Artificial nails per se | |
Containers or accessories specially adapted for handling toiletry or cosmetic substances | |
Diagnosis; Surgery; Identification | |
Dentistry | |
Electrotherapy; Magnetotherapy; Radiation therapy; Ultrasound therapy | |
Mixing, e.g. dissolving, emulsifying or dispersing | |
Essential oils; Perfumes | |
Measuring or testing processes involving enzymes or microorganisms; Compositions or test papers therefore; Processes of preparing such compositions; Condition-responsive control in microbiological or enzymological processes | |
Investigating or analysing materials by determining their chemical or physical properties |
Looping references between A61L and A61K have been identified. CPC will be updated/corrected once this inconsistency is resolved in IPC. The current classification practice in CPC is as follows: Both A61L and A61K will be considered as informative reference (classification in both subclasses is done when needed).
Attention is drawn to the definitions of the groups of chemical elements following the title of section C.
In this subclass, with the exception of group A61K 8/00, in the absence of an indication to the contrary, classification is made in the last appropriate place.
Attention is drawn to the Notes in class C07, for example, the Notes following the title of the subclass C07D, setting forth the rules for classifying organic compounds in that class, which rules are also applicable, if not otherwise indicated, to the classification of organic compounds of subclass A61K.
A composition, i.e. a mixture of two or more ingredients, is classified in the last of groups A61K 31/00 - A61K 47/00 that provides for at least one of these ingredients.
Any part of a composition which is not identified by classification according to the last appropriate place of an active ingredient, which itself is determined to be novel and non-obvious, must also be classified. The part can be either a single ingredient or a composition in itself.
Any part of a composition which is not identified by the classification according to the last appropriate place, which is considered to represent information of interest for search, may also be classified. This can for example be the case when it is considered of interest to enable searching of compositions using a combination of classification symbols. Such non-obligatory classification should be given as "additional information".
This place covers:
Chemical compositions:
- for temporarily or permanently fixing teeth
- for artificial teeth
- for filling or capping teeth
- for dental root treatment
- for taking dental impressions
Apparatus, devices and methods per se for the application of the dental compositions are classified in the subclasses corresponding to their general function, e.g. impression cups or impression trays (A61C 9/00 or A61C 9/0006); impression methods (A61C 9/00); computer assisted-sizing (A61C 13/0004).
Chemical compounds/compositions per se without claimed use as dental composition are to be primarily classified in the subclasses corresponding to their chemical features (e.g. C07C, C07D, C08L, C04B, etc.), with a secondary classification in A61K 6/00 if the use in a dental treatment is further claimed.
A61K 6/90 covers dental compositions for taking dental impressions. However analysis and determination, detecting (2D or 3D) by using computer imaging methods are classified G06T 19/00.
Images-producing devices are classified in A61B 90/00 .
Surgical adhesives or cements are classified in A61L 24/00 and ionomer cements, e.g. glass-ionomer cements are classified A61L 24/12.
Attention is drawn to the following places, which may be of interest for search:
Preparations for care of the teeth, of the oral cavity or of dentures | |
Dentistry | |
Dental machines for boring or cutting | |
Dental tools or instruments | |
Filling or capping teeth | |
Orthodontics, e.g. brackets, arch wires, etc. | |
Dental implants, e.g. means to be fixed to the jawbone | |
Means to be fixed to the jawbone for consolidating natural teeth or for fixing dental prostheses thereon characterised by the material or composition, e.g. ceramics, surface layer, or metal alloy | |
Impression cups, impression methods | |
Dental prostheses | |
Computer-assisted sizing | |
Tools for fastening artificial teeth | |
Fastening dental prostheses in the mouth using adhesive foils or adhesive compositions | |
Fastening of peg-teeth in the mouth | |
Cements | |
Acyclic or carbocyclic compounds | |
Heterocyclic compounds | |
Inorganic or non-macromolecular organic substances as compounding ingredients | |
Resins compositions | |
Alloys |
In the following groups the focus is on the intended use; the subgroups within a class are used to index further characteristics of the composition:
A61K 6/30: for temporarily or permanently fixing teeth
A61K 6/40: as primers
A61K 6/50: for dental root treatment
A61K 6/80: for artificial teeth, for filling or capping teeth
A61K 6/90: for taking dental impressions
The following groups are used as an indexing scheme to classify further features of the chemical composition:
A61K 6/15: characterised by physical properties
A61K 6/802: use of ceramics
A61K 6/84: use of metals or alloys
A61K 6/849: use of inorganic cements
A61K 6/884: use of natural or synthetic resins
In groups A61K 6/30-A61K 6/58 and A61K 6/887-A61K 6/90 the use of specific polymers is indicated by addition of classification symbols of the subclass C08L, creating the correspondent Combination-set, e.g. compositions for taking dental impressions containing alginates are classified in (A61K 6/90, C08L 5/04).
Further details of subgroups
Polymers obtained by reactions only involving carbon to carbon unsaturated bonds are classified in A61K 6/887 in combination with the C08L subclass identifying the specific monomer; if only the general polymerisation mechanism is characterising, and not a specific monomer, documents are to be classified in A61K 6/887 without combination subclass.
In the special case of glass ionomer cements, documents are to be classified in the groups A61K 6/889 (Polycarboxylate cements).
A glass ionomer cement is a dental cement mixture (CaF2-Al2O3-SiO2 + polycarboxylate) of low strength and toughness produced by mixing a powder prepared from a calcium aluminosilicate glass and a liquid prepared from an aqueous solution of polycarboxylates.
Polymers obtained otherwise than by reactions only involving carbon to carbon unsaturated bonds are classified in A61K 6/891 in combination with the C08L subclass identifying the specific polymer; if a variety of polymers is mentioned, no particular type of polymer being characterising, documents are to be classified in A61K 6/891 without combination subclass.
In the special cases of polyurethanes (A61K 6/893), polyorganosilicon compounds (A61K 6/896), polysaccharides (A61K 6/898) documents are to be classified also in said groups.
This place covers:
Compositions characterised by physical properties and physical parameters in general. For example, a composition of a semi-crystalline resin and nanocluster with the physical property of being self-supporting. Furthermore the self-supporting structure has sufficient malleability to be reformed into a second shape, preferably at a temperature of about 15°C to 38°C, more preferably at a temperature of about 20°C to 38°C etc.
Attention is drawn to the following places, which may be of interest for search:
Image-producing devices | |
Acyclic or carbocyclic compounds | |
Heterocyclic compounds | |
Manipulating 3D models or images for computer graphics |
Documents are classified in A61K 6/15 only if the characterising physical property is other than:
- refractive index (A61K 6/16)
- particle size (A61K 6/17)
- retraction (A61K 6/18: compositions causing retraction are able to widen the sulcus, for making dental impressions or removing teeth)
- self-expansion (A61K 6/19: compositions having self-expanding properties are able to overcome the effect of polymeric shrinkage)
This place covers:
Protective coatings for natural or artificial teeth such as anti-abrasive coatings or remineralization coatings with particles, dye coatings for natural appearance, and temporary acid-resistant coatings during treatment as well as varnishes.
This place covers:
Material used for assessing if the surface of a tooth is regular or not, for instance by determining the surface contact points.
This place covers:
Chemical compositions for temporarily or permanently fixing teeth, palates or the like. For example, dental adhesives and primers for dental adhesives.
Surgical adhesive or cements are classified in A61L 24/00.
Attention is drawn to the following places, which may be of interest for search:
Preparations for care of the teeth, of the oral cavity or of dentures |
If the type of polymer is characterized as an adhesive, the polymer should be classified in combination with the appropriate C08L subclass for specifying the kind of polymer. For example, using hydroxy cellulose as adhesive: Combination-set (A61K 6/35, C08L 1/02). The specific use of stabilising dentures in the mouth are to be classified in A61K 6/35.
This place covers:
Primer compositions used before applying a further material or layer thereon.
A primer is a chemical composition for modifying and pre-treatment of (dental) surfaces, resulting in a preparatory surface modification on materials before further treatment, ensuring better adhesion, but not to be confused with dental adhesives, which are classified in A61K 6/30.
This place does not cover:
Chemical means for temporarily or permanently fixing teeth, palates or the like, including adhesive primers |
Primers are to be classified in combination with the appropriate C08L subclass for specifying the kind of polymer, e.g. a primer composition based on homopolymers or copolymers of methyl methacrylate: Combination-set (A61K 6/40, C08L 33/12); if the kind of polymer is not characterizing, they are to be classified in the group A61K 6/40.
This place covers:
Chemical compositions for the treatment of the root canal:
- for cleaning or disinfecting
- for filling or sealing
- for apical treatment
- and in combination with dental implants
This place covers:
Chemical compositions for dental treatment characterised by the presence of organic or organo-metallic additives.
This place covers:
Chemical compositions for dental treatment characterised by the presence of inorganic additives.
This place covers:
Chemical compositions for artificial teeth, for filling or for capping teeth with respect to e.g. crowns, inlays, onlays, and filling material based on polymeric, metallic, ceramic, cement and composite materials.
Documents are to be classified in the group A61K 6/80 only if no more appropriate class can be found according to the material used:
- Ceramics (A61K 6/802), further subdivided into different kinds of ceramics; based on the main metal used, documents are to be classified in the subgroups A61K 6/804 - A61K 6/827.
- Cermets (Ceramics-Metal) composites (A61K 6/829).
- Materials comprising non-metallic elements or compounds thereof (A61K 6/831), further subdivided into: glass-ceramic composites (A61K 6/833); glasses (A61K 6/836); phosphorus compounds, e.g. apatite (A61K 6/838).
- Metals or alloys (A61K 6/84), further subdivided into: rare earth metals (A61K 6/842); noble metals (A61K 6/844), amalgams (A61K 6/847).
- Inorganic cements (A61K 6/849); based on the chemical features of the cement, documents are to be classified in subgroups A61K 6/851 - A61K 6/882.
- Natural or synthetic resins (A61K 6/884).
Specific resins are to be classified in the appropriate class (A61K 6/887 or A61K 6/891, see below) in combination with a C08L class for specifying the kind of polymer; only if the resin used is very general, documents are to be classified in the main group A61K 6/884.
This place covers:
Chemical compositions for taking dental impressions.
Attention is drawn to the following places, which may be of interest for search:
Image-producing devices | |
Impression methods | |
Impression cups or impression trays | |
Analysis and determination, detecting (2D or 3D) - computer assisted-sizing |
The specific chemical compositions for taking dental impressions are to be classified in combination with the appropriate C08L subclass for specifying the kind of polymer, e.g. containing alginates are classified in Combination set (A61K 6/90, C08L 5/04); for general polymer compositions, documents are to be classified in the main group A61K 6/90.
This place covers:
Compositions for making-up the skin e.g. lipsticks, rouge, mascara, foundation or preparations for removing make-up, manicure and pedicure preparations e.g. nail polish and nail coating remover, hair care preparations e.g. Shampoo, preparations for permanent waving or straightening, bleaching, dying or conditioning the hair, preparations for affecting hair growth e.g. treatment of hair loss or for slowing hair grow, Preparations for removing hair e.g. shaving and depilatory preparations, oral care preparations e.g. dentifrices, formulations for perfume preparations, Anti-perspirants or body deodorant, barrier preparations e.g. insect repellents or sunscreens, preparations for care of the skin e.g. whitening, tanning, slimming, anti-aging or cleansing preparations.
Processes of preparing and using the cosmetic or similar toilet preparations.
Use of cosmetics or similar toilet preparations should be further classified in subclass A61Q.
Soap bars (i.e. solid cleansing compositions) are classified in C11D, even though general cleansing compositions which are usually liquid are classified in A61Q and in A61K 8/00.
Compositions which can be used in a therapeutic treatment should be classified in A61K 31/00 - A61K 51/00 and subgroups if they are defined by their ingredients or in A61K 9/00 and subgroups, if they are defined by their physical form.
Ingested compositions like food or dietary supplements having an external cosmetic effect are further classified in A23L. Examples might be dietary supplements to strengthen nails or to act against skin aging, or drops or sweets affecting the oral cavity
Attention is drawn to the following places, which may be of interest for search:
Preparations for dentistry | |
Medicinal preparation characterised by physical form | |
Medicinal preparation characterised by chemical ingredients | |
Artificial eyelash or hair | |
Artificial nails | |
Casings or accessories for storing or handling of solid or pasty toilet or cosmetic substances | |
Artificial skin | |
Deodorisation of air | |
Chemical compounds as such | |
Essential oils or perfumes per-se | |
Bar soap | |
Dyeing of wool |
In each of groups A61K 8/02 and A61K 8/18, in the absence of an indication of the contrary, classification is made in the last appropriate place.
Each relevant compound (i.e. which belong to the core of the invention (see* in Glossary of terms)) is classified according to the last place rule in one of A61K 8/19 - A61K 8/99.
Only relevant compounds or pertinent physical form, i.e. compounds in the composition or specific form (example: emulsion, foam) linked to the effect aimed, i.e. "core of the invention" (see * in Glossary of terms). To classify a patent document, the claims, the description and the examples have to be checked to assess the core of the invention (see* in Glossary of terms).
-> example I
Only specific compounds, i.e. those present in the exemplified compositions, are classified, not a general concept or a general formula.
-> example II
Attention is drawn to the Notes in class C07, for example the Notes following the title of subclass C07D, setting forth the rules for classifying organic compounds in that class, which rules are also applicable, if not otherwise indicated, to the classification of organic compounds in group A61K 8/00.
Salts or complexes of organic compounds are classified according to the base compounds. If a complex is formed between two or more compounds, classification is made in both places.
Further "properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects" are further classified in A61K 2800/00 and subgroups.
Class combinations
This group is always combined with the subclass A61Q (i.e. principal uses (see** in Glossary of terms)) and can be further classified in A61K 2800/00 and/or A61Q.
Properties of cosmetic compositions or active ingredients thereof or formulation aids used therein and process related aspects | A61K 2800/00 e.g. A61K 2800/522 (Antioxidants) |
The Indexing Codes of this scheme are to be used mainly with classification A61K 8/00 and A61Q and is considered to be obligatory supplementary classification of subject matter already classified in A61K 8/00 and A61Q if applicable.
Compositions or compounds characterised by specific properties |
A61Q is considered as additional information (see** in Glossary of terms), i.e. obligatory supplementary classification of subject matter already classified in A61K 8/00 and A61Q if applicable. This subclass concerns cosmetic uses that are indicated as eventual application(s) for the composition.
->Example III
Reference is further made to annotations in subclasses where it has been considered relevant and helpful.
Example I: "classify only relevant compounds"
A sunscreen composition stabilised with a specific compound will be classified as follow: A61Q 17/04 and under A61K 8/00 for the compound; no class is attributed for the sunscreen agent used in the example, i.e. redundant information. In case where the technical problem of the patent document is linked to a specific compound, the compound is classified!
The same can be observed for compositions containing hair oxidative or direct dyes or skin tanning agent and dealing with an other technical problem, i.e. viscosity, cosmetic properties where the type of cosmetic agent is not pertinent.
Example II: "General formula /specific compound"
A patent document claims the use of an amino acid (group title of A61K 8/44) but the single compound used in the examples is Histidine (i.e. A61K 8/4946) or Cysteine (A61K 8/447). Classes are made only for the concrete compounds explicitly disclosed or present in exemplified compositions in the patent document.
The same is applicable for ingredients described by a general formula (i.e. "Markush formula"), only the concrete compounds are classified, not the common chemical concept.
Example III: principal or additional use(s)
A skin moisturising composition which is further used in a anti-ageing preparation or as lip cream, would be classified in A61Q 19/00, A61Q 19/08 and A61Q 19/001.
If the active is a new sunscreen and an anti-ageing preparation which contains this new compound is exemplified, it should be classified in A61Q 17/04 and A61Q 19/08.
If the active is a compound to condition the hair and a shampoo or a colouring composition (non-permanent hair dyeing containing direct dyes) is exemplified, it should be classified in A61Q 5/12, A61Q 5/02 and A61Q 5/065
In this place, the following terms or expressions are used with the meaning indicated:
(*) Core of the invention | Compounds in the composition or specific form (example: emulsion, foam) linked to the effect aimed. This means that not all compounds present in a composition are classified. To classify a document, the claims, the description and the examples have to be checked. Only specific components, i.e. those present in the exemplified compositions, are classified not a general concept or a general formula. |
(**) Principal use vs. additional use | The principal use is linked to the effect intended in the document and is classified in A61Q. In the claims or examples further uses or types of compositions are often described which should be classified in A61Q. For example: a mixture of compounds is directed to an anti-aging purpose ( A61Q 19/08) which is further introduced in sunscreen composition ( A61Q 17/04 ). |
This place covers:
Films; injections; cotton-swab applicators (Q-tip); paracrystaline or
multiphases; Oral administration
This place does not cover:
Hexagonal or cubic phases being liquid crystals |
Attention is drawn to the following places, which may be of interest for search:
Compositions characterized by special physical form should be classified in group A61K 8/02 together with the appropriate Indexing Code under A61K 2800/00.
This place covers:
Towelettes; impregnated napkins; impregnated sponges; impregnated
non-woven fabrics.
Attention is drawn to the following places, which may be of interest for search:
This place covers:
Packs
Attention is drawn to the following places, which may be of interest for search:
Masks for cosmetics treatment of the face |
This place covers:
Rod-like micelles; globular micelles.
On a phase diagram, a multiple emulsion (classified in A61K 8/066), liquid crystals (classified in A61K 8/0295) and micelles can coexist.
This place covers:
Lyotropic phases; mesophases; mesomorphic phases; lamellar, cubic
(isotropic) or hexagonal phases; nematic, smectic or cholesteric phases;
bilayer lamellar gel network (WO0119343).
Attention is drawn to the following places, which may be of interest for search:
Liquid crystals in medicinal preparations |
Attention is drawn to the following places, which may be of interest for search:
Use of substances as emulsifying, wetting, dispersing or foam-producing agent |
This place covers:
Dispersions of a macromolecular compound in colloidal form in a liquid dispersing phase.
This place covers:
Foamable compositions; self foaming (CO2 releasing) compositions;
sprays; post-foaming compositions.
Effervescent compositions should be classified in subclass A61K 8/046 together with the appropriate Indexing Code under A61K 2800/00.
This place covers:
Direct emulsions (O/W); inverse emulsions (W/O); Pickering emulsions.
This place covers:
Transparent emulsions; o/w nanogel (US2003012759).
This place covers:
Encapsulated compositions having a particle size bigger than 100
micrometers.
This place covers:
Nanosomes; nanovesicles.
Nanosomes and nanovesicles should be classified in subclass A61K 8/14 together with the appropriate Indexing Code under A61K 2800/00.
This place covers:
Perborates; persalts; H 2O 2.
This place covers:
Persulphates; thiosulphates.
This place covers:
Tripolyphosphates; Fluorophosphates (NaMFP).
This place covers:
Glass beads; sand; talc (Mg silicate); Chrysotile (Mg silicate).
This place covers:
Clays in general (aluminosilicates): Bentonite, hectorite, montmorillonite, kaolin; mica; zeolites.
Quaternary ammonium clays should be classified in subclass A61K 8/26 together with subclass A61K 8/416.
This place covers:
Aluminium-zirconium compounds.
This place covers:
Paraffins, e.g. Permethyl 99A (isododecane) or Isopar E (mixture of C 8-C 9 aliphatic hydrocarbons); Squalane; Patrolatum (Vaseline); Carotenes, e.g. lycopene.
This place does not cover:
Chlorofluorocarbons (Freons) |
Attention is drawn to the following places, which may be of interest for search:
Polymeric Chlorofluorocarbons (polymeric Freons) |
Attention is drawn to the following places, which may be of interest for search:
Hydroquinone |
This place covers:
Salts and anhydrides thereof; Dicarboxylic acids.
This place covers:
Salts and anhydrides thereof; Gluconic acids.
This place covers:
Salts and anhydrides thereof.
This place covers:
Carbonic acid ester, e.g. dialkyl carbonates.
This place covers:
Polyglycerins containing from 2 to 10 repeating units in the same chain.
This place covers:
Imine R=N-H; Oximes R-CH=N-OH; Hydrazones R-CH=N-NH 2; Azines
R-CH=N-N=C; Nitriles R-CN; Isocyanates R-N=C=O; Cyanates R-O-CN;
Amine oxides; Quinoneimines; Azo compounds; imides RR'C=NH.
This place covers:
Alkanolamines; Cyanamides NC=NH 2; Sphingosines.
This place does not cover:
Anthraquinones |
This place covers:
Urea (H 2N-C(=O)-NH 2 ) is also included in this group
This place covers:
Betaines; Carbamates.
Attention is drawn to the following places, which may be of interest for search:
Asparagine | |
Glutamine | |
Proline | |
Tryptophan | |
Histidine | |
Peptides, i.e. polymers of 2 or more aminoacids |
This place covers:
Amidobetaines; Cocoamphoacetates; Aspargine; Glutamine.
This place covers:
Sulfonamides; Sulfosuccinates; Sultaines, e.g. Cocoamidopropylhydroxysultaine; Taurine; Isethionates.
This place covers:
Thiazole, pyrrole-oxazole, morpholine; Heterocyclic compounds containing having at least two different hetero atoms in the same or
different hetero ring, condensed or not and/or in the same or different ring system.
This place covers:
Heterocyclic compounds containing one or several hetero rings, condensed or not, in the same or different ring system, each hetero ring
having only one nitrogen as the only hetero atom.
This place covers:
Pyrrole; Pyrrolidine; Proline; Heterocyclic compounds containing one or several not condensed hetero rings, in the same or different ring system, each hetero ring having only one nitrogen as the only hetero atom and having five membered hetero rings.
This place covers:
Phthalimides; Pyrrocoline; Isatin; Trytophan; Heterocyclic compounds containing one or several condensed hetero rings, in the same or different ring system, each hetero ring having only one nitrogen as the only hetero atom and having five membered hetero rings.
This place covers:
Piperidine; Pyridine; Acridine; Quinoline; Heterocyclic compounds containing one or several condensed or not hetero rings, in the same or different ring system, each hetero ring having only one nitrogen as the only hetero atom and having six-membered hetero rings.
Attention is drawn to the following places, which may be of interest for search:
Nicotinic acid |
This place covers:
Heterocyclic compounds containing one or several hetero rings, condensed or not, in the same or different rings systems, each hetero ring having only one nitrogen as the only hetero atom and having sulphur as an exocyclic substituent of the hetero ring.
This place covers:
Pyrazole; Piperazine; Pyridazine; Pyrazine; Heterocyclic compounds containing one or several hetero rings, condensed or not, in the same or different rings systems, and at least one hetero ring having more than one nitrogen as the only hetero atom.
This place covers:
Allantoin (imidazolidinyl urea derivative); Histidine.
This place covers:
Condensed derivatives of the pyrimidine ring; Purines; Orotic acid;
Adenine; Guanine.
Attention is drawn to the following places, which may be of interest for search:
Folic acid |
This place covers:
Oxetane; sorbitan esters; Heterocyclic compounds containing one or several hetero rings, condensed or not, in the same or different rings systems, each hetero ring having oxygen as the only hetero atom.
This place covers:
Genistein; Heterocyclic compounds containing one or several hetero rings, condensed or not, in the same or different rings systems, each hetero ring having oxygen as the only hetero atom and having six membered hetero rings.
This place covers:
Thiophene; Lipoic acid; Compounds containing one or several hetero rings, condensed or not, in the same or different rings systems, each hetero ring having sulphur as the only hetero atom.
This place covers:
Condensed derivatives; Polysorbates.
Attention is drawn to the following places, which may be of interest for search:
Alkoxylated derivatives containing 11 or more oxyalkylene groups in thesame chain |
This place covers:
Organometallic compounds.
This place covers:
Silanes; Silisesquioxanes; Cyclomethicones, e.g. Hexamethylcyclotrisiloxane, Octamethylcyclotetrasiloxane, Decamethylcyclopentasiloxane; Linear volatile siloxanes; Chlorophyll.
Attention is drawn to the following places, which may be of interest for search:
Polysiloxanes |
This place covers:
Oligosaccharides up to 5 monomeric units; Aminosugars, e.g. glucosamine; Uronic acids, e.g. glucuronic acid; Erythrulose.
Attention is drawn to the following places, which may be of interest for search:
Reduced sugar derivatives, e.g. erythritol, glucitol or xylitol | |
Straight-chain acids, e.g. gluconic acid | |
Ethers of disaccharides and a steroid |
This place covers:
Glucosides; Galactosides.
In this place, the following terms or expressions are used with the meaning indicated:
Glycoside | acetal derivative of a sugar. |
This place covers:
Alkylmonoglycosides, oligoglycosides and -polyglycosides.
Attention is drawn to the following places, which may be of interest for search:
Alkoxylated derivatives containing 11 or more oxyalkylene groups in thesame chain |
This place covers:
Cytochromes; glycoproteines; antibodies.
Attention is drawn to the following places, which may be of interest for search:
Polyaminoacids formed from one up to three repeating aminoacid units | |
Medicinal preparations containing peptides | |
Medicinal preparations containing antigens or antibodies |
In this place, the following terms or expressions are used with the meaning indicated:
Peptide | Compounds containing at least two aminoacids units which are bound through at least one normal peptide link. |
Normal peptide link | The link between an alpha-amino group of an amino acid and the carboxyl group - in position 1 - of an other alphaaminoacid. |
Cyclosporins should be classified according to their structures.
This place covers:
Derivatives of ascorbic acid.
This place covers:
Derivatives of tocopherol.
This place covers:
Synthetized pseudoceramide derivatives which are characterized by structures having both amide or nitrogen bonds and hydroxyl groups as hydrophilic units, as well as two long chains; Alkanolamides from sphingosine and a fatty acid; Glycoceramides.
This place covers:
Organic fluorides and Chlorofluorocarbons (Freons).
This place covers:
Natural rubber; Latex; Melanins; Dendrimers; Supra-molecular polymers.
This place does not cover:
Alkylpolyglycosides | |
Nucleic acids | |
Proteins |
This place covers:
Polymers of 6 or more saccharide units, e.g. dextran.
This place covers:
(Malto) dextrin.
Attention is drawn to the following places, which may be of interest for search:
Cyclodextrins |
This place covers:
Locust bean gum; Tara; Ceratonia siliqua.
Attention is drawn to the following places, which may be of interest for search:
Cyclodextrins in medicinal preparations |
This place covers:
Unless otherwise specified in this classification, these group and its subgroups cover the polymers as defined in C08L 23/00 - C08L 49/00.
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
This place covers:
Polydecene.
This place covers:
Sodium polystyrene sulfonate; Polymers obtained from divinylbenzene.
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Homopolymers or copolymers of esters, e.g. (meth)acrylic acid esters per se |
This place covers:
Polymers obtained from polyacrylamidomethylpropane sulphonic acid
(AMPS).
Attention is drawn to the following places, which may be of interest for search:
Such homopolymers or copolymers of amides or imides per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
This class is given only if the nitrogen atom or the nitrogen containing heterocycle ring is directly linked to the polymer forming double bond.
This place covers:
Polyvinylsulfonates; Polythiophenes.
This place does not cover:
Polystyrene sulphonic acid | |
AMPS polymers | |
Polyquaternium-44 |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
This class is given only if the sulphur atom or the sulphur containing heterocycle ring is directly linked to the polymer forming double bond.
This place covers:
Polymers obtained from monomers having conjugated double bonds, e.g. butadiene, isoprene and chloroprene.
This place does not cover:
Polydecene | |
Divinylbenzene | |
Allylmethacrylate | |
Diallylamines, e.g. Polyquaternium-6, Polyquaternium-7, Polyquaternium-22 and Polyquaternium-39 |
Attention is drawn to the following places, which may be of interest for search:
Compositions of such homopolymers or copolymers per se |
This place covers:
Unless otherwise specified in this classification, these group and its subgroups cover the polymers as defined in C08L 59/00 - C08L 87/00;
Polyureas; Polyurilene resin.
This place covers:
Alkoxylated derivatives containing 11 or more oxyalkylene groups in the same chain.
Attention is drawn to the following places, which may be of interest for search:
Alkoxylated derivatives, i.e. derivatives containing from 2 to 10 groups |
Attention is drawn to the following places, which may be of interest for search:
Organo silicon compounds not being polymers |
In this place, the following terms or expressions are used with the meaning indicated:
Saturated | polysiloxanes containing silicone atoms bound to saturated or aromatic group. |
This place covers:
Polysiloxanes derivatives containing from 2 to 10 alkoxy or aryloxy groups; Dimethicone PEG-8 benzoate.
This place covers:
Polysiloxanes derivatives containing 11 or more oxyalkylene groups.
This place covers:
Crosspolymers obtained from vinyl dimethicone.
This place covers:
Mixtures of several compounds of different nature and being oils, fats or waxes.
This place covers:
Humus; Tar; Charcoal; Saponins; Tannins with no specific chemical structure.
Attention is drawn to the following places, which may be of interest for search:
Condensed tannins, e.g. proanthocyanidines | |
Hydrolysable tannins, e.g. glycosides |
No document should be classified in this subgroup. Every document concerning material or animal origin should be classified in A61K 8/981 and subgroups or in A61K 8/987 and subgroups.
This place covers:
Material from fish or insects; Silk; Natural sponges; Pearls from oysters;
Mother-of-pearl (nacre).
This place covers:
Cell cultures; Yeasts; Plankton; Lower fungi; Bacteria; Krill; Lichens;
Undifferentiated plant seed cells.
Fermentated products should be classified according to their origin together with an Indexing Code, e.g. A61K 2800/85.
This place covers:
Pharmaceutical compositions which are characterised by the following galenical aspects:
- The form (e.g. tablets) (see also "Special rules" section)
- The site of application, i.e. the body location where they are administered (e.g. oral, nasal, rectal compositions)
- The drug release technique (e.g. effervescent compositions, osmotic delivery systems)
In addition, the following is also classified:
- Processes of making such compositions
- Medical uses characterised by any of the above galenical aspects (dosage form, site of application, release technique)
- Excipients for use in a specific dosage form (e.g. tableting excipients)
In A61K "Galenic" form" relates to pharmaceutical (drug delivery) compositions in general. "Galenical" aspects relate to aspects of pharmaceutical technology of pharmaceutical compositions. (i.e. aspects of pharmaceutical compositions other than the active ingredient per se, e.g. physical form, excipients, dosage etc.
It is sufficient to say pill, capsule, particle etc. for classification. The pill or the like does not require further elaboration to warrant classification in A61K 9/00. Any concrete, well-defined pharmaceutical composition disclosed in the examples is considered to be characterized by a physical form. Also classified are independent claims defining galenical aspects of a pharmaceutical composition or a medical use. For example the following forms are considered to be characterized by a physical form:- a suppository, ointment, solution, dispersion, emulsion, aerosol, foam, liposome, powder, granulate, micro/nanosphere, pill, tablet, capsule, micro/nanocapsule, web, sheet, filament, etc. This list is not exhaustive. A borderline case is an animal test where the galenical aspect of the composition is not further defined (e.g. intravenous injection, per os administration), unless it is absolutely clear that the test represents the intended mode of administration.
Galenical aspects of pharmaceutical compositions are usually classified by giving a combination of classes in A61K 9/00 and A61K 47/00. The last place rule does not apply between A61K 9/00 and A61K 47/00 - A61K 47/46. Excipients can be classified in A61K 47/00 or in A61K 9/00, depending on the situation: A61K 47/00 is used to classify excipients in compositions for which A61K 9/00 does not provide information on excipients. No A61K 47/00 is given if A61K 9/00 already provides information on excipients (e.g. tablet excipients are only classified in A61K 9/20...). New excipients per se are (in addition) classified in A61K 47/00.
Conjugates, i.e. compounds comprising a non-active ingredient bound to the active ingredient, are classified in A61K 47/50. Pharmaceutical compositions comprising conjugates may in addition be classified in A61K 9/00.
The active ingredients in pharmaceutical compositions are classified in A61K 31/00 - A61K 45/00, or A61K 48/00 - A61K 51/00.
Bandages for treatment of wounds are classified in A61L. These bandages may comprise active agents, e.g. anti-inflammatory, antibacterial agents to enhance the action of the bandage. Compositions comprising an active agent for wound healing which are neither bandages, nor form bandages (e.g. spray-on bandages) are classified in A61K 9/00. These compositions, e.g. lotions, are classified according to the site of application, A61K 9/0014 and, where relevant, according to the form e.g. A61K 9/06, A61K 9/08 and/or excipients used (A61K 47/00). Bandages for wound treatment should not be confused with transdermal patches, the main function of which is (usually systemic) drug delivery rather than the treatment of wounds. Transdermal patches are classified in A61K 9/7023 and subgroups. Bandages also should not be confused with medicated film (forming) compositions that are not for wound healing; these are classified in A61K 9/7007 (and A61K 9/7015)
This place does not cover:
Nuclear magnetic resonance contrast preparations or magnetic resonance imaging contrast preparations | |
Preparations containing radioactive substances |
Classified are concrete, well-defined pharmaceutical compositions disclosed in the examples. Also classified are independent claims defining galenical aspects of a pharmaceutical composition or a medical use.
All relevant galenical aspects must be classified.
The one dot groups of A61K 9/00 do not follow the last place rule
In principle all examples are classified, also 'standard' examples in documents describing e.g. a new medical use.
However, systematically classifying all excipients in the examples is not necessary, and often undesirable. In any case classified are excipients which are described as being important for the invention, or which the reader can identify as having an important function, e.g. for sustained release. For 'standard' compositions the examiner should choose one or a few excipients to classify. (Note: A 'standard' example is an example that is simple and does not appear to be part of the invention. For instance in a document relating to the new medical use of a (new) chemical compound, often some compositions are given which are 'standard' (if not hypothetical): a tablet with e.g. lactose, microcrystalline cellulose and magnesium stearate, an injection solution with NaCl, etc.
In any case such examples should be classified, whether considered 'standard' or not. In how far all excipients in such compositions are classified is left to the classifier's discretion).
When there are too many examples, they can sometimes be covered by a more general class. However, head groups should not be used for this.
( A "head" group does not have the same meaning as a main group. With "head group" is meant a group which is further subdivided in such a way that classification can always be made in one of the lower groups. For instance A61K 9/2004 is a head group, A61K 9/0012 is a head group, as well as A61K 9/0002. In principle such head groups should be empty.
A61K 9/0002 is the head group for the drug release techniques (see Definition statement). Only its subgroups are used for classification, when relevant. A61K 9/0002 is empty; there is no general sustained release group.
Animal tests to study pharmacokinetic properties of a drug are not classified, unless it is absolutely clear that they represent the intended mode of administration. (Note: What is meant here, are pharmacokinetic tests in animals, e.g. by injection or gavage. Such tests usually say nothing about the final intended dosage forms, but are necessary e.g. for regulatory purposes. The value of their pharmaceutical/galenical information is therefore very low. Exceptions are perhaps inhalation tests in animals, as these are normally only done with drugs intended to be inhaled).
Processes for preparing a composition, even when claimed, are only classified if they appear of interest.
The description and dependent claims are not classified. However, if the document as a whole focuses on one clearly preferred embodiment, this embodiment may be classified, even in the absence of relevant examples or independent claims. The intention here is primarily to avoid that all lists in the dependent claims are fully classified (e.g. all tablet excipients for sustained release, while only one is used in the examples).
If a specific subcombination is claimed, such a subcombination will usually be reflected in the examples, which should in any case be classified. And if this subcombination is not reflected in examples, but clearly forms the invention (following e.g. the description), then it also should be classified. In all other cases, the specific subcombination is probably not inventive, so no need to classify.
In general, information relating to the invention is classified using invention information symbols, while additional information is classified using additional information symbols. This is largely up to the discretion of the examiner. Please note however the following special situations:
- Normally only final compositions are classified, not intermediates. However, it may be useful to classify intermediates as additional information (e.g. a tablet comprising microcapsules; a multicoated microparticle). If the intermediates are claimed separately, they must be classified as invention information.
- If, in the classification scheme, a group refers out to another group, an additional information symbol may still be given for the first group (e.g. oral mucoadhesive film).
In this place, the following terms or expressions are used with the meaning indicated:
Microemulsion | any emulsion with a particle size below 1 μm |
Microparticle | particle having a size between 1 μm and about 3 mm |
Microsphere | homogenous or multi-nuclear particle having a size between 1 μm and about 3 mm |
Microcapsule | capsule or coated particle having a size between 1 μm and about 3 mm |
Nanoparticle, nanocapsule | (coated) particle or capsule having a size below 1 μm |
This place does not cover:
Hollow drug-filled fibres |
Attention is drawn to the following places, which may be of interest for search:
Bandages, dressings or absorbent pads | |
Chemical aspects thereof |
This place covers:
Medicinal formulations or compositions per se containing organic therapeutically active ingredients.
Organic active compounds for use in any first or further medical application.
Use of an organic active ingredient for the manufacture of a medicament for the treatment of a pathological condition.
When a compound is only and exclusively defined in the description and claims by means of a functional definition, and no specific examples at all are provided, and it is unclear whether the functionally defined compound would be a compound according to any of A61K 31/00, A61K 33/00, A61K 35/00, A61K 36/00, A61K 38/00 or A61K 39/00, the main group A61K 45/00 is to be assigned.
For mixtures containing one or more active ingredients without chemical characterisation (i.e. only expressed in term of a functional feature, e.g. antihistamines, PDE4 inhibitors, neuroprotectors, serotonin receptor agonists, etc.), the symbol A61K 45/06 is also to be given in addition to the A61K 31/00 subgroup corresponding to the well defined component(s) of the mixture.
In the case of peptidic enzyme inhibitors wherein the chemical structure is well defined and can be classified under an A61K 31/00 subgroup (e.g. apicidin), that A61K 31/00 subgroup is assigned in addition to the appropriate A61K 38/00 symbol. This applies in the rare cases where a compound could be classified in both groups and thus could be retrieved either via the A61K 31/00 classification scheme or the A61K 38/00 classification scheme, bearing in mind that no information should be lost during the classification process.
Cosmetic preparations which can be used for cosmetic as well as therapeutic indications are classified in both A61K 8/00 and A61K 31/00.
Compositions with a therapeutic application in the form of food, beverages or dietary supplements are further classified in A23L.
Organic active compounds containing non-radioactive isotopes, e.g. deuterium, N15 or C13, are to be classified in A61K 31/00 and not in A61K 51/00.
Contrary to IPC , no A61K 31/00 symbol is given for novel compounds, i.e. compounds claimed per se (including polymorphs and highly pure compounds) falling under subclasses C07C - C07J, even when the set of claims further contains claims relating to their medical use. For those compounds, classification is only made in the relevant subclasses C07C - C07J according to the structure. This exception does not apply to novel compounds falling outside C07C - C07J. Thus, novel polymers also claimed for their therapeutic use will be classified under C08 as well as the corresponding symbol under A61K 31/00.
However, when the invention also discloses medicinal preparations containing said novel compounds, falling under C07C - C07J, in admixture with one or more active organic ingredients, then said novel compounds are also classified in the appropriate A61K 31/00 subgroup followed by symbol A61K 2300/00 to create the corresponding Combination-set, e.g. (A61K 31/60, A61K 2300/00), in addition to the C07 classification.
When a document discloses materials or extracts from algae, lichens, fungi or plants as well as the active component which has been isolated from said material or extract, and said active component has a well defined chemical structure falling under A61K 31/00, a class under A61K 36/00 together with the appropriate A61K 31/00 class is given. This applies unless the isolated active compound is a novel compound falling under C07C - C07J.
This place does not cover:
Empty galenic formulations, i.e. only comprising carriers, additives, excipients | |
Organic active compounds forming salts with heavy metals (unless otherwise specified) | |
Organic compounds claimed per se, i.e. allegedly novel organic compounds | |
General methods in organic chemistry | |
Acyclic and carbocyclic compounds per se | |
Heterocyclic compounds per se | |
Sugars per se | |
Steroids per se |
Attention is drawn to the following places, which may be of interest for search:
In addition to the classification in A61K 31/00, a document has to be forwarded for classification in the following fields if (also) relating to:
Preparations for dentistry | |
Cosmetics, unless a therapeutic effect is also implied | |
Galenic formulations characterized by special physical form | |
Medicaments containing inorganic active ingredients | |
Medicaments containing active ingredients of undetermined constitution | |
Medicaments containing active ingredients from algae, lichens, fungi or plants | |
Medicaments containing peptides or proteins | |
Medicaments containing antigens or antibodies, vaccines, adjuvants | |
Homeopathy, thermotherapy, photodynamic therapy, photoactivable drugs | |
Mixtures of active ingredients without chemical characterization | |
Galenic formulations characterized by the non-active ingredients used | |
Conjugates of active ingredients with a non-active ingredient | |
Medicinal preparations containing genetic material, gene therapy | |
Preparations for testing in vivo, e.g. screening, contrast agents, ultrasound | |
Preparations containing radioactive substances | |
Biocides, pest repellants or attractants | |
Animal feeding-stuffs | |
Food or functional food (nutraceuticals) | |
Prosthese; orthopedic, nursing or contraceptive devices; protections of eyes or ears; bandages, dressing or absorbent pads, first-aid kits | |
Biomaterials | |
Electrotherapy | |
Magnetotherapy | |
Radiation therapy | |
Organic compounds claimed per se | |
General methods in organic chemistry | |
Acyclic and carbocyclic compounds per se | |
Heterocyclic compounds per se | |
Sugars per se | |
Steroids per se | |
Antibodies | C07K/16 |
Detergent compositions | |
Undifferentiate human, animal or plant cells, e.g. cell lines; Tissues; Culture media | |
Coding and non-coding nucleic acids, e.g. ribozymes, antisenses | |
In vitro diagnosis |
General rules
- In the absence of an indication to the contrary, a compound is classified in the last appropriate place (last place rule), i.e. by identifying the portion of molecule which is identified in the lowest possible place in the classification scheme (i.e. the lowest place in print order), and assigning the corresponding subgroup.
- In this group no distinction is made between Invention information and Additional information
What is classified?
- Classified are the concrete, well-defined organic pharmaceutically active compounds disclosed in the claims and/or in the examples. In the absence of specific compounds, generic formulae (Markush formulae) are to be classified.
- In principle all specific compounds mentioned in the claims are classified. However, when there are too many compounds, as in the case of lengthy lists, classification is to be limited to a reasonable number of assignments, i.e. 10-15 compounds, covering e.g. the compounds tested, or the more significant compounds. Any generalisation to the next hierarchically higher level is to be avoided.
How are compounds classified?
When a compound is only and exclusively defined in the application by means of functional definitions, and no specific examples at all are provided, the main group A61K 31/00 will be assigned provided it is clear that the intended compound is an organic compound falling under A61K 31/00. However, should the application refer to said functionally defined compounds as being e.g. inorganic compounds, peptides, proteins, antigens or antibodies, then A61K 31/00 is not to be assigned.
Combinations or mixtures of pharmaceutically active compounds are classified in the appropriate A61K 31/00 subgroup followed by A61K 2300/00 in the corresponding Combination-set for each active ingredient. This also applies to mixtures comprising novel compounds receiving C07 classification. However, the combination of an organic therapeutically active compound with a specified excipient (e.g. crospovidone and mannitol) is not to be considered as a mixture of two therapeutically active ingredients and therefore no Combination-set is to be created. In this case, the assignment of the appropriate symbol in A61K 47/00 for the excipient(s) may be required.
Salts or complexes of organic active compounds are classified according to the corresponding free compounds. However, salts or complexes formed between two or more organic active compounds are classified according to all compounds forming the salts or complexes followed by with A61K 2300/00 to create the corresponding Combination-set (i.e. as a mixture of active organic compounds).
According to the last place rule, salts formed between an organic active compound and heavy metals should be classified in A61K 33/24 - A61K 33/38 and not in subgroups A61K 31/28 - A61K 31/32, A61K 31/555 or A61K 31/714. This does not apply to complexes with heavy metals, as apparent from the A61K 31/00 scheme, wherein the complexes hemin and hematin are classified in A61K 31/555 and cyanocobalamin in A61K 31/714.
If a subgroup title is the name of a specific compound or a group of specific compounds (e.g. A61K 31/203 "Retinoic acid", A61K 31/375 "Ascorbic acid", A61K 31/4415 "Pyridoxine") only exactly the compounds named are classified in this group (not derivatives thereof). For instance A61K 31/203 covers retinoic acid, but not derivatives thereof. However, salts of retinoic acid or ascorbic acid still fall under the respective subgroup for the acid.
On the other hand, when a compound is mentioned in the group title only as an example ("e.g."), such as in A61K 31/5375 "1,4-oxazines, e.g. morpholine", the scope is not limited to this example.
A fusion heteroatom, i.e. a heteroatom which is shared by two (or more) rings in a fused ring system is to be counted for all adjacent rings. Example: indolizine is correctly classified in A61K 31/437.
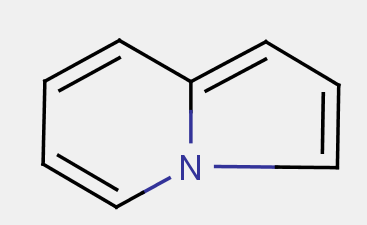
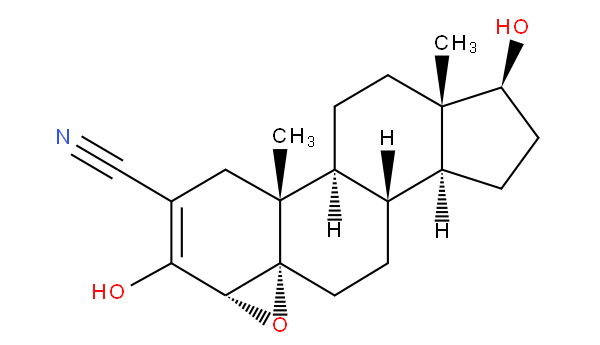
Unless otherwise specified (e.g. when distinguishing between non-condensed pyridines in A61K 31/44 and non-condensed piperidines in A61K 31/445), a class definition using a ring name encompasses all hydrogenated, (partially) dehydrogenated, oxidized (i.e. carrying a keto-group) and/or substituted derivatives. Examples: A61K 31/404 "Indoles" also covers fully hydrogenated derivatives, e.g. trandalopril
A61K 31/4709 "Non condensed quinolines and containing further heterocyclic rings" covers e.g. clinafloxacin
In this place, the following terms or expressions are used with the meaning indicated:
Unless explicitly defined otherwise, all chemical terms have their universally accepted meaning as understood by a chemist, e.g. as defined in the IUPAC Gold Book.
Alkyl | does not encompass alkenyl, alkynyl or cycloalkyl |
Arylalkylamine (A61K 31/137) | - does not encompass arylcycloalkyl-amine; - does not encompass arylalkenyl-amine; - does not encompass N-aryl,N-alkylamine; - encompasses hydroxy-substituted, keto-substituted or halo-substituted arylalkylamine as well as bis-arylalkylamine. |
Aryloxyalkylamine (A61K 31/138) | indicates the sequence Ar-O-Alk-N- and as above, allows substitutions on the Ar and Alk portion. |
Steroids | see definition given in the Note following the title of C07J. |
Carbohydrates and sugars | see definitions given in the Notefollowing the title of C07H. |
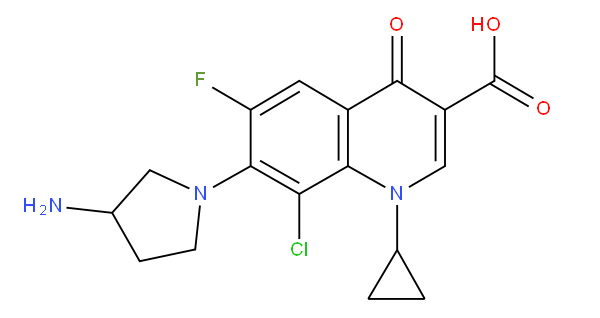
Condensed ring system | Two rings are "condensed" if they share at least one ring member, i.e. not only ortho- and peri-, but also "bridged" and "spiro" are considered as condensed, as for example irbesartan, which is correctly classified in A61K 31/4184 |
Attached to | directly attached, not via a linker, thus for example cyclopentolate is to be classified in A61K 31/216 and not A61K 31/235 |
Bridged ring system | a system which contains interlocking rings, i.e. a ring system where some of the rings constitute a fused ring system with ortho- and/or peri-condensation, and the remaining rings are created by one or more bridges, e.g. amantadine and hexamine |
" Containing carbocyclic rings", or "containing heterocyclic rings" | the plural form does not require the presence of more than one such ring, as for example in trilostane, which is correctly classified in A61K 31/58 |
" Containing heterocyclic rings" [(for compounds classified according to the presence of a specific carbocyclic ring (system)], or"containing further heterocyclic rings" [for compounds classified according to the presence of a specific heterocyclic ring (system)] | does not require these further rings to be part of the specific ring (system) used as primary criterion for classification. Thus for example vecuronium bromide is to be classified in A61K 31/58 |
Condensed with heterocyclic ring system | allows the condensation to occur via the non-heterocyclic ring of the heterocyclic ring system. |
" containing further heterocyclic rings" and "condensed with heterocyclic rings" | also cover compounds having two or more identical heterocyclic rings, e.g. the compounds wherein the further heterocyclic ring is the same as the primary ring. |
"containing" vs. "having" | in the definitions for all heterocyclic compounds, the term "having" requires that the feature indicated in the subgroup is possessed by or is on the heterocycle, whereas the term "containing" relates to an additional feature which can be in any part of the molecule. |
Further details of the subgroups
The term "Arylalkylamines" in A61K 31/137:
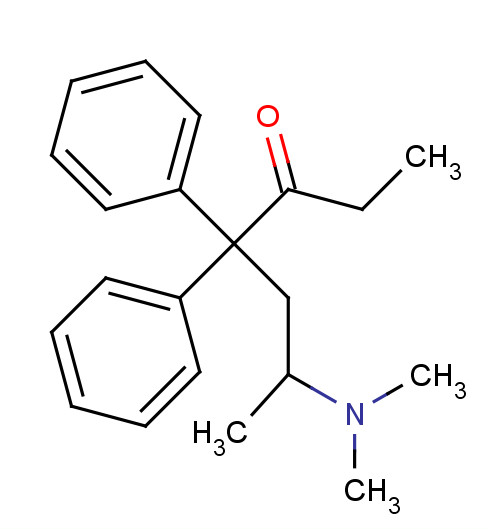
- - encompasses bis-arylalkylamine; for example methadone
- - encompasses hydroxy-substituted, keto-substituted or halo-substituted arylalkylamine; for example clenbuterol and bupropion
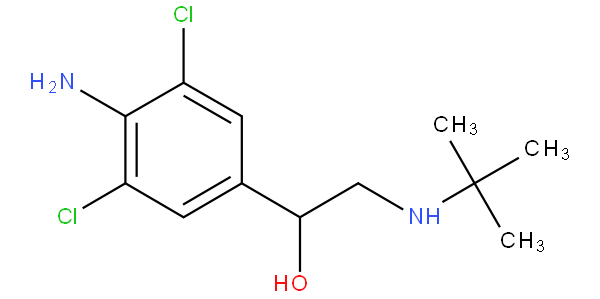
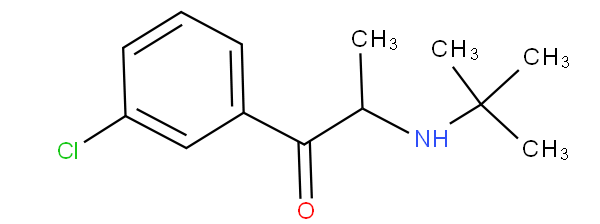
are to be classified in A61K 31/137.
However, if the keto-substitution is adjacent to the amino-group to give an amide, e.g. as in guanfacine
 ,
,
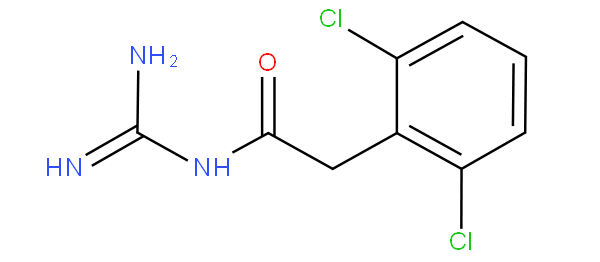
then the amido group would take precedence, thus the compound is not to be classified in A61K 31/137 nor in A61K 31/155 (guanidine), but in A61K 31/165 according to the last place rule.
- - does not encompass arylcycloalkylamine;
for example ketamine and tranylcypromine
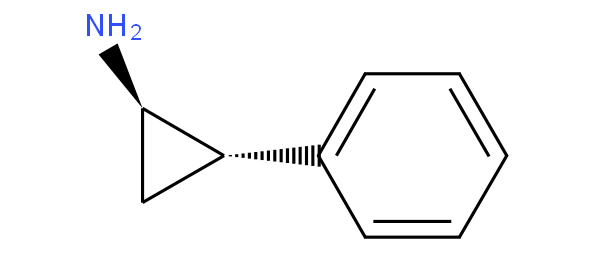
are to be classified in A61K 31/135 not in A61K 31/137.
- - does not encompass arylalkenylamine; for example notriptyline
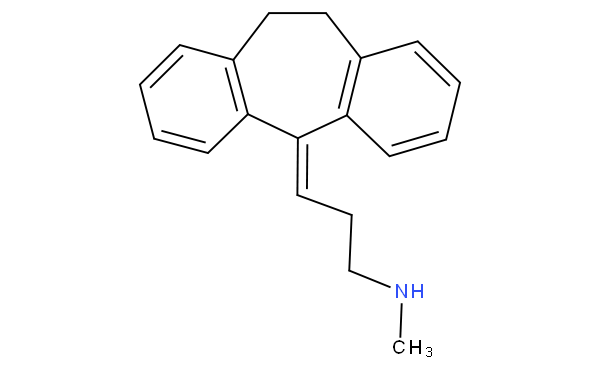
is to be classified in A61K 31/135 not in A61K 31/137.
does not encompass N-aryl,N-alkylamine; for example aprindine
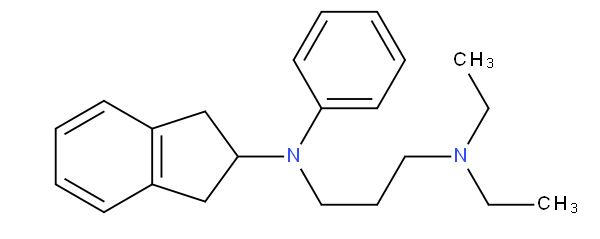
is to be classified in A61K 31/136 not in A61K 31/137.
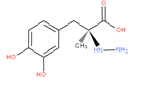
This subgroup also encompasses amino acid derivatives such as e.g. carbidopa, which is seen as an N-amino-substituted amino acid.
Heterocyclic compounds having e.g. "sulfur as a ring heteroatom" and "having five-membered rings" (A61K 31/381), means that the sulfur containing ring must be a five-membered one. For example zileuton, which contains a carbocyclic six-membered ring, is to be classified in A61K 31/381 and not in A61K 31/382.
The same applies to all subclasses relating to heterocyclic compounds.
Ergoline derivatives include a class of compounds characterized by the ring system
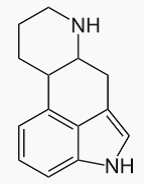
As explicitly mentioned in the scheme, the subclass for the ergoline ring system A61K 31/48 takes precedence over A61K 31/495 "six membered rings with two nitrogen atoms as the only ring heteroatoms" and corresponding subclasses. For example bromocriptine is to be classified in A61K 31/48 and not in A61K 31/495.
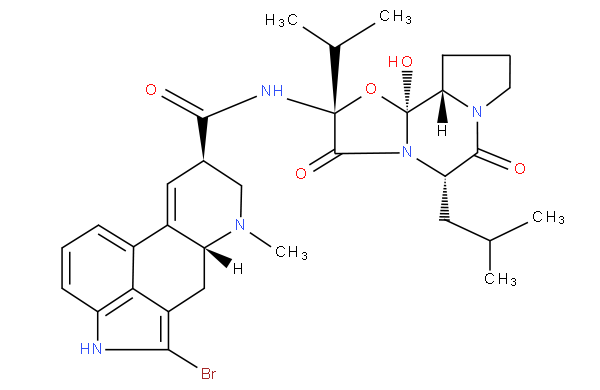
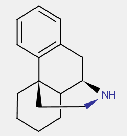
Morphinan derivatives include a class of compounds characterized by the ring system below (with or without an oxygen bridge)
e.g. butorphanol
 ;
;
; dihydrocodeine
Chinconane derivatives are characterized by the ring system
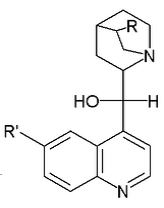
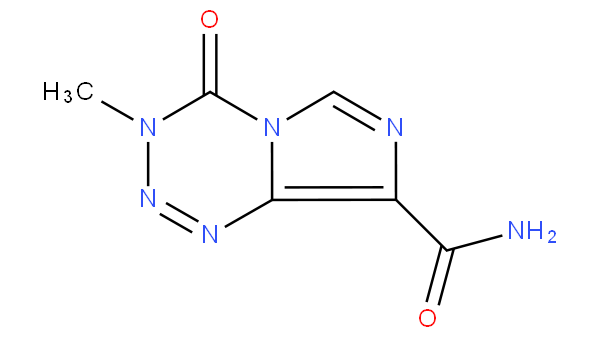
This subgroup also covers compounds having six-membered rings with four nitrogens as the only ring heteroatoms, i.e. tetrazine and tetrazine-containing compounds. For example temozolomide is to be classified in this subgroup.
Barbituric acid derivatives share the structure below
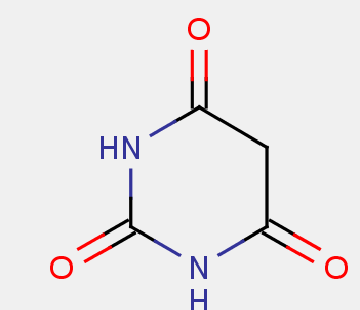
Therefore, primidone is not to be considered a barbituric acid derivative since it is missing a carbonyl group (primidone is to be correctly classified in A61K 31/513).
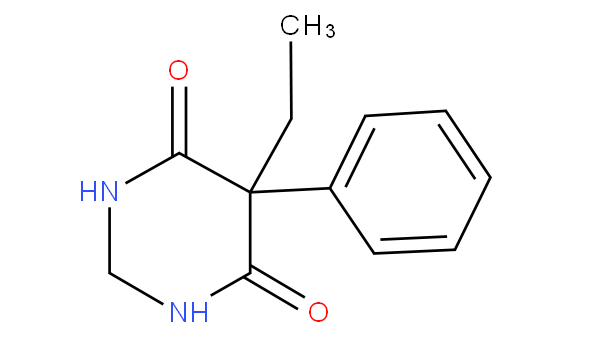
Moreover, the presence of the core structure of barbituric acid may not be sufficient per se in order to identify a compound as a barbituric acid derivative. In this subgroup, the classifier will not only take into account the extent of modification but also whether the compound shares the same pharmacological effect of barbituric acid, i.e. any of sedative, anaesthetic, anxolytic, hypnotic and anticonvulsant effect.
In addition to the examples explicitly referred to in the scheme, other examples e.g. carboplatin, oxaliplatin and sodium stibogluconate are also to be classified here (not in A61K 33/00 nor in A61K 31/282 or A61K 31/29), since they are heterocyclic active compounds forming a complex with a heavy metal.
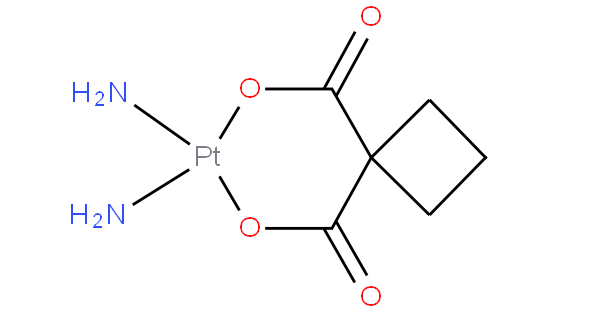
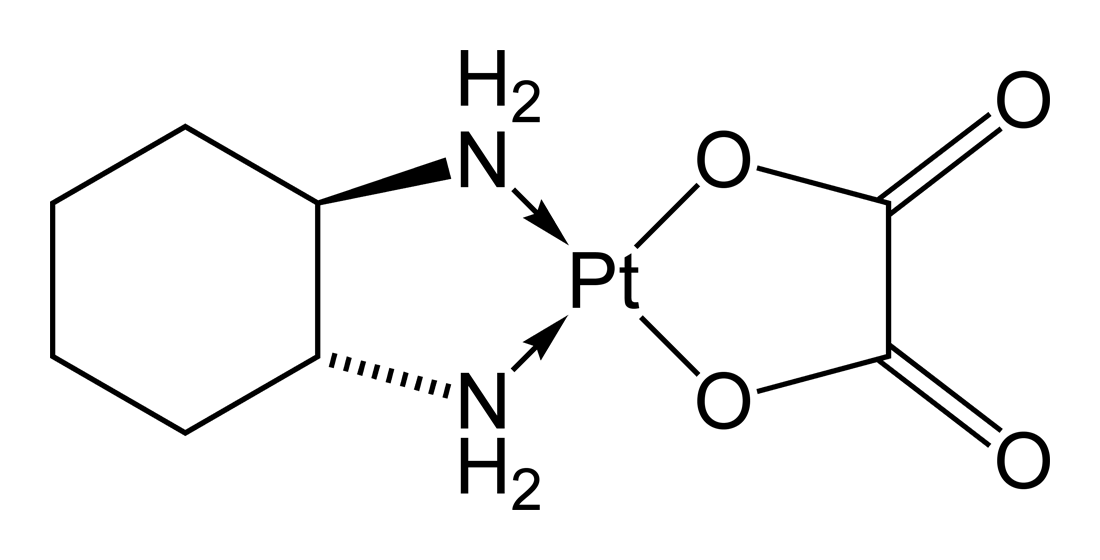
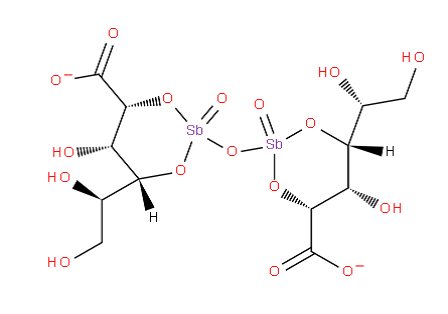
The compounds in these subgroups have the following skeleton
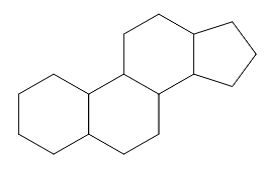
and are further subdivided according to the structure of
estranes
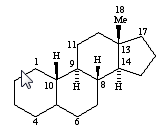
gonanes
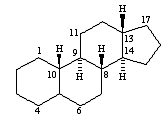
androstanes
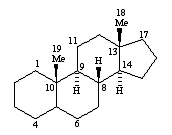
pregnanes
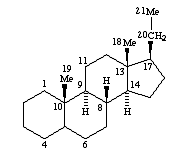
cholanes
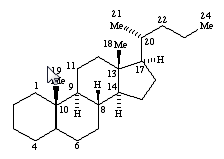
as well as according to the presence or absence of substitutions in position 10, 13, 17 and/or 21.
In subgroups A61K 31/58 and A61K 31/585, the additional ring can be isolated or condensed to the cyclopentanoperhydrophenantrene ring system.
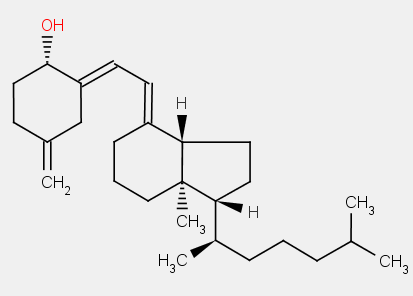
The compounds in these subgroups have the following skeleton
the difference between 9,10-secoergostane derivatives and 9,10-secocholestane derivatives being a double bond in the side chain.
ergocalciferol
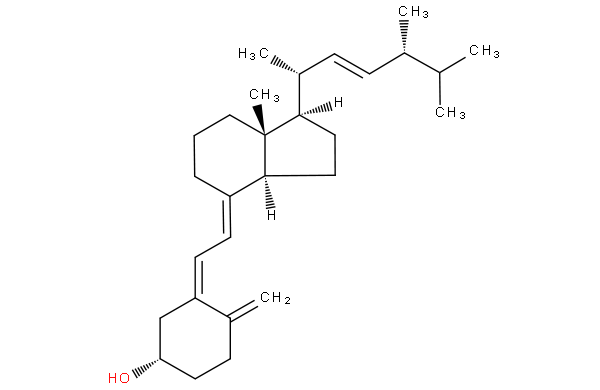
cholecalciferol
These subgroups cover salicylic acid and derivatives thereof.
The presence of the core structure of the 2-hydroxybenzoic acid, i.e.
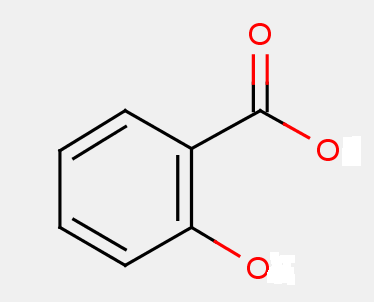
may not be sufficient per se in order to identify a compound as a salicylic acid derivative. In this subgroup, the classifier will not only take into account the extent of modification but also whether the compound shares the same pharmacological effect of salicylic acid, i.e. any of analgesic, antipyretic and antiinflammatory effect. Derivatives with a substitution on the benzene ring are also included in this group (e.g. diflunisal).
For instance, certain compounds are not to be considered salicylic acid derivative due to the extent of the modification and because they do not possess the pharmacological profile of salicylates, e.g. for example the compounds labetalol (A61K 31/166), cisapride (A61K 31/4468), flecainide (A61K 31/4458), metoclopramide (A61K 31/166) and remoxipride (A61K 31/40).
The compounds in this subgroup share the core structure of tetracycline
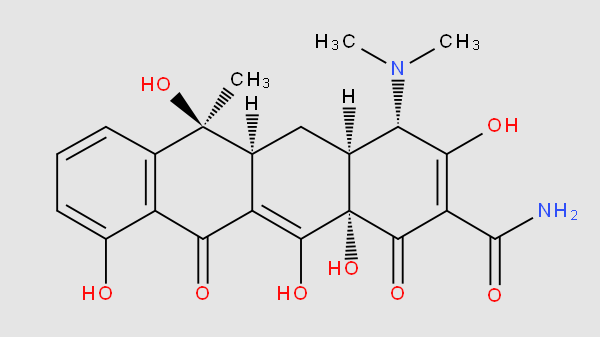
This subgroup also includes non-modified miRNA and snRNA; shRNA and ribozymes.
This subgroup also includes siRNA, pRNA, sd-rxRNA, recombinant nucleic acids, as well as modified miRNA and snRNA.
Antisense nucleotides and non-coding nucleic acids are classified in C12N 15/00.
This place covers:
Medicinal preparations containing inorganic active ingredients either alone or in a mixture with other active ingredients. Organic active compounds forming salts or complexes with heavy metals are also classified in A61K 33/00 according to the last place rule, unless explicit reference to the contrary is made in the A61K 31/00 scheme (see below)
This place does not cover:
Empty galenic formulations, i.e. only comprising carriers, additives, excipients | |
Hemin and hematin | |
Cyanocobalamin | |
Biomaterials | |
Disinfecting or sterilising contact lenses | |
For bandages, dressings or absorbent pads | |
Surgical sutures | |
Surgical adhesives or cements | |
For wound dressings, bandages, also liquid, gel, powder | |
For (coating of) grafts, prostheses | |
For other surgical articles, e.g. stents, embolization | |
Detergent compositions | |
Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefor |
Attention is drawn to the following places, which may be of interest for search:
Pharmaceuticals | |
Organic active ingredients | |
Active ingredients of undetermined constitution (i.e. natural products) | |
Active ingredients from algae, lichens, fungi or plants | |
Peptides, proteins | |
Antigens or antibodies, vaccines, adjuvants | |
Homeopathy, thermotherapy, photodynamic therapy, photoactivable drugs | |
Mixtures of active ingredients without chemical characterisation | |
Characterized by non-active ingredients e.g. carriers, inert additives, excipients... | |
Conjugates; targeted drugs | |
Gene therapy |
Diagnosis
Testing in vivo, e.g. screening, contrast agents, ultrasound | |
Radioactive substances | |
Medicine/pharmacy - mechanical aspects | |
Stents | |
Contraceptive devices | |
Ophthalmic implants | |
Bandages, dressings, absorbent pads | |
Making transdermal patches | |
Tampons (also medicated) | |
Electrotherapy | |
Iontophoresis | |
Magnetotherapy | |
In vitro diagnosis |
Other medical
Other human necessities
Cosmetics | |
Biocides, pest repellants or attractants | |
Animal feeding-stuffs | |
Food or functional food i.e food containing ingredients performing an additional function e.g. disease prevention (neutraceuticals) |
General chemistry
Quaternary ammonium compounds | |
Phosphatides | |
Dendrimers | |
Macromolecular gels |
In this group classification is according to the last place priority rule.
In this group no distinction is made between Invention and Additional information.
In other words all information, whether it be invention or additional, is classified in as far as it relates to material which the classifier considers likely to be of importance for future reference. Trivial or general state-of-the-art disclosure is not classified.
Therapeutic use: an A61K 33/00 classification symbol is given only if the inorganic ingredient has a physiological, pharmacological or biological effect.
Combinations/mixtures: Give a classification symbol followed by A61K 2300/00 for each active ingredient in the mixture. For each mixture containing at least one active ingredient without chemical characterisation (e.g. functional feature), A61K 45/06 is additionally to be given.
This place covers:
Medicinal preparations containing various tissues, cells, organisms, materials or reaction products thereof, also with undetermined constitution, as well as their first or further medical use.
An A61K 35/00 symbol is given only if the therapeutic effect is clearly attributed to the "active substance" of the medicinal preparation, and is an essential part of the disclosure.
The main group A61K 35/00 may overlap with many other subclasses or main groups relating to medicinal preparations, or preparations comprising the tissues, cells organisms or materials, where the effect is neither mainly nor only pharmaceutical. The most relevant areas are genetically modified cells, viral or cellular vaccines, and antibodies.
- Documents relating to microorganisms and cells used for vaccination where there is no other therapeutic use or therapeutic effect are not classified in A61K 35/00 class.
- Genetically modified cells and documents relating to gene therapy:
Gene therapy: A61K 48/00. Genetically modified cells: C12N 5/10.
- Isolated cells: If cells are used as hosts or vectors (no therapeutic activity), no A61K 35/00 class is given. Cells that are transplanted after a culturing step are classified in A61K 35/00 and C12N 5/00.
- Lymphocytes: Overlap with vaccines: Compositions comprising cells of the myeloid line and lymphocytes, when activated by a specific antigen (for example antibodies), are classified in A61K 39/00, since this represents a vaccine. However, therapeutic combinations of antibodies (or fragments thereof) and blood derived cells that have no vaccine effect are classified in A61K 35/00, A61K 39/395 and C07K 16/00 because this is a combination of a vaccine and a therapeutically used cell.
Example of where an A61K 35/17 class is given in regard to lymphocytes: A method of enhancing normal healing in individuals comprising administering to a site in need thereof an effective amount of autologous B cells immediately before, at the time of or immediately after tissue injury.
- Viruses: Pharmaceutical compositions comprising a virus (and its medical use) are classified in A61K 35/00 if it is not a viral vaccine. If the therapeutic effect is clearly and exclusively a vaccine effect, no A61K 35/00 class is given.
Documents relating to viruses per se (without medical use) are classified in C12N 7/00; viral proteins per se C07K 14/005; use of virus as a vector C12N 15/86; use of virus or part thereof as vaccine A61K 39/12; therapeutic use of a viral protein A61K 38/16.
Preparations comprising a modified virus are classified in C12N 7/00; the ICO code A61K 35/13 is given if a therapeutic activity (not vaccine) is suggested. If a therapeutic activity (not vaccine) is disclosed for the modified virus, it is also classified in A61K 35/00.
- Bacteria: If bacteria are claimed per se, they are classified in C12N 1/00 and the CPC symbol A61K 2035/11 is given.
- Toxins of bacteria, snakes, scorpions, fish etc: documents related to protein toxins of these animals only are classified in A61K 38/00. No A61K 35/00 class is given unless the animal or part thereof is also part of the therapeutic composition or use.
- When the composition comprises a special galenic form, it is also classified in A61K 9/00.
- Combinations with other substances: are classified in all appropriate subgroups. For example, combination of lactobacillus and a protein: give A61K 35/74 symbol and also classify in A61K 38/00.
- For each mixture containing at least one ingredient without chemical characterization (e.g. functional feature), A61K 45/06 is to be given.
- When a compound is only and exclusively defined in the application by means of a functional definition, and no specific examples at all are provided, and it is unclear whether the functionally defined compound would be a compound according to any of A61K 31/00, A61K 33/00, A61K 35/00, A61K 36/00, A61K 38/00 or A61K 39/00, the main group A61K 45/00 is to be assigned.
This place does not cover:
Medical use of chemical compounds | |
Plants | |
Algae, Fungi, e.g. yeast | |
Medical use of peptides | |
Microorganisms used for vaccination | |
Vaccines | |
Viruses per se, purification; virus vaccines | |
Medicinal preparations obtained by treating materials with wave energy or particle radiation | |
Compound defined by means of a functional definition | |
Drug delivery systems | |
Transgenic animals | |
Food | |
Orthopaedic methods for treatment for bones or joints | |
Bandages, dressings, surgical articles | |
Chemical compounds as such | |
Bacteria claimed per se | |
Genetic engineering of cells, cell lines per se | |
Screening methods |
Attention is drawn to the following places, which may be of interest for search:
Furthermore, a class in addition to a A61K 35/00 from the list below might be necessary depending on the subject-matter to be classified:
Cosmetic preparations | |
Medicinal preparation characterised by physical, galenic form | |
Medicinal preparations containing inorganic active ingredients | |
Medical use of peptides originating from organisms falling under A61K 35/00 | |
Food or "functional" food, e.g. probiotic bacterial where a medical use and a food is claimed. | |
Therapeutic activity of chemical compounds | |
Modified cells with medical use | |
Modified virus with medical use (not vaccine) |
- Relevant subject-matter that is exemplified, but not claimed, should also be classified. For very long, exhaustive lists of actives claimed for a therapeutic use, if it appears from the dependent claims/description/examples that only few actives are preferred/well described, only these substances will be classified.
The last place rule does not apply in A61K 35/00. Each component of a pharmaceutical composition (if an active ingredient and not a vaccine) that is falling under the definitions of A61K 35/00 is classified
- Mixtures/Combinations comprising several actives classified in A61K 35/00:
A classification symbol is given for each active ingredient in the mixture followed by A61K 2300/00 to create the corresponding Combination-set.
For example, a composition comprising lactobacilli and propolis extract will be allocated the symbols A61K 35/74 and A61K 35/644, each followed by the symbol A61K 2300/00.
For each mixture containing at least one ingredient without chemical characterisation (e.g. functional feature), A61K 45/06 is to be given.
- Compositions comprising cells or non-embryonic stem cells: if the cells are characterized, give the symbol of the corresponding tissue, i.e. Pancreatic stem cells are classified in A61K 35/39, Pancreas. The most relevant types of stem cells relating to different tissues are specifically indicated by the respective symbols.
- Isolated cells that are transplanted without a culturing step, in order to obtain a therapeutic effect are classified in A61K 35/12 if the cell type is not specified. Otherwise classify under the respective tissue.
- Mesenchymal stem cells are always classified in A61K 35/28, irrespective of the origin.
- Important aspects of the invention mentioned only in the description deserve classification as well.
In this place, the following terms or expressions are used with the meaning indicated:
Blue algae | cyanobacteriae |
Bacteriophage | virus |
Protozoa | single cell animal-like eukaryotic organisms, e.g. flagellates (Giardia), amoeboids, sporozoans (Plasmodium) |
Indexing codes used in A61K 35/00:
For additional information purposes:
A61K 35/00 Medicinal preparations comprising living biological materials, e.g. virus, cells, tissue, organs
A61K 2035/11 Medicinal preparations comprising living procariotic cells
A61K 2035/115: Probiotics (used in cases of nutritional compositions with probiotics, where a medical use is not clearly shown but implied, or where the probiotic bacteria are not disclosed and a specific A61K 35/74 class cannot be assigned.
With respect to materials from mammals:
A61K 35/12 Medicinal preparations comprising living eukaryotic cells
A61K 2035/122..for inducing tolerance or suppression of immune responses
A61K 2035/124.. the cells being haematopoietic, bone marrow derived or blood cells
A61K 2035/126.. immunoprotecting barriers, e.g. jackets, diffusion chambers
A61K 2035/128… capsules, e.g. microcapsules
A61K 35/13 Medicinal preparations comprising living viruses
This place covers:
Medicinal preparations containing material from algae, lichens, fungi or plants, or derivatives thereof, e.g. traditional herbal medicines, also of undetermined constitution.
A61K 36/00 is given only if a therapeutic effect is clearly attributed to the "active substance" of the medicinal preparation, and is an essential part of the disclosure. In particular:
Medicinal formulations or compositions per se containing therapeutically active material from algae, lichens, fungi or plants
Material from algae, lichens, fungi or plants for use in any first or further medical application
Use of a material from algae, lichens, fungi or plants for the manufacture of a medicament for the treatment of a pathological condition
Purified active components: if the active component has been isolated from material from algae, lichens, fungi or plants and the chemical structure is well-defined and can be classified under A61K 31/00, a class under A61K 36/00 together with the appropriate A61K 31/00 class is given.
Purified proteins, peptides or lectins: documents related to isolated plant peptides or proteins are classified in A61K 38/00. An additional A61K 36/00 class is given if the source of the purified compound can be clearly attributed to a specific material from algae, lichens, fungi or plants.
Claims to a new galenic formulation of a known material for use in therapy are classified in A61K 9/00. An additional A61K 36/00 class is given for the material only in case of a specific material from algae, lichens, fungi or plants. No A61K 36/00 class is given if only unspecific material (for example "plant extracts", "essential oil") is mentioned.
Cosmetic preparations containing material from algae, lichens, fungi or plants are classified in A61K 8/00. An additional A61K 36/00 class is given for the material only in case of a therapeutic effect.
Food containing material from algae, lichens, fungi or plants is classified in A23L 5/00. An additional A61K 36/00 class is given for the material only in case of a therapeutic effect (functional food).
Bacteria: If bacteria are claimed per se, they are classified in C12N 1/00, medicinal preparations with bacteria are classified in A61K 35/74.
Blue algae (Spirulina) are cyanobacteriae and classified in A61K 35/74.
Honey, beeswax, propolis: A61K 35/644
Genetically modified cells: Compositions comprising genetically modified cells are classified in A61K 48/00 (Gene therapy), or C12N 5/10 (Genetically modified cells).
Separation procedures as such, not relating to a medicinal preparation or a therapeutic use, are classified in B01D.
- When a compound is only and exclusively defined in the application by means of a functional definition, and no specific examples at all are provided, and it is unclear whether the functionally defined compound would be a compound according to any of A61K 31/00, A61K 33/00, A61K 35/00, A61K 36/00, A61K 38/00 or A61K 39/00, the main group A61K 45/00 is to be assigned.
This place does not cover:
Cosmetic preparations containing material from algae, lichens, fungi or plants | |
Medical use of chemical compounds | |
Medicinal preparations containing material of undetermined constitution | |
Medical use of peptides | |
Microorganisms used for vaccination | |
Vaccines | |
Viruses per se, purification; virus vaccines | |
Medicinal preparations obtained by treating materials with wave energy or particle radiation | |
Compound defined by means of a functional definition | |
Drug delivery systems | |
Food | |
Bandages, dressings, absorbent pads containing material from algae, lichens, fungi or plants | |
Separation or extraction processes as such | |
Chemical compounds as such | |
Bacteria claimed per se | |
Genetic engineering of cells, cell lines per se | |
Screening methods |
Attention is drawn to the following places, which may be of interest for search:
Cosmetic preparations e.g. plant material or extracts where a medical and a cosmetic use is claimed | |
Medicinal preparation characterised by physical, galenic form | |
Medicinal preparations containing inorganic active ingredients | |
Medical use of peptides originating from organisms falling under A61K 36/00 | |
Food or "functional" food, e.g. plant material or extracts where a medical use and a food is claimed | |
Modified cells with medical use |
Each specific active component is classified according to its systematic name in A61K 36/00. The last place rule does not apply.
A class within this group is only given for the use of specific compounds, i.e. those present in the claims or exemplified in the description, and therapeutically active agents (which is not always the case, e.g. essential oils used as perfume or taste masking compounds).
Relevant subject-matter that is exemplified, but not claimed, should also be classified. Important aspects of the invention mentioned only in the description deserve a class as well.
Classification should be restricted to the relevant or essential part of the disclosure. For very long lists (10 and more) of material claimed for a therapeutic use, and if it appears from the dependent claims/description/examples that only few plants are preferred/well described, classification should be limited to the compounds tested, or the more significant compounds.
A general concept (e.g. "Compound X for use in treating disease Y, wherein X is a plant, an antibody or a chemical compound" with no specific compounds given) does not get any A61K 36/00 class.
The medicinal preparation can also be in the form of a product by process claim, reflecting a specific extraction process.
If the extraction process is claimed and considered relevant or an essential part of the disclosure, at least one additional Indexing Code in A61K 2236/00 is given.
Combinations/mixtures: a Combination-set is generated having after "," the symbol A61K 2300/00 for each active ingredient in the mixture, for example (A61K 31/60, A61K 2300/00)
For each mixture containing at least one ingredient without chemical characterisation (e.g. functional feature), A61K 45/06 is to be given.
For fermented preparations the Indexing Code A61K 2236/19 is to be added:
A medical preparation with red fermented rice will get A61K 36/899 (rice), A61K 36/062 (Monascus) and A61K 2236/19 (fermentation process)
Pollen (not further specified): A61K 36/00
Wood tar, sap or resin (not further specified): A61K 36/00
This place covers:
Peptides, proteins or their fragments (from dipeptides onwards), or the corresponding nucleic acids encoding these peptides/proteins, when claimed for use in the therapy of humans or animals, i.e. when claimed as therapeutically active components.
In particular:
Medicinal formulations or compositions per se containing therapeutically active peptides or proteins.
Peptides or proteins for use in any first or further medical applications.
Use of a peptide or protein for the manufacture of a medicament for the treatment of a pathological condition.
Proteins or peptides claimed per se, are classified under the proper C07K subgroup; within the same document, further claims to their use in therapy are given the additional information symbol A61K 38/00. An invention information classification should be given in A61K 38/00+ only for documents claiming the further medical use of proteins or peptides without claiming the proteins or peptides per se (Note: The A61K 38/00 scheme is mostly (with exceptions) aligned with the C07K 2/00 - C07K 14/00 schemes, however with less subdivisions. Hence to find the appropriate A61K 38/00 classification subgroup to be given to a protein used in therapy, the procedure consists of checking the place of said protein in the C07K scheme and apply the corresponding place, if correct and justified, in the A61K 38/00 scheme, for the sake of consistency between schemes).
The use of proteins or peptides of known source in therapy, or therapeutic compositions, is classified as additional information in the A61K 35/00 or A61K 36/00 subgroups if the source is considered an important aspect of the invention, as well as in the appropriate A61K 38/00 subgroup for known sequences or A61K 38/02 for unknown sequence. If the source is not known, only a classification in A61K 38/00+ is needed.
When a protein or peptide mixture for use in therapy contains at least one active ingredient without chemical characterisation (e.g. functional feature such as antiphlogistics, anti-cancer agent), invention information classifications should be given in A61K 38/00+ and A61K 45/06.
When a compound is only and exclusively defined in the description and claims by means of a functional definition, no specific examples are provided, and it is unclear whether the functionally defined compound is a compound according to any of A61K 31/00, A61K 33/00, A61K 35/00, A61K 36/00, A61K 38/00 or A61K 39/00, then invention information classification in A61K 45/00 is assigned. For example a general concept (e.g. "Compound X for use in treating disease Y, wherein X is a protein, an antibody or a chemical compound" with no specific compounds given, i.e. the document does not give a single, concrete example of peptidic or proteic compounds) is not classified under A61K 38/00 but under A61K 45/00.
Preparations comprising enzymes are classified under A61K 38/43 - A61K 38/54, followed by a C12Y invention information symbol for the specified enzyme(s). For example, a preparation comprising alpha-galactosidase is classified under A61K 38/47 (i.e. Hydrolases acting on glycosyl compounds) and C12Y 302/01022 (i.e. alpha-galactosidase EC 3.2.1.22).
Claims to "enzyme inhibitors" for use in therapy, without any specific example or without an identified peptidic structure, i.e. "reach-through" claims, are not covered by A61K 38/00 but are classified in C12Q 1/00 or G01N 33/00 as appropriate. At first glance this may appear somewhat of an anomaly however claims of this type are usually a result of a screening process e.g. A compound identified by the method of claim 1 for treatment of disease X. Claim 1 would typically be something like A method for identifying an inhibitor for cholesterol esterase comprising ...etc. As the method for identification of such a compound would be classified in C12Q and no provision is made in A61K 38/00 for classification of compounds without an identified peptidic structure, classification in C12Q alone is deemed sufficient. The same applies to documents classified in G01N for screening purposes. (Note: A "reach-through" claim is defined as a claim attempting to obtain protection for a chemical products' uses, compositions thereof, etc. by defining that product by its functionality in terms of its action e.g. an agonist, antagonist, inhibitor of a biological target such as an enzyme or receptor).
Stabilisation of a specific active protein or peptide with an inert additive (i.e. inactive carrier, targeting agent, potentiator, adjuvant) for use in therapy is classified under the appropriate A61K 38/00 symbol for the therapeutically active protein or peptide and under A61K 47/00. For example, a therapeutic composition comprising growth hormone together with albumin or collagen, the latter being present in the composition for its function as stabiliser of the hormone, will be classified under A61K 38/27 (growth hormone) as well as A61K 47/42 (albumin, collagen) but not under A61K 38/38 (albumin used as therapeutically active agent) nor A61K 38/39 (collagen used as therapeutically active agent).
Proteins or peptides for use in therapy, characterised by the special physical form (i.e. new galenic formulation of a known protein or peptide for use in therapy) may require classification under A61K 9/00. An A61K 38/00 classification is given for the protein or peptide only in case of a specified protein or family of proteins. No A61K 38/00 classification is given as invention information to an exhaustive list of unrelated and unspecific proteins. Instead A61K 38/00 is given as additional information.
For gene therapy, an appropriate A61K 38/00 classification is given for the protein or peptide used in the gene therapy method, together with an A61K 48/00 symbol. The same applies for the use of a nucleic acid encoding a specific protein, or of cells modified to produce a specific protein, for use in gene therapy: an invention information is given both in A61K 38/00+ and A61K 48/00+.
For vaccines comprising peptides or fragments thereof, if the therapeutic effect is clearly and exclusively a vaccine effect, no A61K 38/00 classification is given. The vaccine is classified in A61K 39/00 or one of its subgroups as appropriate.
This place does not cover:
Single amino acids or single nucleic acids for use in therapy | |
Medical use of non-coding nucleic acids, e.g. ribozymes, antisenses | |
Vaccines | |
Stabilisation of proteins in general | |
Peptides forming the non active part of a conjugate | |
NMR contrast preparations containing peptides | |
Preparations containing radioactive peptides for use in therapy | |
Antibodies | |
Enzymes per se | |
Non-coding nucleic acids, e.g. ribozymes, antisenses |
Attention is drawn to the following places, which may be of interest for search:
Cosmetic preparations containing proteins orpeptides | |
Medicinal preparations characterised by physical form | |
Medicinal preparations containing material of undetermined constitution | |
Proteins or peptides used in food | |
Proteins or peptides involved in bandages, dressings | |
Proteins or peptides involved in surgical articles | |
Proteins or peptides involved in prostheses or coatings | |
Proteins or peptides involved in catheters | |
Proteins or peptides involved in antithrombogenic treatment of surgical articles | |
Proteins or peptides per se | |
Methods for the preparation of peptides | |
Enzymes per se |
Each specific active component in a medical use claim is classified according to the last place rule.
Classification within this group is only given for the use of peptides or proteins (i.e. those present in the claims or exemplified in the description) as active agents.
The sole symbol for additional information classification is A61K 38/00, without subdivisions. An invention information classification with the head symbol A61K 38/00 should be avoided.
A fragment of a protein in a medical claim is classified with its parent, if known. If the protein from which the fragment derives is not identified in the document to be classified, the fragment will be classified according to its length. Protein fragments less than 5 amino acids long are additionally classified under A61K 38/043 - A61K 38/07.
A nucleic acid encoding a protein in a medical use claim is classified under the protein it encodes.
In principle all specific compounds mentioned in the claims are classified. However, when there are too many compounds, as in the case of lengthy, exhaustive lists of proteins or peptides claimed for a therapeutic use, classification should be limited to a reasonable number of assignments covering e.g. the compounds tested or exemplified. Any generalisation which would lead to the next hierarchically higher level should be avoided. The therapeutic use of undefined proteins or peptides (no peptidic structure described) is not classified under A61K 38/02 which is for the medical use of compounds of peptidic or proteic nature but of undefined length, e.g. polymers of repeated motifs of aminoacids of undefined length, but nevertheless disclosed in the document.
Enzyme inhibitors in a medical use claim are classified within the A61K 38/005 (enzyme inhibitors) or A61K 38/55 (protease inhibitors) groups only if they are of peptidic structure. A61K 38/005 or A61K 38/55 should be assigned with another, more informative classification symbol: if the inhibitor is directly derived from the enzyme sequence itself, like for example an inhibitory enzyme fragment, it is also classified under the enzyme from which it is derived (A61K 38/43 - A61K 38/54); if the inhibitor is not disclosed in the document as being derived from a known enzyme, for example the inhibitor is synthetically produced and is clearly constituted of a sequence of amino acids (defined or not), it is classified under its length. The concept "enzyme inhibitors" without any specific example or given peptidic structure does not require classification within this group.
For mixtures or combinations: a classification symbol is given for each active ingredient in a medical use claim, followed by A61K 2300/00 (Additional Information Symbol for mixtures) to create the corresponding Combination-set. This applies also if one mixture component is claimed per se.
Fusion peptides in a medical use claim are classified under A61K 38/00 with the symbols of their components.
Important aspects of the invention mentioned only in the description should also be classified.
This place covers:
The use of antigens or antibodies in medicinal preparations for immunization..
Gene therapy: An appropriate A61K 39/00 symbol is given when the in vivo expression of a protein arising from the delivery of a nucleic acid encoding the protein, and the generation of an immune response against the encoded protein results in a prophylactic or therapeutic effect. The same applies for the use of cells modified to produce a specific antigen/antibody, for use in gene therapy. An A61K 48/00 symbol may also be given, if applicable, as specified under the A61K 48/00 section definitions
This place does not cover:
Gene therapy | |
Material for immunoassay |
Attention is drawn to the following places, which may be of interest for search:
Viral peptides having more than 20 amino acids | C07K 14/005 and/or C12N7xx/xxxx22 defining the origin of the viral peptide |
Bacterial peptides having more than 20 amino acids | C07K 14/195 and subgroups further defining the origin of the bacterial peptide |
Fungal peptides having more than 20 amino acids | C07K 14/37 and subgroups further defining the origin of the fungal peptide |
Animal/human peptides having more than 20 amino acids | C07K 14/435 and subgroups further defining the origin of the animal/human peptide |
In CPC, an important limitation for the classification in A61K 39/00 has been introduced, in comparison with the IPC:
The medicinal use of proteinaceous antigens hypothetically useful for vaccination is classified only in C07K 14/00 or C12N 9/00, according to the origin of the protein, with addition of the Indexing Code A61K 39/00.
Guidance for the classification of medicinal preparations containing antibodies (A61K 39/395 and subgroups) as active ingredients is given in a separate definition concerning antibodies (C07K 16/00).
What follows below is the classification strategy for the medicinal use of antigens for which vaccination has been sufficiently disclosed.
Documents relating to the medicinal use of antigens are classified in A61K 39/00, mainly according to the origin of the antigen.
Indexing Codes (A61K 2039/51 - A61K 2039/64) are used for classifying further relevant and sufficiently disclosed aspects of the immunogenic compositions.
Preparations containing archeal antigens for vaccination: A61K 39/0001
Preparations containing fungal antigens for vaccination: A61K 39/0002
Preparations containing invertebrate antigens for vaccination: A61K 39/0003
Preparations containing vertebrate antigens for vaccination:
from A61K 39/0005 - A61K 39/0012
A61K 39/0006 :contraceptive vaccins, vaccins against sex hormones
A61K 39/0007 : preparations containing nervous system antigens or prions
A61K 39/0008 : preparations containing antigens related to auto-immune diseases and preparations to induce self-tolerance
A61K 39/001 : preparations to induce tolerance to non-self, e.g. prior to transplantation
A61K 39/0011 : preparations containing cancer antigens
A61K 39/0012 : preparations containing lipid or lipoprotein antigens
Preparations containing protozoa antigens: from A61K 39/002 - A61K 39/018 according to the origin of the antigen
A61K 39/005 : preparations containing Trypanosoma antigens
A61K 39/008 : preparations containing Leishmania antigens
A61K 39/012 : preparations containing Coccidia antigens
A61K 39/015 : preparations containing Hemosporidia antigens e.g. Plasmodium antigens
A61K 39/018 : preparations containing Babesia antigens e.g. Theileria antigens
Preparations containing bacterial antigens: from A61K 39/02 - A61K 39/118 according to the origin of the antigen
A61K 39/0208 : preparations containing antigens from specific bacteria, which cannot be classified in A61K 39/0216 - A61K 39/118
A61K 39/0216 : preparations containing antigens from Bacteriodetes, e.g. Bacteroides, Ornithobacter, Porphyromonas
A61K 39/0225 : preparations containing antigens from Spirochetes, e.g. Treponema, Leptospira, Borrelia
A61K 39/0233 : preparations containing antigens from Rickettsiales, e.g. Anaplasma
A61K 39/0241 : preparations containing antigens from Mollicutes, e.g. Mycoplasma, Erysipelothrix
A61K 39/025 : preparations containing antigens from Enterobacteriales, e.g. Enterobacter, Yersinia
A61K 39/0258 : preparations containing antigens from Escherichia
A61K 39/0266 : preparations containing antigens from Klebsiella
A61K 39/0275 : preparations containing antigens from Salmonella
A61K 39/0283 : preparations containing antigens from Shigella
A61K 39/04 : preparations containing antigens from Mycobacterium, e.g. Mycobacterium tuberculosis
A61K 39/05 : preparations containing antigens from Actinobacteria (e.g. Actinomyces, Streptomyces, Nocardia, Bifidobacterium,Gardnerella), Corynebacteria, Propionibacteria. Preparations containing antigens from Mycobacterium are classified in A61K 39/04
A61K 39/07 : preparations containing antigens from Bacillus
A61K 39/08 : preparations containing antigens from Clostridium, e.g. Clostridium tetanus
A61K 39/085 : preparations containing antigens from Staphylococcus
A61K 39/09 : preparations containing antigens from Lactobacillales, e.g. Aerococcus, Enterococcus, Lactobacillus, Lactococcus
A61K 39/092 : preparations containing antigens from Streptococcus
A61K 39/095 : preparations containing antigens from Neisseria
A61K 2039/10 : not used
A61K 39/098 : preparations containing antigens from Brucella
A61K 39/099 : preparations containing antigens from Bordetella, e.g. Bordetella pertussis
A61K 39/102 : preparations containing antigens from Pasteurellales, e.g. Actinobacillus, Pasteurella, Haemophilus
A61K 39/104 : preparations containing antigens from Pseudomonadales, e.g. Pseudomonas
A61K 39/1045 : preparations containing antigens from Moraxella
A61K 2039/106 : not used
A61K 39/098 : preparations containing antigens from Delta proteobacteriales, e.g. Lawsonia, from Epsilon proteobacteriales, e.g. campylobacter, Helicobacter
A61K 39/114 : preparations containing antigens from Fusobacterium
A61K 39/116 : preparations containing antigens from more than one bacteria; preparation containing a mixture of bacterial and viral antigens are classified in A61K 39/295
A61K 39/118 : preparations containing antigens from Chlamydiaceae, e.g. Chlamydia trachomatis or Chlamydia psittaci
Preparations containing viral antigens: A61K 39/12 AND C12N 7/00 in combination with an Indexing Code in the C12N 2710/00 - C12N 2796/00 series according to the origin of the viral antigen.
The specific Indexing Codes have the format C12N7xx/xxxx34
The first part (xxx/xxxx)of the code indicates the specific virus from which the antigen has been derived. The first four digits after the "/" represent the taxonomic location of the virus and the last place rule applies.
C12N 2710/00 double stranded DNA virus
C12N 2720/00 double stranded RNA virus
C12N 2730/00 reverse transcribing DNA virus
C12N 2740/00 reverse transcribing RNA virus
C12N 2750/00 single stranded DNA virus
C12N 2760/00 single stranded RNA virus negative-sense
C12N 2770/00 single stranded RNA virus positive-sense
C12N 2780/00 viroids and subviral agents
C12N 2790/00 naked RNA Virus
C12N 2792/00 archaeabacteria virus
C12N 2795/00 bacteriophage
The second part of the Indexing Code(34) indicates the use, namely that the virus or viral component is used as vaccine
Preparations containing multiple antigens of which at least one is a viral antigen: A61K 39/295 AND C12N 7/00 in combination with an Indexing Code in the C12N 2710/00 - C12N 2796/00 series according to the origin of the viral antigen(s).
Preparations containing allergens for vaccination: from A61K 39/35 - A61K 39/36
A61K 39/36 : preparations containing allergens
A61K 39/36 : preparations containing allergens from pollen
Preparations containing antigens from snakes for vaccination: A61K 39/38
Preparations characterized by haptens or antigens bound to a carrier: A61K 39/385
Documents disclosing a new carrier will be classified within A61K 39/385. In addition, specific examples will also be classified according to the nature of the antigen with the addition of an Indexing Code from the A61K 2039/60 series to characterise the carrier.
Documents relating to compositions containing specific antigens bound to a carrier are preferably classified according to the nature of the antigen in groups A61K 39/00 - A61K 39/38 with the addition of an Indexing Code from the A61K 2039/60 series to characterise the carrier and possibly also an Indexing Code from the A61K 2039/62 series to characterise the link between the carrier and the antigen and an Indexing Code from the A61K 2039/64 series to characterise the architecture of the carrier-antigen complex.
Preparations characterized by the immunostimulating additive or adjuvant: A61K 39/39
Documents disclosing a new adjuvant will be classified within A61K 39/39 and the specific examples will also be classified according to the nature of the antigen with the addition of an Indexing Code from the A61K 2039/555 series to characterise the adjuvant.
Documents relating to compositions containing specific antigens in the presence of an immunostimulating additive/adjuvant are classified according to the nature of the antigen in groups A61K 39/00 - A61K 39/38 with the addition of an Indexing Code from the A61K 2039/555 series to characterise the adjuvant.
Preparations containing DNA encoding an antigen for vaccination
Documents disclosing a DNA vaccine will be classified according to the nature of the antigen in groups A61K 39/00 - A61K 39/38 with the addition of the Indexing Code A61K 2039/53.
Preparations containing an antigen for vaccination in combination with another/other active ingredient(s) are classified in the corresponding A61K 39/00 class with the addition of the Indexing Code A61K 2300/00
The following relevant and sufficiently disclosed aspects of the vaccines are further classified using Indexing Codes:
vaccines comprising whole cells, animal cells, bacterial cells or viruses: A61K 2039/51 and subgroups thereof
vaccines characterised by the route of administration: A61K 2039/54 and subgroups thereof
vaccines characterised by the dose, timing or administration schedule: A61K 2039/545
vaccines characterised by the host/recipient: A61K 2039/55 and subgroup A61K 2039/552
vaccines characterised by a specific combination antigen/adjuvant: A61K 2039/555 and subgroups thereof for characterising the adjuvant
vaccines characterised by the type of immune response: A61K 2039/57
vaccines characterised by the carrier linked to the antigen: A61K 2039/60 and subgroups thereof
vaccines characterised by the link between antigen and carrier: A61K 2039/62 and subgroups thereof
vaccines characterised by the architecture of the carrier-antigen complex: A61K 2039/64 and subgroup A61K 2039/645
Attention is drawn to the following places, which may be of interest for search:
Mucosal route or intranasal |
Attention is drawn to the following places, which may be of interest for search:
Medicinal preparations containing animal cells expressing foreign proteins | |
Medicinal preparations containing bacterial, fungal or protozoal cells expressing foreign proteins |
This place covers:
Isolated cells of the immune system presenting or targeting a specific antigen or a mix of antigens for use in therapy against, e.g. cancer, infectious diseases or auto-immune diseases.
The antigen presented/targeted may be a single epitope or a mix of epitopes or antigens.
Antigens may also be undefined like in the case of TILs or LAKs derived lymphocytes which will target several unidentified antigens.
Due to their structure, cells used in cellular immunotherapy may warrant classification of subject-matter in various main groups, beyond the allocation in A61K 40/00 and subgroups.
If the structure of the antibody portion of the construct is an important aspect, then classification in C07K 16/00 and subgroups should be considered. The same applies to the type of protein expressed (TCR, CAR, cytokine…), which can be classified in C07K 14/00 and subgroups.
Genetically modified cells or T lymphocytes for immunotherapy may be classified in C12N 5/0634 - C12N 5/064 with added classification in A61K 40/00 and subgroups, and indexing codes A61K 2239/00 - A61K 2239/59. Documents relating to the in vitro cell culture/expansion methods or to specific types/subtypes of immune cells may also be classified in C12N 5/00 and subgroups.
Cells for cellular immunotherapy may be given symbols in A61K 35/15 (e.g. dendritic cells) and/or A61K 35/17 (e.g. lymphocytes; B-cells; T-cells; natural killer cells; interferon-activated or cytokine-activated lymphocytes) for the cell and A61K 39/00 and subgroups for the therapeutic aspect of the antigen/antibody, as well as in C07K 14/00 (when a novel peptide is claimed and exemplified) and/or C07K 16/00 and subgroups (for further, specific classification of the antibody and/or when the antibody is novel).
Classification in A61K 35/17 should be considered if the effect of the lymphocyte is not due to its antigen specificity (e.g. the administration of B cells to treat tissue injury). A61K 35/15 or A61K 35/17 need not be given by default to immune cells directed to a given antigen (or to a mixture of antigens).
This place does not cover:
Medicinal preparations containing antigens or antibodies |
Attention is drawn to the following places, which may be of interest for search:
Medicinal preparations containing blood or cells from blood | |
Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy | |
T-cell receptor (TcR)-CD3 complex | |
Immunoglobulins [IGs], e.g. monoclonal or polyclonal antibodies | |
Fusion polypeptide (containing a transmembrane segment) | |
Culture or maintenance of cells from the blood or the immune system | |
Screening and/or testing using cells |
In this group, classification of the cell type and of the antigen specificity is compulsory, provided the document contains credible evidence that the effect has been or can be achieved.
Further relevant aspects, like those characterising the structure of the chimeric antigen receptor [CAR], the target(s) and the route of administration, should also be classified.
The cell type and the type of immune response is to be classified in subgroups A61K 40/10 - A61K 40/24.
These subgroups relate to immune cells, including antigen-presenting cells or T lymphocytes. Further aspects relate to the type of immune responses, which can be for instance, immunosuppressive or immunostimulatory (A61K 40/20 - A61K 40/24).
Aspects relating to the nature of the receptors or molecules expressed recombinantly by the immune cell are to be classified in subgroups A61K 40/30 - A61K 40/36. CAR receptors are for instance, classified in A61K 40/31.
The former subgroups should all be used in combination with the subgroups relating to the target antigen(s), which are to be found in subgroups A61K 40/40 - A61K 40/482.
The additional subgroups A61K 2239/00 - A61K 2239/59 should also be considered for a comprehensive classification of cellular immunotherapy.
These subgroups concern the structure of the chimeric antigen receptor [CAR], as well as the mechanisms enabling the control of the function or activity of the CAR or of the CAR-expressing cells in vivo.
In case of multiple targets, classification in the subgroups A61K 2239/27 - A61K 2239/30 can also be considered.
The route of administration, the schedule and the adjuvant(s) used can be classified in A61K 2239/31 - A61K 2239/39.
Finally, and in view of the wide use of cellular immunotherapy against cancer, specific subgroups for the cancer organ can be found in subgroups A61K 2239/46 - A61K 2239/59. These subgroups can be used in combination with the antigen subgroups to more precisely identify documents relating to some specific cancers.
Combination Sets (C-Sets) classification:
- In groups A61K 40/00 - A61K 40/482, the combined use of cellular immunotherapy with other therapeutic elements classified in A61K 31/00 - A61K 41/00 are classified in the form of C-Sets.
- While multiple drugs are used in an invention, only the essential components of the invention that differentiate them from the prior art are given C-Sets.
C-Sets syntax rules:
- Each C-Set shall contain exactly two symbols, in which the base symbol represents one therapeutic drug belonging to A61K 31/00 - A61K 41/00, and followed by a subsequent symbol of A61K 2300/00.
- Two or more separate C-Sets are made when two or more drugs are used in combination with cellular immunotherapy. See examples below.
C-Sets examples:
- Example 1: The treatment of blood cancer with anti-CD19 CAR-T cells and venetoclax is classified as (A61K 40/4211, A61K 2300/00).
- Example 2: The treatment of blood cancer with anti-CD19 CAR-T cells and exogenous IL-7 is classified as (A61K 40/4211, A61K 2300/00).
- Example 3: The treatment of cancer with anti-CEA CAR-T cells combined with anti-PD-L1 antibodies is classified as (A61K 40/4266, A61K 2300/00).
This place covers:
- Medicinal preparations guided in vivo through the body by a magnetic field: A61K 41/00.
- A61K 41/0004: preparations which are obtained by using homeopathic procedures, procedures for dynamisation, or esoteric preparations; homeopathy; vitalisation; resonance; dynamisation; esoteric applications; oxygenation of blood.
A61K 41/0023: preparations for use in therapy during which wave energy or particle radiation is administered, in order to "activate" the agent, e.g. photodynamic therapy, or for releasing a pharmacologically active agent, e.g. thermosensitive liposomes, photolabile linkers are classified in A61K 41/0023 - A61K 41/0095
- aggression treatment or altering: of a medicinal preparation prior to administration to the human/animal (e.g. altering a binding specificity of a monoclonal antibody used in a medicinal agent with an oxidizing agent or an electric potential); of a tissue/organ prior to graft (e.g. destroying immunodominant epitopes); the permeability of cell membranes or biological barriers in vivo (e.g. by ultrasound) prior to the administration of a medicinal preparation to the animal/human; for inducing the production of stress response proteins or heat shock proteins in order to reduce subsequent response to injuries.
A61K 41/0028: disruption (e.g. by heat or ultrasounds), sonophysical or sonochemical activation; e.g. thermosensitive or heat-sensitive liposomes, disruption of calculi with a medicinal preparation and ultrasounds.
A61K 41/0033: sonodynamic cancer therapy with sonochemically active agents/sonosensitizers, having their cytotoxic effects enhanced through application of ultrasounds (ultrasound therapy per se is classified in A61N 7/00).
A61K 41/0038: radiosensitizing, i.e. administration of pharmaceutical agents that enhance the effect of radiotherapy (radiotherapy per se is classified in A61N 5/10).
A61K 41/0042: photocleavage of drugs in vivo (e.g. cleavage of photolabile linkers in vivo by UV radiation for releasing the pharmacologically-active agent from the administered agent); photothrombosis or photoocclusion.
A61K 41/0047: sonopheresis (i.e. ultrasonically-enhanced transdermal delivery), electroporation of a pharmacologically active agent (NB: to be classified in A61K 9/0009 when it is in relation to the galenic form).
A61K 41/0052: thermotherapy; hyperthermia; magnetic induction; induction heating therapy (NB: simple magnetic guidance of drugs in vivo is to be classified in A61K 41/00, and in A61K 47/6941).
A61K 41/0057: photodynamic therapy with a photosensitizer, i.e. agent able to produce reactive oxygen species upon exposure to light or radiation, e.g. UV or visible light; photocleavage of nucleic acids with an agent.
A61K 41/0061: 5-aminolevulinic acid-based PDT (5-ALA-PDT involving porphyrins or precursors of protoporphyrins generated in vivo from 5-ALA).
A61K 41/0066: psoralene-activated UVA photochemotherapy (PUVA-therapy), e.g. for treatment of psoriasis or eczema, extracorporeal photopheresis with psoralens (fucocoumarins).
A61K 41/0071: PDT with porphyrins having 20 carbon atoms and 4 nitrogen atoms forming the ring system (i.e. based on the non-expanded tetrapyrrolic ring system, e.g. bacteriochlorin, chlorin e6, or phthalocyanines).
A61K 41/0076: PDT with expanded (i.e. having more than 20 carbon atoms and/or 4 nitrogen atoms forming the ring system) (metallo)porphyrins, e.g. texaphyrins, sapphyrins, hexaphyrins, pentaphyrins, porphocyanines.
A61K 41/008: two-photon or multi-photon PDT, e.g. with two-photon upconverting dyes or photosensitizers.
A61K 41/0085: mossbauer effect therapy based on mossbauer effect of a material (i.e. re-emission of gamma rays after absorption of gamma rays by the material); selective radiation therapy (i.e. involving re-emission of ionizing radiation upon exposure to a first ionizing radiation).
A61K 41/009: neutron capture therapy, e.g. using uranium or non-boron material.
A61K 41/0095: boron neutron capture therapy (BNCT), e.g. using boronated porphyrins.
A61K 41/10: preparations which are obtained by treating materials with wave energy, e.g. U.V. light, or particle radiation, prior to administration, for decontamination:
- inactivation or decontamination of a medicinal preparation prior to administration to the animal/human, e.g. : inactivation of viruses or bacteria for vaccines, sterilisation by electromagnetic radiation (see A61K 41/17 for the specific method; A61L 2/0029 if the invention lies in the method of sterilization of the medicinal preparation rather than the sterilized medicinal preparation.
A61K 41/13: by ultrasonic waves.
A61K 41/17: by ultraviolet [UV] or infrared [IR], X-rays or gamma rays.
Galenical aspects of pharmaceutical compositions are also classified by giving a combination of classes in A61K 9/00 and A61K 47/00 to and including A61K 47/50.
The active ingredients in pharmaceutical compositions are classified in A61K 31/00 - A61K 48/00.
Preparations for testing in vivo using radioactive substances, or substances for use in therapy labeled with a radioactive isotope, are classified in A61K 51/00.
Preparations for testing in vivo, or for use in an in vivo diagnostic imaging method (e.g., by luminescence, fluorescence, X-ray imaging, ultrasound imaging, echography, MRI) are classified in A61K 49/00 and its subgroups.
Attention is drawn to A61L 2/0029, A61L 2202/21 and/or A61L 2202/22 if the invention lies in the method of sterilization of the medicinal preparation rather than the sterilized medicinal preparation.
Attention is drawn to the following places, which may be of interest for search:
In A61K 41/10 and its subgroups, pharmaceutical compositions are classified that are decontaminated prior to use, by applying radiation, or of which one of the constituents is thus decontaminated.
Photosensitizers used in photodynamic therapy (the photosensitizer being considered as the therapeutically active part) and modified by another compound (e.g. polymer or an antibody) to be classified in A61K 41/0071 or A61K 41/0076 and according to the A61K 47/50 subclass of the modifying agent.
Attention is drawn to A61L 2/0029 if the invention lies in the method of sterilization of the medicinal preparation rather than the sterilized medicinal preparation.
Attention is drawn to the following places, which may be of interest for search:
Pharmaceuticals | |
Galenic aspects | |
Organic active ingredients | |
Inorganic active ingredients | |
Active ingredients of undetermined constitution (i.e. natural products) | |
Active ingredients from algae, lichens, fungi or plants | |
Peptides, proteins | |
Antigens or antibodies, vaccines, adjuvants | |
Homeopathy, thermotherapy, photodynamic therapy, photoactivable drugs | |
Characterized by non-active ingredients e.g. carriers, inert additives, excipients... | |
Conjugates; targeted drugs | |
Gene therapy | |
Medicine/pharmacy - mechanical aspects | |
Stents | |
Contraceptive devices | |
Ophthalmic implants | |
Bandages, dressings, absorbent pads | |
Making transdermal patches | |
Tampons (also medicated) | |
Biomaterials | |
Disinfecting or sterilising contact lenses | |
For bandages, dressings or absorbent pads | |
Surgical sutures | |
Surgical adhesives or cements | |
For wound dressings, bandages, also liquid, gel, powder | |
For (coating of) grafts, prostheses, also with active | |
For other surgical articles, e.g. stents, embolization | |
Electrotherapy | |
Iontophoresis | |
Magnetotherapy |
Diagnosis
Testing in vivo, e.g. screening, contrast agents, ultrasound | |
Radioactive substances | |
In vitro diagnosis |
Other medical
Dentistry | |
Sterilization methods | |
Undifferentiated human, animal or plant cells, e.g. cell lines; Tissues; Cultivation or maintenance thereof; Culture media therefore | |
Contact lenses | |
Healthcare informatics |
Other human necessities
Cosmetics | |
Biocides, pest repellants or attractants | |
Animal feeding-stuffs | |
Food or functional food (neutraceuticals) |
General chemistry
Quaternary ammonium compounds | |
Phosphatides | |
Dendrimers | |
Macromolecular gels | |
Detergent compositions |
1) No distinction is made between Invention and Additional information.
2) Therapeutic use: the A61K 45/06 classification symbol should mainly be reserved for mixtures containing at least one active ingredient without chemical characterisation.
It is also used in the case that there are too many exemplified combinations.
This place covers:
- Pharmaceutical compositions characterised by the excipients, i.e. the non-active ingredients.
- New excipients per se.
- Conjugates, i.e. compounds comprising a non-active ingredient chemically bound to the pharmaceutically active ingredient.
It should be noted that in A61K 47/00 the invention resides in the use of at least one particular compound as a biologically inert additive or excipient, for example to enhance the stability of a pharmaceutical formulation or to enhance the bioavailability of a biologically active ingredient in the body. The invention therefore does not reside in what the active ingredient does but in what at least one of the biologically inert ingredients does. It may be that this inert ingredient is a separate chemical entity from the biologically active ingredient or it may be that the inert ingredient is chemically bound to the biologically active ingredient. Thus any novelty that resides in the said inert compound per se will warrant classification in the appropriate area in class C01 for inorganic compounds or classes C07 or C08 for organic or polymeric compounds respectively.
Galenical aspects of pharmaceutical compositions are classified in A61K 9/00 and in A61K 47/00. The last place rule does not apply between A61K 9/00 and A61K 47/00 - A61K 47/46.
Excipients can be classified in A61K 47/00- A61K 47/46 or in A61K 9/00, depending on the situation: A61K 47/00 is used to classify excipients in compositions for which A61K 9/00 does not provide information on excipients. No A61K 47/00 is given if A61K 9/00 already provides information on excipients (e.g. tablet excipients are only classified in A61K 9/20...). New excipients per se are (in addition) classified in A61K 47/00.
Conjugates, i.e. compounds comprising a non-active ingredient bound to the active ingredient, are classified in A61K 47/50. Pharmaceutical compositions comprising conjugates may in addition be classified in A61K 9/00.
The active ingredients in pharmaceutical compositions are classified in A61K 31/00 - A61K 45/00, or A61K 48/00 - A61K 51/00.
Attention is drawn to the following places, which may be of interest for search:
Nuclear magnetic resonance contrast preparations or magnetic resonance imaging contrast preparations | |
Preparations containing radioactive substances |
Classified are concrete, well-defined pharmaceutical compositions disclosed in the examples. Also classified are independent claims defining galenical aspects of a pharmaceutical composition or a medical use.
IIn principle all examples are classified, also 'standard' examples in documents describing e.g. a new medical use.
However, systematically classifying all excipients in the examples is not necessary, and often undesirable. In any case classified are excipients which are described as being important for the invention, or which the reader can identify as having an important function, e.g. for sustained release. For 'standard' compositions the examiner should choose one or a few excipients to classify.
Animal tests are not classified, unless it is absolutely clear that they represent the intended mode of administration.
The description and dependent claims are not classified. However, if the document as a whole focuses on one clearly preferred embodiment, this embodiment may be classified, even in the absence of relevant examples or independent claims.
In general, information relating to the invention is classified using invention information symbols, while additional information is classified using additional information symbols. This is largely up to the discretion of the examiner. Please note however the following special situations:
- Normally only final compositions are classified, not intermediates. However, it may be useful to classify intermediates as additional information (e.g. a tablet comprising microcapsules; a multicoated microparticle). If the intermediates are claimed separately, they must be classified as invention information. If, in the classification scheme, a group refers out to another group, an additional information symbol may still be given for the first group (e.g. oral mucoadhesive film).
A61K 47/02 covers inorganic compounds but does not cover polymeric inorganic materials, which are covered by A61K 47/30 - macromolecular (organic or inorganic) compounds – e.g., inorganic polyphosphates.
A61K 47/06 covers hydrocarbon materials of both natural and synthetic sources. Natural examples include petrolatum, mineral oil and ozokerite – i.e., there are no functional groups present in such materials, i.e., ester, ketone, hydroxyl or carboxylic groups. A61K 47/06 also covers mixtures of hydrocarbons as well as pure hydrocarbons.
A61K 47/10 covers alcohols such as aliphatic alcohols, phenols or salts thereof; polyalkylene glycols [PEG], [PPG]; poloxamers; PEG/POE alkyl ethers. This subgroup does not cover sugar alcohols (A61K 47/26) or copolymers comprising polyalkylene glycols or poloxamers (A61K 47/34).
A61K 47/34 does not cover polyalkoxylated compounds, which are classified according to the compounds being derivatised. The following list gives examples of such polyalkoxylated compounds together with the relevant group.
- POE alkyl ethers - A61K 47/10
- PEG fatty acid esters – A61K 47/14
- Poloxamines – A61K 47/18
- Polysorbates – A61K 47/26
- POE castor oil – A61K 47/44
A61K 47/44 covers materials that comprise mixtures of chemically distinct components, i.e., more than one of the groups A61K 47/02-A61K 47/42, i.e., carboxylic acids and esters. Materials that comprise such mixtures may include natural or modified oils, fats or waxes such as olive oil, castor oil, montan wax, lignite, shellac, lanolin and beeswax. Materials that comprise only a mixture of different carboxylic acids – i.e., of different carbon chain lengths or a mixture of aromatic and aliphatic carboxylic acids – will be classified in A61K 47/12.
A61K 47/64 covers the situation where peptides, proteins or polyamino acids are chemically bonded directly to drugs or are chemically bonded to the drug via a linker. A61K 47/62 is more appropriate where peptides, proteins or polyamino acids are bound or complexed to special galenical forms of a drug, e.g., a liposome or a nanoparticle modified on its surface by a peptidic modifying agent. In these latter situations, classification in A61K 47/69 would also be appropriate.
If the modifying agent in the conjugate is some kind of inclusion complex, e.g., a cyclodextrin, then classification in A61K 47/69 will be appropriate. If cyclodextrin is used as a simple excipient then classification in A61K 47/40 will be required.
In this place, the following terms or expressions are used with the meaning indicated:
Targeting agent | Natural or artificial substances that enhance absorption of a biologically active agent in a particular part of the body – i.e., in a particular organ or to a particular cell type. |
Pre-targeting system | An example of this is an immuno-conjugate that has a part that recognizes a target antigen and a part that recognizes a further substance to which a therapeutic agent is attached. When said conjugate is administered it binds to target cells, furnishing said cells with binding sites for the therapeutic agent. |
Modifying agent | Natural or artificial substances that enhance the physio-chemical properties of a biologically active agent – i.e., that improve long term storage stability or enhance bioavailability when delivered orally. |
Macromolecular | A chemical moiety (organic or inorganic) that is linked to identical sub-units in numbers of greater than 5. Thus, polysiloxanes are classified in A61K 47/34, polyphosphazines in A61K 47/34 and inorganic species such as polyphosphate A61K 47/30 |
Chemically bound | When two distinct chemical entities are linked to one another by co-valent bonds. An alternative situation may arise when both chemical entities are linked using ionic bonds, for example using organic/inorganic ions that complex with one another, e.g., see A61K 47/52 |
Codrug | A dimer, oligomer or polymer of one or more pharmacologically or therapeutically active compounds. e.g. polyaspirin |
This group does not cover polyalkoxylated compounds, which are classified according to the derivatized compounds. The following list of references provides examples of such polyalkoxylated compounds together with their relevant group.
This place does not cover:
POE alkyl ethers |
Attention is drawn to the following places, which may be of interest for search:
PEG fatty acid esters | |
poloxamines | |
polysorbates | |
POE castor oil |
This place covers:
Medicinal preparations containing conjugates. A conjugate is meant to define a pharmacologically/therapeutically-active agent or drug chemically bound (by covalent bonds or by complexation) to a modifying agent. The classification in this subgroup is based on this modifying agent. The "pharmacologically/therapeutically-active agent" covers a molecule used as the drug and linked to the modifying agent, or a molecule used as the drug and encapsulated/linked to a special physical/galenical form. The modifying agent is for example used to:
- modify the physico-chemical properties of the pharmacologically/therapeutically-active agent, for example to increase its solubility in bodily fluids,
- modify the pharmacokinetic properties, for example to increase the time of residence in the blood,
- modify the pharmacological activity (in case of e.g. codrugs or mutual drugs), or
- target specific sites in the body for delivery, i.e. receptors, cells, tissues or organs.
Attention is drawn to the following places, which may be of interest for search:
Medicinal preparations containing organic active ingredients | |
Organic chemistry in general | |
Labelling of peptides or proteins | |
Enzymes or proenzymes |
In the subgroups of A61K 47/50, the classification is based on the non-active ingredient, i.e. the modifying agent.
However, for the conjugates of an antibody, the pharmacologically/therapeutically-active agent of the conjugate is also classified, in the subgroups of A61K 47/68. The modifying group must be part of a well-defined class of compounds.
The last place priority rule does not apply in group A61K 47/50, i.e. all aspects of the invention are classified. For example, a liposome modified on its external surface by a modifying agent, is classified both in A61K 47/6911 and in the appropriate subgroup of A61K 47/50, e.g. in A61K 47/62 for a peptide/protein, and in the appropriate subgroup of A61K 47/6835 for an antibody.
Targeted drug delivery systems as defined in A61K 47/555, A61K 47/66 and A61K 47/6891 comprise more than one component. For example, in antibody-directed enzyme prodrug therapy [ADEPT], one component carries the enzyme to its target, and the other the prodrug. Although less detailed, the classification of conjugates in which the modifying component is a peptide follows a classification similar to that in the field of new peptides or proteins, i.e. C07K 14/00 and subgroups. Similarly, the classification of conjugates in which the modifying component is an antibody, the classification of the characterising antibody follows a classification similar to that of new antibodies, C07K 16/00 and subgroups, again less detailed. For the specificity of the antibody, the same rules are followed as for the classification in C07K 16/00. For the specificity of the antibody, if the antibody is new, the corresponding class in C07K 16/00 and subgroups is also given.
The active agent is also classified in A61K 47/50 in two situations:
- if the modifying agent is also active: A61K 47/55, A61K 47/551, A61K 47/552, and in the case of sugars A61K 47/549;
- if the active agent is attached to an antibody as modifying agent: A61K 47/68.
In this place, the following terms or expressions are used with the meaning indicated:
ADEPT | Antibody-Directed Enzyme-Prodrug Therapy |
VDEPT | Virus-Directed Enzyme-Prodrug Therapy |
PDEPT | Polymer-Directed Enzyme-Prodrug Therapy |
ECTA | Enzyme-Catalyzed Therapeutic Agent |
Attention is drawn to the following places, which may be of interest for search:
Classic ion pairs of medicinal agents |
Attention is drawn to the following places, which may be of interest for search:
Fatty acid conjugates | |
He Cholesterol conjugates |
Attention is drawn to the following places, which may be of interest for search:
Porphyrins used as photosensitizers in photodynamic therapy | |
Porphyrins used as fluorescent diagnostic optical agents administered in vivo |
Porphyrins used as photosensitizers in photodynamic therapy, where the photosensitizer is considered as the therapeutically active part, and modified by another compound, e.g. polymer or an antibody, should be classified in A61K 41/0071 or A61K 41/0076 in addition to the appropriate subgroup A61K 47/50 according to the modifying agent.
Attention is drawn to the following places, which may be of interest for search:
Paramagnetic chelates used in MRI and conjugated to another compound, e.g. a polymer, a peptide, a protein, an antibody, a small molecule like a sugar | |
Paramagnetic chelates used in MRI and conjugated to another compound not being used as therapeutic agent, according to the nature of the modifying agent | |
Paramagnetic chelates used in MRI and not linked to by further compound, e.g. polymer, peptide, protein, antibody, small molecules like sugars | |
Radiolabelled chelates | |
Radiolabelled chelates being linked to a further molecule, e.g. an organic compound, polymer, peptide, protein or polyamino acid, antibody |
Attention is drawn to the following places, which may be of interest for search:
Nucleic acid carriers |
This place covers:
Nucleic acids can be coding, non-coding, nucleic acid which being therapeutically-active or not, e.g. oligonucleotides, DNA, RNA, siRNA, and nucleic acid aptamers.
Attention is drawn to the following places, which may be of interest for search:
Sugar, nucleoside, nucleotide, nucleic acid |
This place covers:
Cholesterol
Codrugs of pharmacologically active/therapeutically-active steroids are classified in this group and also in A61K 47/55.
This place covers:
A targeting agent able to target specific cells or receptors in the body (T) being an organic compound, not being a peptide, protein or antibody.
The concept of "pre-targeting" covers the administration of the modifying agent, which is an agent able to target specific cells in the body, and of the pharmacologically or therapeutically active agent, e.g. drug D, in several steps, their "binding" occurring at the in vivo targeted site.
It involves administration in at least two steps, for example, (i) a conjugate T-A corresponding to a targeting agent able to target specific cells or receptors in the body (T) linked to a compound A, and (ii) a conjugate D-M corresponding to the drug linked to a modifying agent M able to target the compound A.
The sequence involves the administration of T-A and then D-M. Between step (i) and step (ii), a further compound able to bind to A and M may also be administered, e.g. during a clearing step.
Classification is made according to the nature of T in the subgroups of A61K 47/555, A61K 47/66, and A61K 47/6891.
In A61K 47/555 and its subgroups, if T is an organic compound, other than a peptide, protein or antibody, classification is also made according to the nature of organic compound T in the appropriate A61K 47/54 subgroup. If T is a peptide, protein or antibody, classification is made in the corresponding A61K 47/66 (peptide or protein) or A61K 47/6891 (antibody) group.
This place covers:
Enzymes being used as group A, and being first targeted to specific cells via administration of the conjugate T-A. Then, the conjugate M-D which being a substrate for A being administered. The enzyme A being able to cleave the conjugate M-D, which can be a prodrug. The drug D being thus released through enzymatic cleavage at particular targeted cells.
This place covers:
Chemical reactions inducing the cleavage of the pharmacologically or therapeutically active agent from the carrier while at the same time producing light.
Attention is drawn to the following places, which may be of interest for search:
Conjugates being cleaved through activation by light in vivo in order to release the drug | |
Dyes or luminescent agents for photodynamic therapy | |
Dyes or luminescent agents for optical diagnostic imaging |
Attention is drawn to the following places, which may be of interest for search:
Block copolymers | |
Peptides, proteins, polyamino acids | |
Antibodies |
Attention is drawn to the following places, which may be of interest for search:
Proteoglycans as modifying agents attached to the pharmacologically or therapeutically active agents | |
Cyclodextrin being used to complex the drug |
Attention is drawn to the following places, which may be of interest for search:
Peptidic linkers used to connect a drug and a modifying agent | |
Antibodies or immunoglobulins |
Special physical or galenic forms modified by covalent attachment or complexation of a protein, peptide or polyamino acid, are classified in A61K 47/62 and additionally in A61K 47/69, if considered relevant. E.g. a liposome modified on its surface by a peptide is classified in A61K 47/6911 and in A61K 47/62, a PLGA nanoparticle modified on its surface by a peptide is classified in A61K 47/6937 and in A61K 47/62.
This place covers:
Conjugates, wherein a peptide or protein being the pharmacologically or therapeutically active agent is linked to another peptide or protein being the modifying agent via chemical methods.
Chemically-produced peptides or protein-peptides or protein conjugates, the peptides or proteins used as modifying agents.
Attention is drawn to the following places, which may be of interest for search:
The connection of the drug to the peptide, protein or polyamino acid can be by a direct covalent linkage or through a linker Fusion/chimeric proteins genetically produced |
Attention is drawn to the following places, which may be of interest for search:
Haptens, e.g. conjugates of morphine or nicotine and KLH inducing an immune response |
Haptens, e.g. conjugates of morphine or nicotine and KLH inducing an immune response, are classified both in this group and additionally in A61K 47/643.
This place covers:
The concept of "pre-targeting" covers the administration of the modifying agent, i.e. an agent able to target specific cells in the body, and of the pharmacologically or therapeutically active agent (drug D) in several steps, their "binding" occurring at the in vivo targeted site.
It involves administration in at least two steps, for example, (i) a conjugate T-A corresponding to a targeting agent T able to target specific cells or receptors in the body (T) linked to a compound A, and (ii) a conjugate D-M corresponding to the drug D linked to a modifying agent M, able to target the compound A.
T being a peptide or protein, not being an antibody.
The sequence involves the administration of T-A and then D-M. Between step (i) and step (ii), a further compound able to bind to both A and M may also be administered, e.g. during a clearing step.
Attention is drawn to the following places, which may be of interest for search:
M being biotin and A being a (strept)avidin or a derivative thereof |
Classification is made according to the nature of T in subgroups of A61K 47/555, A61K 47/66, and A61K 47/6891.
This place covers:
Enzyme prodrug therapy, e.g. gene directed enzyme drug therapy [GDEPT], VDEPT. An enzyme is used as group A in the sense of this group, being first targeted to specific cells via administration of the conjugate T-A. Then, the conjugate M-D, which is a substrate for A, is administered. The enzyme A is able to cleave the conjugate M-D, which can be a prodrug. The drug D is thus released through enzymatic cleavage at particular targeted cells.
This place covers:
Drugs conjugated to an antibody or immunoglobulin, e.g. cisplatin-antibody conjugates. The modifying part is an antibody or immunoglobulin bearing at least one antigen-binding site.
Antibodies per se are classified in C07K 16/00.
Attention is drawn to the following places, which may be of interest for search:
Conjugates comprising a polymer or a polyamino acid |
In this group, classification is made according to the nature of the drug, i.e. the pharmacologically or therapeutically active agents in the antibody conjugate. If the nature of the antibody in a specific conjugate is known, it is additionally classified in A61K 47/6835. If the conjugate comprises also a polymer or a polyamino acid, then classification is also made in A61K 47/6883 or A61K 47/6885.
This place covers:
Modifying agents being a well-defined antibody or immunoglobulin bearing at least one antigen-binding site.
Attention is drawn to the following places, which may be of interest for search:
Porphyrins used as photosensitizers in photodynamic therapy, the photosensitizer being considered as the therapeutically active part, and modified by an antibody |
According to the nature of the antibody, classification is additionally made in the appropriate groups of A61K 47/6835. The pharmacologically or therapeutically active agent in the antibody conjugate is additionally classified in A61K 47/6803, whenever considered appropriate.
This place covers:
Pre-targeting systems involving an antibody for targeting specific cells. The concept of "pre-targeting" covers the administration of the modifying agent, i.e. an agent able to target specific cells in the body, and of the pharmacologically or therapeutically active agent (drug D) in several steps, their "binding" occurring at the in vivo targeted site.
It involves administration in at least two steps, for example, (i) a conjugate T-A corresponding to a targeting agent able to target specific cells or receptors in the body (T) linked to a compound A (wherein T is an antibody), and (ii) a conjugate D-M corresponding to the drug linked to a modifying agent M, able to target the compound A.
The sequence involves e.g. the administration of T-A and then D-M. Between step (i) and step (ii), a further compound able to bind to A and M may also be administered, e.g. during a clearing step.
Classification is additionally made according to the nature of T in A61K 47/555, A61K 47/66, and A61K 47/6893. Classification is also made according to the nature of the antibody in A61K 47/6835. If M and A form a pair of biotin and (strept)avidin, or derivatives of biotin and (strept)avidin, then classification is also made in A61K 47/6898.
This place covers:
Avidin-biotin systems, wherein at least one avidin- or biotin-conjugated antibody is used in a two- or three-steps pretargeting system, e.g. wherein M and A in form a pair of biotin and (strept)avidin, or derivatives of biotin and (strept)avidin.
This place covers:
Antibody Directed Enzyme Prodrug Therapy [ADEPT]. An enzyme is used as group A and is first targeted to specific cells via administration of the conjugate T-A. Then, the conjugate M-D, which is a substrate for A, is administered. The enzyme A is able to cleave the conjugate M-D, which can be a prodrug. The drug D is thus released through enzymatic cleavage at particular targeted cells.
This place covers:
Conjugates characterized by a special physical or galenical form.
The conjugates correspond either (i) to a pharmacologically or therapeutically active agent complexed/covalently linked to the special physical or galenical form, e.g. on the surface of a polymeric nanoparticle or liposome, or to polymeric chains in the matrix of a polymeric gel, or (ii) to a special physical or galenical form encapsulating the pharmacologically or therapeutically active agent and modified on its surface or matrix by a modifying agent.
In case of (i) above, classification is made according to the nature of the special physical or galenical form in this group. Additionally the compound, to which the pharmacologically or therapeutically active agent is linked, is classified in the relevant subgroups of A61K 47/50 e.g. A61K 47/544 in case of a drug linked to a phospholipid and inserted in the bilayer surface of a liposome.
In case of (ii) above, classification is made according to the nature of the modifying agent. Physical or galenical forms not modified by a modifying agent and/or wherein the pharmacologically or therapeutically active agent is not complexed/covalently linked to said forms, are not classified in A61K 47/50, but in A61K 9/00.
Attention is drawn to the following places, which may be of interest for search:
Encapsulation in cells | |
Encapsulation in a virus capsid |
Attention is drawn to the following places, which may be of interest for search:
Simple encapsulation of a drug in micelle |
Micelles modified by a polymer because they incorporate a polymer-lipid conjugate are only classified in this group, if the polymer modifying the lipid is unusual. Micelles, which are pegylated because they incorporate a pegylated lipid, are not classified in this group, but in A61K 9/1075.
In this place, the following terms or expressions are used with the meaning indicated:
Microemulsion | means that the dispersed phase being in the form of globules having a diameter above or equal to 1 micrometer. |
Nanoemulsion | means that the dispersed phase being in the form of globules having a diameter below 1 micrometer. |
Micelles | comprise a monolayer of surfactant molecules that are aggregated head-to-head and tail-to-tail, thus forming a small spherical particle; micelles can be normal, i.e. the surfactant heads are hydrophilic, or inverse. |
This place covers:
Liposomes, i.e. bi-layered vesicles, having its surface modified by covalent attachment or complexation of the pharmacologically or therapeutically active agent and/or modifying agent.
Attention is drawn to the following places, which may be of interest for search:
Encapsulation of a drug, which is not functionalised on its surface by a modifying agent | |
Antibodies |
Liposomes, which are modified by a polymer because they incorporate a polymer-lipid conjugate are only classified in this group, if the polymer modifying the lipid is unusual. Liposomes, which are PEGylated because they incorporate a PEGylated lipid, are not classified in this group, but in A61K 9/1271.
When the surface of the liposome is functionalised by a modifying agent, classification is also made according to the nature of this modifying agent, e.g. a liposome modified on its surface by a peptide is classified in this group and additionally in A61K 47/605. Liposomes, wherein the pharmacologically or therapeutically active agent is linked to a phospholipid of the liposomal surface, are classified in this group and additionally in A61K 47/544.
Classification is also made according to the nature of the antibody in the appropriate subgroup of A61K 47/6835.
This place covers:
Polymersomes, i.e. a liposome with polymerisable or polymerized bilayer-forming substances.
Liposomes comprising polymers grafted on their surface are not classified in this group, but in A61K 47/6911, if the polymer is unusual, or in A61K 9/1271.
This place covers:
Inorganic particles, e.g. ceramic particles, silica particles, ferrite, synsorb
When the inorganic particle is a magnetic particle, being guided from outside the body with the means of a magnetic field, classification is additionally made in A61K 41/00.
This place covers:
Microcapsules, nanocapsules, microbubbles or nanobubbles, i.e. a hollow or gas-filled microparticle or nanoparticle or sphere, a gas-filled microparticle or nanoparticle for use in therapy
Attention is drawn to the following places, which may be of interest for search:
Micro- or nanobubbles used for ultrasound imaging |
Pharmacologically or therapeutically active agents released from a microcapsule or nanocapsule by acoustic/ultrasound activation are also classified in A61K 41/0028 and A61K 9/0009.
This place covers:
Solid microparticles having no hollow or gas-filled core, wherein its size or diameter is higher or equal to 1 micrometer.
This place covers:
Nanoparticles, e.g. immuno-nanoparticles, wherein its size or diameter is smaller than 1 micrometer.
Classification is also made according to the nature of the antibody with the appropriate subgroup of A61K 47/6835.
This place covers:
Genetic material encoding proteins, the in vivo expression of which results in an in vivo therapeutic effect being attained. Cells can be used as delivery vehicle following ex vivo transfection with a nucleic acid encoding a therapeutic protein, enzyme or peptide if the therapeutic effect is a consequence of the ex vivo transfection step, i.e. arising as a result of the modification carried out. The protein in question may be expressed directly from the delivered nucleic acid sequence, or may, for example, be involved in a recombination process with a mutant, 'defective' gene, protein expression from the 'repaired' gene then ensuing at a time thereafter. The expression of the protein may be to replace / complement a 'defective protein', or may be to provide an entity (such as an enzyme) which is therapeutic (for example in the context of cancer therapy).
Transgenic animals are classified in A01K 67/027.
Proteins and peptides per se, encoded by the genetic material, are classified in the appropriate C07K subgroups, unless the protein is an enzyme. In this case the appropriate subgroup in C12N 9/00 should be used. Then classification of the specific enzyme is also made in C12Y as invention.
If therapy arises only as a consequence of the cells per se being delivered, and not due to the manner in which the cells have been modified ex vivo, classification in the subclass for gene therapy should not be made.
Attention is drawn to the following places, which may be of interest for search:
Liposomes | |
Medicinal preparations comprising compounds having three or more nucleosides or nucleotides | |
Medicinal preparations containing materials or reaction products thereof with undetermined constitution | |
Medical use of proteins | |
Medical use of vaccines or antibodies | |
Medicinal preparations characterised by the non-active ingredient | |
Specific therapeutic activities | |
Proteins | |
Antibodies | |
Cells | |
Viruses | |
Genetic engineering | |
Antisense nucleic acids, siRNA | |
Vectors for animal cells | |
Viral vectors |
An invention classification symbol should be given in A61K 38/00 for the protein or peptide if the expression results from the nucleic acid delivered via gene therapy.
Enabling subject matter should be classified. More specifically, subject matter that pertains to the technical effect in question which the skilled person would be able to carry out based on the teachings of the application without undue burden, should be classified. Subject matter which is enabling but not clearly a technical contribution over the art should also be classified. Subject matter which is purely hypothetical or theoretical should not be classified. An example is when a particular substance is stated to be suitable for the treatment of a list of diseases but for which no data, even in an in vitro or animal model setting, is made available. On the other hand, subject matter supported by data which may be for example derived only from an in vitro setting but which lends at least partial credibility to an in vivo treatment should be classified.
This place covers:
Contrast agents for use in diagnostic imaging methods performed in vivo, or for detecting in vivo.
An apparatus used for in vivo imaging is classified in A61B.
In the preparation of new organic compounds and their use in X-ray contrast preparations, classification is only made in the relevant subclasses C07C - C07J according to the type of compound.
Galenical aspects of pharmaceutical compositions: if this is an important aspect of the invention, it may also be classified in A61K 9/00 or A61K 47/00 - A61K 47/46.
Attention is drawn to the following places, which may be of interest for search:
Preparations for testing in vivo using radioactive substances, or substances labeled with a radioactive isotope used for in vivo imaging or detection | |
Preparations for testing in vitro or ex vivo |
In this place, the following terms or expressions are used with the meaning indicated:
In vivo | in the living animal or performed on/in contact with the living animal |
Ex vivo | in a sample taken from the living animal |
In vitro | means literally: in glass. Tests in vitro are performed outside the living or dead body, e.g. in a test tube or a Petri dish. |
This place covers:
General contrast agents are e.g. chelating agents for which several imaging methods are exemplified and/or claimed, e.g., an antibody labeled with a chelating group, where the chelating group is used to complex either gadolinium (for MRI) or a heavy metal (for X-ray imaging). This subgroup also includes conjugates of a targeting agent, linked to a chelating group, wherein the application mentions several imaging techniques, without an actual example.
Multifunctional or multimodal agents are preparations, in which the contrast agent is suitable for more than one imaging technique, e.g. agents for dual mode imaging, e.g., containing a moiety for MRI and a fluorescent moiety.
The classification codes of the individual imaging techniques are also given.
This place covers:
- compositions used for assessing biological conditions or parameters of a patient or mammal.
- skin tests.
- screening methods performed in vivo using non-human animal models wherein the animal model is especially adapted for this screening, in order to find new agents for treating a disorder or disease.
Attention is drawn to the following places, which may be of interest for search:
Medicinal preparations containing organic active ingredients | |
New breeds of vertebrates, e.g. animal models |
Non-human animal models (e.g. transgenic model for heart failure, Alzheimer disease animal model) are only classified in subgroup A61K 49/0008 if they are used in a method for testing/screening agent in vivo and if this testing/screening for therapeutic usefulness in vivo is the invention or part of the invention.
This subgroup is not used for those documents in which the examples are using a well-known animal model to verify if an agent does indeed exert the effect it is supposed to, where the actual invention is the compound or its use.
This place covers:
Diagnostic compositions containing an agent detected in vivo using fluorescence, phosphorescence, (chemo)luminescence or dying/colouring/staining.
If a fluorescent group is complexed or covalently linked to a carrier, classification is also made according to the nature of the carrier in the appropriate A61K 49/005 subgroup.
If the physical or galenical form containing a fluorescent agent is modified by a particular agent, classification is also made according to the nature of this agent in the appropriate A61K 49/005 subgroup.
Classification is also made according to the nature of the fluorescent group in the appropriate subgroup of A61K 49/0019.
If the dye used for staining is fluorescent, classification is also given for the appropriate subgroup of A61K 49/0019.
Quantum dots modified on their surface by an antibody are also classified in A61K 49/0058.
When the formulation aspect is the invention or part of the invention (e.g. liposomal formulations), or when the physical form is the fluorescence-active part (e.g. quantum dots), classification is also made according to the appropriate A61K 49/0063 group.
In the fluorescence subgroups, the compound can be characterised by the fluorescing group, by the carrier/targeting group and/or the physical/galenical form. If the three aspects are present, the three groups must be allocated.
Microemulsion means that the dispersed phase is in the form of globules having a diameter above or equal to 1 micrometer. Nanoemulsion means that the dispersed phase is in the form of globules having a diameter below 1 micrometer.
Micelles comprise a monolayer of surfactant molecules that are aggregated head-to-head and tail-to-tail, thus forming a small spherical particle; micelles can be normal, i.e. the surfactant heads are hydrophilic, or inverse.
When the surface of the liposome encapsulating a fluorescent agent and used in vivo is functionalised by a modifying agent, classification is also made according to the nature of this modifying agent: e.g. a liposome modified on its surface by a peptide is classified in A61K 49/0084 and A61K 49/0056. Liposomes encapsulating a fluorescent agent, used in vivo and modified on their surface by a polymer because they incorporate a polymer-lipid conjugate, are only additionally classified in A61K 49/0054 if the polymer modifying the lipid is unusual. Liposomes encapsulating a fluorescent agent which are pegylated because they incorporate a pegylated lipid are only classified in A61K 49/0084, not in A61K 49/0054.
When the surface of the microparticle encapsulating a fluorescent agent and used in vivo is functionalised by a modifying agent, classification is also made according to the nature of this modifying agent, e.g. a microparticle modified on its surface by a peptide is classified in A61K 49/0091 and A61K 49/0056.
In this place, the following terms or expressions are used with the meaning indicated:
Luminescence | light emanating from the compound or composition when used in vivo |
This place covers:
compositions used for enhancing the contrast of an X-ray image taken in vivo or in contact with the living animal/patient.
A61K 49/0404 (2 dots): containing barium sulphate.
A61K 49/0409 (2 dots): physical forms, mixtures of two different X-ray contrast-enhancing agents, containing at least one X-ray contrast-enhancing agent which is not a halogenated organic compound.
A61K 49/0414 (3 dots): particles, beads, capsules, spheres.
A61K 49/0419 (4 dots): microparticles, microbeads, microcapsules, microspheres (i.e. having a size or diameter higher or equal to 1 micrometer).
A61K 49/0423 (4 dots): nanoparticles, nanobeads, nanospheres, nanocapsules (i.e. having a size or diameter smaller than 1 micrometer).
A61K 49/0428 (5 dots): surface-modified nanoparticles, e.g. immuno-nanoparticles.
A61K 49/0433 (2 dots): containing an organic halogenated X-ray contrast-enhancing agent.
A61K 49/0438 (3 dots): organic X-ray contrast-enhancing agent comprising an iodinated group or an iodine atom, e.g. iopamidol.
A61K 49/0442 (3 dots): polymeric X-ray contrast-enhancing agent comprising a halogenated group.
A61K 49/0447 (3 dots): physical forms, mixtures of two different X-ray contrast-enhancing agents, containing at least one X-ray contrast-enhancing agent which is a halogenated organic compound.
A61K 49/0452 (4 dots): solutions, e.g. for injection.
A61K 49/0457 (4 dots): semi-solid forms, ointments, gels, hydrogels.
A61K 49/0461 (4 dots): dispersions,colloids, emulsions, suspensions.
A61K 49/0466 (5 dots): liposomes, lipoprotein vesicles (e.g. HDL and LDL lipoproteins), phospholipidic or polymeric micelles.
A61K 49/0471 (5 dots): perflubron (i.e. perfluoroctylbromide, C8F17Br) emulsions
A61K 49/0476 (4 dots): particles, beads, capsules, spheres.
A61K 49/048 (5 dots): microparticles, microbeads, microcapsules, microspheres (i.e. having a size or diameter higher or equal to 1 micrometer).
A61K 49/0485 (5 dots): nanoparticles, nanobeads, nanospheres, nanocapsules (i.e. having a size or diameter smaller than 1 micrometer).
A61K 49/049 (6 dots): surface-modified nanoparticles, e.g. immune-nanoparticles.
A61K 49/0495 (4 dots): intended for oral administration
This place covers:
Contrast agents used for enhancing the (diagnostic) imaging or detection in vivo, i.e., in the living animal or patient. Such contrast agents are active in MRI because they bear an NMR-active nucleus (e.g. 1H, 13C, 31P, 19F), or because they have a magnetisable group (e.g. iron oxide).
Attention is drawn to the following places, which may be of interest for search:
Microcapsules containing magnetic carrier material, e.g. ferrite for drug targeting | |
Magnetic targeting of therapeutic agents | |
Magnetic compositions used for therapeutic heating of a living body part | |
Pharmaceutical compositions in which the active agent is chemically bound to inorganic nanoparticles | |
Detecting, measuring or recording for diagnosis involving electronic or nuclear magnetic resonance | |
Introduction of isotopes of elements into organic compounds (e.g. deuterium, 13C) |
The classification is made according to the carrier which is covalently linked/complexed to the MRI-active nucleus that is responsible for the NMR/MRI signal (e.g. Gd3+), and according to the galenical aspect or physical form (in A61K 49/18 subgroups).
The complex-forming compound (e.g. chelating group) of an NMR/MRI-active metal ion is classified only if it is an uncommon agent that is the real contribution to the claimed invention, or if it is the only carrier (i.e. no targeting part like e.g. a peptide further linked to the chelating group). In the A61K 49/101 subgroups, the MRI-active nucleus being complexed to a complex-forming compound, e.g. chelating group. Classification being made according to the nature of this complex-forming agent, if it being either an uncommon or new complexing agent (not the usual DTPA, DOTA, DOTP, etc. groups) that forms the real contribution to the claimed MRI invention, or if it being not conjugated to any further molecule, e.g. which being not conjugated to a polymer, peptide, protein or antibody. In that latter case, the MRI probe being e.g. a paramagnetic metal chelate.
Chelates (e.g. Gd-DOTA) conjugated to a further molecule are classified in A61K 49/085 and, additionally, according to the nature of this further molecule, e.g. an MRI contrast agent being Gd-DOTA conjugated to glucose is classified in A61K 49/085 and A61K 49/10, a MRI contrast agent comprising a plurality of Gd-DOTA appending to a linear polymer backbone is classified in A61K 49/085 and A61K 49/128. MRI contrast agents which are based on MRI-active nanoparticles (e.g. iron oxide nanoparticles, MRI nanoparticles) are classified in the appropriate A61K 49/1818 subgroups. If the nanoparticles have a super/para/magnetic core which is coated or functionalised with different compounds, classification is made according to the nature of all these different compounds (e.g. a nanoparticle having a super/para/magnetic core which is coated with an organic silane linked to a peptide is classified in A61K 49/1848 and A61K 49/1866). MRI contrast agents containing free radicals are classified in A61K 49/20.
When the carrier is a peptide or protein, it is classified in A61K 49/14 and subgroups, e.g. a carrier being a polyamino acid is classified in A61K 49/146 and not in A61K 49/16 that concern antibodies. If the MRI-active nucleus being linked to the peptide or protein or polyamino acid via a complexing or chelating group, the subgroup A61K 49/085 should also be given. If the peptide or protein or polyamino acid being a dendrimer, a dendron, or hyperbranched, then the A61K 49/124 is also given.
When the carrier is an organic macromolecular compound, it concerns an oligomeric, polymeric, dendrimeric molecule, not a peptide, protein, polyamino acid (see A61K 49/00) or an antibody (see A61K 49/00 or A61K 49/16).
Dendrimeric, dendronised or hyperbranched polyamino acids used as carriers are also classified in A61K 49/146.
If the MRI-active nucleus is linked to the peptide or protein or polyamino acid via a complexing or chelating group, the subgroup A61K 49/085 should also be allocated. If the peptide or protein or polyamino acid is a dendrimer, a dendron, or hyperbranched, then the A61K 49/124 is also allocated.
If the MRI-active nucleus is linked to the antibody via a complexing or chelating group, the subgroup A61K 49/085 should also be allocated.
If the paramagnetic metal complexes are covalently linked to the bilayered membrane, then the A61K 49/085 subgroup is also allocated. Liposomes modified on their external surface by a targeting agent, e.g. an antibody are classified in A61K 49/1812 without further indication for the targeting agent.
For nanoparticles, i.e. having a size or diameter smaller than 1 micrometer, the subgroups B82Y 5/00 and B82Y 15/00 are also allocated.
This place covers:
Compositions used for the in vivo diagnosis using ultrasound activation, or wherein the detected signal is an acoustic signal (e.g. optoacoustic imaging).
Attention is drawn to the following places, which may be of interest for search:
Preparations liberating a therapeutically active compound by applying ultrasound | |
Preparations containing sonosensitizers for use in therapy, sonoferese or ultrasonically enhanced transdermal delivery |
This place covers:
Preparations containing radioactive substances or substances that bear a radioactive label, used for therapy in humans or animals, or used for testing in vivo, diagnosis in vivo or diagnostic imaging in vivo.
Liposomes encapsulating the radioactive probe and/or having no radiolabelled phospholipids are classified in A61K 51/1234.
Galenical aspects of pharmaceutical compositions: if this is an important aspect of the invention, it may also be classified in A61K 9/00 or A61K 47/00 - A61K 47/46.
Preparations for testing in vitro or ex vivo are classified in G01N or C12Q, depending on the techniques used.
Radioactive compounds that are new are also classified in C07B 59/00.
Attention is drawn to the following places, which may be of interest for search:
The use in vivo of substances containing a non-radioactive isotope, such as deuterium or 13C. | |
Preparations for testing in vivo using non-radioactive substances | |
Introduction of isotopes of elements into organic compounds | |
Labelling of peptides | |
The use of preparations containing a radioactive substance or a substance that bears a radioactive label, used for diagnosis ex vivo or used for diagnosis or testing in vitro. Therefore, the use in testing in bacteria on, e.g. a Petri dish is excluded. |
Compositions that are well defined and disclosed in the examples or in the claims are classified in this group. Thus, radioactive isotopes mentioned only in the description as being possibly linked to an active agent are not classified here.
The classification is made according to the carrier which is covalently linked, or complexed to the radionuclide, and according to the galenical aspect(s) or physical form(s).
The classification of conjugates in which the carrier is an antibody, the classification of the characterising antibody follows a classification similar to that of new antibodies, C07K 16/00 and subgroups, although less detailed. For the specificity of the antibody, the same rules are followed as for the classification in C07K 16/00.
If the radionuclide is complexed using a chelating group, the classification according to this chelating group is only made in two situations:
- if the chelating group is an uncommon agent, that is the real contribution of the claimed invention, or
- if it is the carrier per se.
If a common chelating group is linked/conjugated to another carrier (e.g. a targeting group, a peptide, or an active agent), classification takes place according to this other carrier, and not according to the common chelating group.
Under A61K 51/041 the last place priority rule is followed.
Porphyrins or texaphyrins used as complex-forming compounds, i.e. wherein the nitrogen atoms forming the central ring system complex the radioactive metal, are classified in A61K 51/0485.
Liposomes encapsulating the radioactive probe or having no radiolabelled phospholipids are classified in A61K 51/1234.
Liposomes modified on their external surface by a targeting agent, e.g. an antibody, are not additionally classified with the symbol of the targeting agent.
Pretargeting is the administration of an agent X bearing the radioisotope or radioactive nucleus and of an agent Y capable of binding X and a cell Y in several steps, e.g. the radiolabelled agent is a radiolabelled biotin and the agent Y is a (strept)avidin molecule targeting specific cells. Classification is also made according to the nature of the carrier bearing/linked to the radioactive nucleus, e.g. an antibody.
In case of a conjugate comprising a complex-forming compound (chelating group) complexing a radioactive metal linked to the carrier (organic compound in A61K 51/0497), the nature of this complex-forming compound is not classified except if the complexing/chelating group is the subject of the invention and is uncommon, e.g. 111In-DTPA-glucose is classified in A61K 51/0497 (not in A61K 51/048) and in A61K 51/0491.
In case of a conjugate comprising a complex-forming compound (chelating group) complexing a radioactive metal linked to the carrier (organic macromolecular compound in A61K 51/065), the nature of this complex-forming compound is not classified except if it is the real contribution of the claimed invention and it is an uncommon complexing/chelating group, e.g. 111In-DTPA-PEG is classified in A61K 51/065 and new DTPA-like derivatives conjugated to PEG and complexing 111In for use in vivo is classified in A61K 51/0478 and A61K 51/065.
In case of a conjugate comprising a complex-forming compound (chelating group) complexing a radioactive metal linked to the carrier (peptide, protein or polyamino acid in A61K 51/088), the nature of this complex-forming compound is not classified except if it is the real contribution of the claimed invention and it is an uncommon complexing or chelating group, e.g. 111In-DTPA-interleukin 2 is classified in A61K 51/088; new DTPA-like derivatives conjugated to interleukin 2 and complexing 111In for use in vivo is classified in A61K 51/0478 and A61K 51/088.
In case of a conjugate comprising a complex-forming compound (chelating group) complexing a radioactive metal linked to the carrier (antibody in A61K 51/1093), the nature of this complex-forming compound being not classified except if it being the real contribution of the claimed invention and it being an uncommon complexing/chelating group, e.g. 111In-DTPA-herceptin being classified in A61K 51/1093 and A61K 51/1051, new DTPA-like derivatives conjugated to herceptin and complexing 111In for use in vivo being classified in A61K 51/0478, A61K 51/1093 and A61K 51/1051.
If the disclosed compound/composition is used for therapy classification is also given in A61K 2121/00.
If the disclosed compound/composition is used for testing in vivo classification is also given in A61K 2123/00.
In patent documents, the following abbreviations are often used:
PET | Positron Emission Tomography |
SPECT | Single Photon Emission Computed Tomography |
Attention is drawn to the following places, which may be of interest for search:
Fermentation or enzyme-using processes in general |
Attention is drawn to the following places, which may be of interest for search:
Extraction at elevated pressure or temperature, e.g. pressurized solvent extraction [PSE], supercritical carbon dioxide extraction or subcritical water extraction |