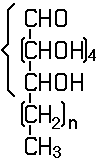Version: 2024.01
Loading Scheme...
CPC | COOPERATIVE PATENT CLASSIFICATION | |||
| C07H | SUGARS; DERIVATIVES THEREOF; NUCLEOSIDES; NUCLEOTIDES; NUCLEIC ACIDS (derivatives of aldonic or saccharic acids C07C, C07D; aldonic acids, saccharic acids C07C 59/105, C07C 59/285; cyanohydrins C07C 255/16; glycals C07D; compounds of unknown constitution C07G; polysaccharides, derivatives thereof C08B; DNA or RNA concerning genetic engineering, vectors, e.g. plasmids, or their isolation, preparation or purification C12N 15/00; sugar industry C13) [2020-05] NOTES
WARNING
|
C07H 1/00 | Processes for the preparation of sugar derivatives [2013-01] |
C07H 1/02 | . | Phosphorylation [2013-01] |
C07H 1/04 | . . | Introducing polyphosphoric acid radicals [2013-01] |
C07H 1/06 | . | Separation; Purification [2013-01] |
C07H 1/08 | . . | from natural products [2013-01] |
C07H 3/00 |
C07H 3/02 | . | Monosaccharides [2013-01] |
C07H 3/04 | . | Disaccharides [2015-07] |
C07H 3/06 | . | Oligosaccharides, i.e. having three to five saccharide radicals attached to each other by glycosidic linkages [2015-07] |
C07H 3/08 | . |
C07H 3/10 | . | Anhydrosugars, e.g. epoxides [2015-07] |
C07H 5/02 | . | to halogen [2013-01] |
C07H 5/04 | . | to nitrogen [2013-01] |
C07H 5/06 | . . | Aminosugars [2015-07] |
C07H 5/08 | . | to sulfur, selenium or tellurium [2013-01] |
C07H 5/10 | . . | to sulfur [2013-01] |
C07H 7/00 | Compounds containing non-saccharide radicals linked to saccharide radicals by a carbon-to-carbon bond [2015-07] |
C07H 7/02 | . | Acyclic radicals [2015-07] |
C07H 7/027 | . . | Keto-aldonic acids [2015-07] |
C07H 7/033 | . . | Uronic acids [2015-07] |
C07H 7/04 | . | Carbocyclic radicals [2013-01] |
C07H 7/06 | . | Heterocyclic radicals [2013-01] |
C07H 9/00 | Compounds containing a hetero ring sharing at least two hetero atoms with a saccharide radical [2013-01] |
C07H 9/02 | . | the hetero ring containing only oxygen as ring hetero atoms [2013-01] |
C07H 9/04 | . . | Cyclic acetals [2013-01] |
C07H 9/06 | . | the hetero ring containing nitrogen as ring hetero atoms [2013-01] |
C07H 11/00 |
C07H 11/02 | . | Nitrates; Nitrites [2013-01] |
C07H 11/04 | . | Phosphates; Phosphites; Polyphosphates [2015-07] |
C07H 13/00 | Compounds containing saccharide radicals esterified by carbonic acid or derivatives thereof, or by organic acids, e.g. phosphonic acids [2015-07] |
C07H 13/02 | . | by carboxylic acids [2015-07] |
C07H 13/04 | . . | having the esterifying carboxyl radicals attached to acyclic carbon atoms [2015-07] |
C07H 13/06 | . . . | Fatty acids [2013-01] |
C07H 13/08 | . . | having the esterifying carboxyl radicals directly attached to carbocyclic rings [2015-07] |
C07H 13/10 | . . | having the esterifying carboxyl radicals directly attached to heterocyclic rings [2015-07] |
C07H 13/12 | . | by acids having the group -X-C(=X)-X-, or halides thereof, in which each X means nitrogen, oxygen, sulfur, selenium or tellurium, e.g. carbonic acid, carbamic acid [2015-07] |
C07H 15/02 | . | Acyclic radicals, not substituted by cyclic structures [2015-07] |
C07H 15/04 | . . | attached to an oxygen atom of the saccharide radical [2015-07] |
C07H 15/06 | . . . | being a hydroxyalkyl group esterified by a fatty acid [2015-07] |
C07H 15/08 | . . . | Polyoxyalkylene derivatives [2015-07] |
C07H 15/10 | . . . | containing unsaturated carbon-to-carbon bonds [2015-07] |
C07H 15/12 | . . | attached to a nitrogen atom of the saccharide radical [2015-07] |
C07H 15/14 | . . | attached to a sulfur, selenium or tellurium atom of a saccharide radical [2015-07] |
C07H 15/16 | . . . | Lincomycin; Derivatives thereof [2015-07] |
C07H 15/18 | . | Acyclic radicals, substituted by carbocyclic rings [2015-07] |
C07H 15/20 | . | Carbocyclic rings [2015-07] |
C07H 15/203 | . . | Monocyclic carbocyclic rings other than cyclohexane rings; Bicyclic carbocyclic ring systems [2017-08] |
C07H 15/207 | . . | Cyclohexane rings not substituted by nitrogen atoms, e.g. kasugamycins [2015-07] |
C07H 15/22 | . . | Cyclohexane rings, substituted by nitrogen atoms [2015-07] |
C07H 15/222 | . . . | Cyclohexane rings substituted by at least two nitrogen atoms [2015-07] |
C07H 15/224 | . . . . | with only one saccharide radical directly attached to the cyclohexyl radical, e.g. destomycin, fortimicin, neamine [2013-01] |
C07H 15/226 | . . . . | with at least two saccharide radicals directly attached to the cyclohexane rings [2015-07] |
C07H 15/228 | . . . . . | attached to adjacent ring-carbon atoms of the cyclohexane rings [2015-07] |
C07H 15/23 | . . . . . . | with only two saccharide radicals in the molecule, e.g. ambutyrosin, butyrosin, xylostatin, ribostamycin [2015-07] |
C07H 15/232 | . . . . . . | with at least three saccharide radicals in the molecule, e.g. lividomycin, neomycin, paromomycin [2015-07] |
C07H 15/234 | . . . . . | attached to non-adjacent ring carbon atoms of the cyclohexane rings, e.g. kanamycins, tobramycin, nebramycin, gentamicin A2 [2017-08] |
C07H 15/236 | . . . . . . | a saccharide radical being substituted by an alkylamino radical in position 3 and by two substituents different from hydrogen in position 4, e.g. gentamicin complex, sisomicin, verdamycin [2017-08] |
C07H 15/238 | . . . | Cyclohexane rings substituted by two guanidine radicals, e.g. streptomycins [2013-01] |
C07H 15/24 | . . | Condensed ring systems having three or more rings [2015-07] |
C07H 15/244 | . . . | Anthraquinone radicals, e.g. sennosides [2013-01] |
C07H 15/248 | . . . | Colchicine radicals, e.g. colchicosides [2013-01] |
C07H 15/252 | . . . | Naphthacene radicals, e.g. daunomycins, adriamycins [2015-07] |
C07H 15/256 | . . . | Polyterpene radicals [2015-07] |
C07H 15/26 | . | Acyclic or carbocyclic radicals, substituted by hetero rings [2015-07] |
C07H 17/00 | Compounds containing heterocyclic radicals directly attached to hetero atoms of saccharide radicals [2015-07] |
C07H 17/02 | . | Heterocyclic radicals containing only nitrogen as ring hetero atoms [2013-01] |
C07H 17/04 | . | Heterocyclic radicals containing only oxygen as ring hetero atoms [2013-01] |
C07H 17/06 | . . | Benzopyran radicals [2015-07] |
C07H 17/065 | . . . | Benzo[b]pyrans [2015-07] |
C07H 17/07 | . . . . | Benzo[b]pyran-4-ones [2015-07] |
C07H 17/075 | . . . . | Benzo[b]pyran-2-ones [2015-07] |
C07H 17/08 | . . | Hetero rings containing eight or more ring members, e.g. erythromycins [2013-01] |
C07H 19/00 | Compounds containing a hetero ring sharing one ring hetero atom with a saccharide radical; Nucleosides; Mononucleotides ; Anhydro-derivatives thereof [2015-07] |
C07H 19/01 | . | sharing oxygen [2017-08] |
C07H 19/02 | . | sharing nitrogen [2013-01] |
C07H 19/04 | . . | Heterocyclic radicals containing only nitrogen atoms as ring hetero atom [2015-07] |
C07H 19/044 | . . . | Pyrrole radicals [2015-07] |
C07H 19/048 | . . . | Pyridine radicals [2015-07] |
C07H 19/052 | . . . | Imidazole radicals [2015-07] |
C07H 19/056 | . . . | Triazole or tetrazole radicals [2015-07] |
C07H 19/06 | . . . | Pyrimidine radicals [2015-07] |
C07H 19/067 | . . . . | with ribosyl as the saccharide radical [2015-07] |
C07H 19/073 | . . . . | with 2-deoxyribosyl as the saccharide radical [2015-07] |
C07H 19/09 | . . . . | with arabinosyl as the saccharide radical [2015-07] |
C07H 19/10 | . . . . | with the saccharide radical esterified by phosphoric or polyphosphoric acids [2015-07] |
C07H 19/11 | . . . . . | containing cyclic phosphate [2015-07] |
C07H 19/12 | . . . | Triazine radicals [2013-01] |
C07H 19/14 | . . . | Pyrrolo-pyrimidine radicals [2013-01] |
C07H 19/16 | . . . | Purine radicals [2013-01] |
C07H 19/167 | . . . . | with ribosyl as the saccharide radical [2015-07] |
C07H 19/173 | . . . . | with 2-deoxyribosyl as the saccharide radical [2015-07] |
C07H 19/19 | . . . . | with arabinosyl as the saccharide radical [2015-07] |
C07H 19/20 | . . . . | with the saccharide radical esterified by phosphoric or polyphosphoric acids [2015-07] |
C07H 19/22 | . . . | Pteridine radicals [2013-01] |
C07H 19/23 | . . . | Heterocyclic radicals containing two or more heterocyclic rings condensed among themselves or condensed with a common carbocyclic ring system, not provided for in groups C07H 19/14 - C07H 19/22 [2016-05] |
C07H 19/24 | . . | Heterocyclic radicals containing oxygen or sulfur as ring hetero atom [2015-07] |
C07H 21/00 | Compounds containing two or more mononucleotide units having separate phosphate or polyphosphate groups linked by saccharide radicals of nucleoside groups, e.g. nucleic acids [2016-08] |
C07H 21/02 | . | with ribosyl as saccharide radical [2013-01] |
C07H 21/04 | . | with deoxyribosyl as saccharide radical [2013-01] |
C07H 23/00 | Compounds containing boron, silicon, or a metal, e.g. chelates, vitamin B12 (esters with inorganic acids C07H 11/00; metal salts, see parent compounds) [2015-07] |
C07H 99/00 | Subject matter not provided for in other groups of this subclass [2013-01] |
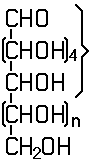 an unbranched sequence of at the most six carbon atoms, having bonds to oxygen as defined in this Note
an unbranched sequence of at the most six carbon atoms, having bonds to oxygen as defined in this Note