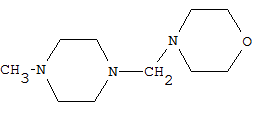
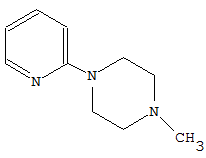
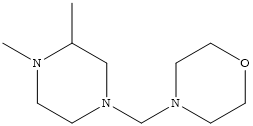
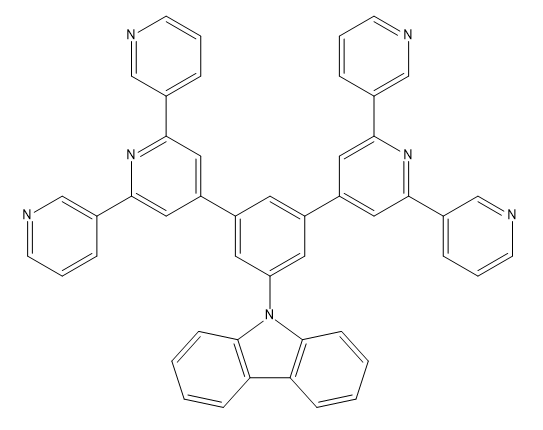
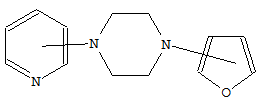CPC Definition - Subclass C07D
This place covers:
Organic compounds containing at least one heterocyclic ring, and with no ring comprising a steroid, saccharide or peptide moiety. Said compounds of C07D contain ring heteroatoms selected from nitrogen, oxygen, sulphur, selenium, tellurium, halogen or a combination thereof.
The compounds may also contain metal atoms, but only as the cations of heterocyclic organic acid salts, alcoholates, phenolates or mercaptides, or as chelating atoms, e.g. in porphyrins.
Preparation of such compounds, including purification, separation, stabilisation or use of additives, unless a separate place is provided elsewhere in the classification scheme.
In class C07, the last place priority rule is used, i.e. in the absence of an indication to the contrary, a compound is classified in the last appropriate subclass. Hence, while individual heterocycle-containing amino acids are classified in this subclass C07D, peptides are generally classified in subclass C07K. Similarly, compounds containing saccharide radicals are classified in subclass C07H, and heterocyclic steroids are classified in subclass C07J. (Detailed instructions which compounds are considered as C07H, C07J or C07K can be found in the corresponding CPC Definitions.) Heterocycles incorporating elements other than C, H, halogen, N, O, S, Se or Te are classified in subclass C07F, but only if the metal-containing compound has a metal carbon bond or if the metal is attached to at least two different ligands. Salts, adducts or complexes formed between two or more organic compounds are classified according to all compounds forming the salts, adducts or complexes (e.g., maleic acid salts of heterocyclic compounds are also classified in C07C).
This subclass is a structure-oriented entry for the compounds themselves and does not cover the application or use of the compounds under the subclass definition.
For classifying such information other entries exist, for example:
- Heterocyclic compounds disclosed as dyes or pigments are classified in subclass C09B.
- Compounds or compositions for preservation of bodies of humans, animals, plants, or parts thereof, as biocides, e.g. disinfectants, pesticides, herbicides, as pest repellents or attractants, and as plant growth regulators are classified in subclass A01N.
- Preparations for medical, dental, or toilet purposes or methods of using compounds for the same purposes are classified in subclass A61K.
Multiple classification:
Biocidal, pest repellent, or plant growth regulatory activity of chemical compounds or preparations is further classified in subclass A01P.
Therapeutic activity of chemical compounds is further classified in subclass A61P.
Uses of cosmetics or similar toilet preparations are further classified in subclass A61Q.
This place does not cover:
Macromolecular compounds |
Examples of places where the subject matter of this place is covered when specially adapted, used for a particular purpose, or incorporated in a larger system:
Pesticides | |
Biocidal activity | |
Therapeutic activity | |
Dyes and Pigments | |
Adhesives | |
Luminescent, e.g. electroluminescent or chemiluminescent materials | |
Use of organic compounds in semiconductors |
Attention is drawn to the following places, which may be of interest for search:
Food or functional food (nutraceuticals) | |
Cosmetics | |
Medicinal preparations containing organic ingredients | |
Physical or Chemical Processes or Apparatus in General | |
Catalysts | |
Generic methods and apparatus therefor used in organic chemistry, such as oxidation, reduction, addition, substitution, purification, separation, stabilisation | |
Heterocyclic organic compounds containing elements other than carbon, hydrogen, halogen, oxygen, nitrogen, sulphur, selenium or tellurium | |
Sugars | |
Steroids | |
Peptides | |
Preparation of heterocyclic organic compounds using enzymes or fermentation processes | |
Electrolytic production of organic compounds | |
Processes for producing compounds in which simultaneously electricity is generated | |
Electrophoretic production of compounds | |
Combinatorial libraries containing organic compounds |
In this subclass, in the absence of an indication to the contrary, a compound is classified in the last appropriate place.
Chemical compounds and their preparation are classified in the groups for the type of compound prepared. The processes of preparation are also classified in the groups for the types of reaction employed, if of interest. The compounds prepared are also classified in the groups for the types of compounds prepared, if of interest.
Salts of a compound, unless specifically provided for, are classified as that compound. Salts, adducts or complexes formed between two or more organic compounds are classified according to all compounds forming the salts, adducts or complexes. Salts, chelates, alcoholates (except Ti/Zr), phenates involving a single ligand are classified as the parent compound (metal containing porphyrin C07D 487/22).
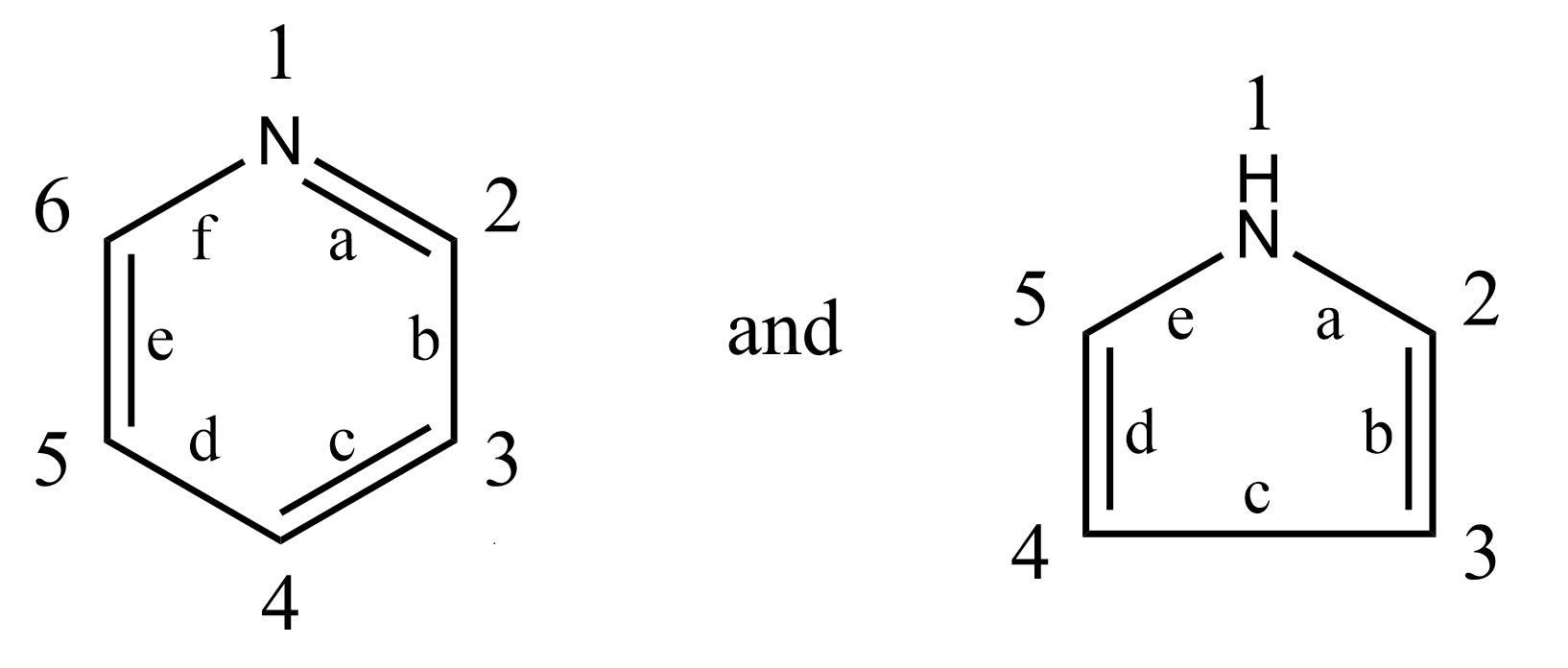
Where a molecule may exist in tautomeric forms, classification is made for the form which appears latest in the scheme. Therefore, double bonds between ring members and non-ring members and double bonds between ring members themselves are considered equivalent in determining the degree of hydrogenation of the ring.
- Compounds containing a single heterocycle are classified in the range C07D 203/00 - C07D 347/00 (cf. Table 1). Compounds containing two or more heterocycles, none of which are condensed among themselves nor condensed with a carbocyclic ring, and all of which are covered by the same main group, are also classified in this range.
- Heterocyclic compounds which contain rings of five or more members, wherein only nitrogen is present as a ring heteroatom, and wherein the ring carbon atoms are fully saturated and only bound to hydrogen atoms, are classified in main group C07D 295/00. 3-azabicyclo[3.2.2]nonanes, piperazines, morpholines and thiomorpholines unsubstituted on the ring carbon atoms are also classified here.
- Compounds containing two or more hetero rings individually covered by different main groups, and not part of the same condensed ring system are classified in the range C07D 401/00 - C07D 421/00 (cf. Table 2). Compounds containing two rings of two different main groups are classified in the relevant subgroups, e.g. C07D 401/04, C07D 401/06, C07D 401/08, C07D 401/10 or C07D 401/12", depending on how they are attached. Compounds containing three or more rings of two or more different main groups are classified in the relevant subgroup, e.g. C07D 401/14.
- Where a compound contains at least one ring covered by group C07D 295/00 and at least one other hetero ring, the hetero ring covered by group C07D 295/00 is ignored and treated as an acyclic chain containing nitrogen atoms for the purposes of classification.
- Compounds containing two or more hetero rings, being part of the same condensed ring system, are classified in the range C07D 451/00 - C07D 519/00 (cf. Table 3 and 4).
The following table shows how classification can change depending on substitution:
| |
| |
| |
| |
| |
| |
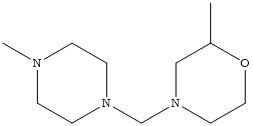
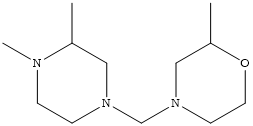
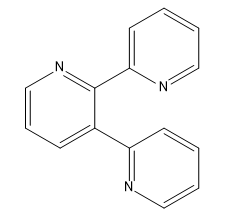
| C07D 213/22 Three occurrences of a pyridine ring in the same compound (one main group) |
| C07D 403/10 Two main groups; Two heterocyclic rings present |
| C07D 401/14 Two main groups; Seven heterocyclic rings present |
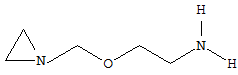
An acyclic side-chain linked to the hetero ring is considered to be terminated by every bond to: 1) an element other than carbon 2) a carbon atom having three bonds to hetero atoms, with at the most one bond to halogen, e.g. ester or nitrile radicals. For example, the following compound is classified in C07D 203/10 and not in C07D 203/12.
Where a heterocycle is linked to a carbocycle by an acyclic chain, and both the chain and the carbocycle are further substituted by either hetero atoms or carbon atoms with three bonds to hetero atoms, not more than one hetero atom being a halogen, the molecule is classified according to the substituents on the acyclic chain. See the following example:
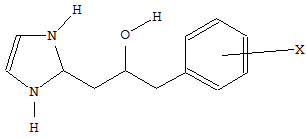
is classified in group C07D 233/22, and the compound
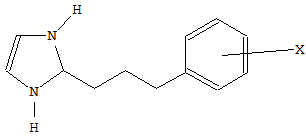
is classified in groups C07D 233/24 and C07D 233/26, where X = - NH2, - NHCOCH3, or - COOCH3.
C07D 451/00 - C07D 517/00 cover compounds containing one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system, with or without other non- condensed hetero rings. For the purpose of classification in groups C07D 451/00 - C07D 519/00, the degree of hydrogenation of the ring system is not taken into consideration. For the purpose of classification in groups C07D 451/00 - C07D 463/00, C07D 473/00 - C07D 477/00, C07D 489/00, C07D 499/00 - C07D 507/00, the wording of the groups has to be understood, in the absence of an indication to the contrary, as including ring systems further condensed with carbocyclic rings or ring systems, but excluding ring systems further condensed with other hetero rings, either directly or through a common carbocyclic ring system, e.g. sparteine is classified in group C07D 471/22 and not in group C07D 455/02. In groups C07D 471/00, C07D 487/00, C07D 491/00 - C07D 498/00 or C07D 513/00 - C07D 517/00, the subdivision is based on the number of relevant hetero rings.
Classification of complex fused ring systems
C07D 451/00 - C07D 517/00 cover heterocyclic compounds containing one system of two or more relevant hetero rings condensed among themselves or condensed with a common carbocyclic ring system, with or without other noncondensed hetero rings. As mentioned in the Glossary of Terms under "number of relevant rings" and "relevant rings", the rings which identify the ring system are determined according to a specific manner and hierarchy of criteria.
Example
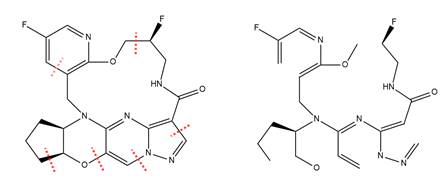
Six scissions are necessary to convert the ring system into an acyclic chain.
The relevant rings, when classified according to the aforementioned hierarchy, are the following: C3N2, C5, C4NO, C4N2, C5N, C10N3O.
The condensed system contains five heterorings, is bridged, and contains at least one hetero ring having nitrogen and oxygen atoms as the only ring hetero atoms.
Thus, the compound should be classified as C07D 498/22.
Classification guidance
What is classified as a compound?
The following compounds are classified: those which are individually named or drawn in the claims; and real examples of compounds disclosed in the specification, that fall within the scope of the claims. A real example is a compound which is prepared or for which physical and/or biological data is given.
The same rules apply for compounds claimed or disclosed as reaction intermediates.
Not classified are the following: generic formulae (Markush formulae, even if they have only one variable group); list(s) of "prophetic compounds" which fall within the scope of the claims but which were not actually prepared; and compounds disclosed in the description/specification but which are not claimed as compounds per se.
What is classified as a process for the preparation of a compound?
Classified are only preparation examples of claimed processes. A process is classified in a process group if a dedicated process group exists (e.g. C07D 201/00 or C07D 301/00). Where there is no process group for making a particular compound, the process is classified according to the product obtained in the claimed process.
Further classification information:
Tables 1-4 provide an overview of the main groups in C07D.
Structural chemical formulae for various subgroups (in particular for specific ring systems as shown in table 3 below) are available in the IPC definitions
Table 1: Overview of main groups C07D 201/00 - C07D 347/00:
Only N: Number of Heteroatoms + Number of
Carbon atoms; Ring Type
Preparation of unsubstituted lactam | |
1N+2C; aziridine, | |
1N+3C; azetidine, | |
1N+4C; non-condensed pyrrole | |
1N+4C; condensed pyrrole | |
1N+5C; hydrogenated | |
1N+5C; aromatic | |
Quinoline | |
Isoquinoline | |
Acridine | |
Other condensed pyridines | |
1N+6C; azepane | |
1N+>6C; azocane and larger | |
Heterocyclic compounds according to more than one of groups C07D 203/00- C07D 225/00 | |
2N+(1C or 2C) | |
2N+3C; 1,2-diazole, | |
2N+3C; 1,3-diazole non-condensed | |
2N+3C; condensed imidazole | |
2N+4C; 1,2-diazine, | |
2N+4C; 1,3-diazine, | |
2N+4C; 1,4-diazine, | |
2N+5C | |
2N+>5C | |
Heterocyclic compounds according to more than one of groups C07D 229/00- C07D 245/00 | |
3N+2C; 1,2,3 or 1,2,4-triazole, | |
3N+3C; 1,3,5-triazine, | |
3N+3C; 1,2,3 or 1,2,4-triazine, | |
Heterocyclic rings with 3N not provided for in C07D 251/00-C07D 253/00 | |
4N | |
>4N |
Only N + O:
1N+1O+3C; 1,2-oxazole, | |
1N+1O+3C; 1,3-oxazole, | |
1N+1O+4C; oxazine, | |
1N+1O+>4C | |
Heterocyclic compounds according to more than one of groups C07D 229/00 - C07D 245/00 | |
2N+1O+2C, | |
Heterocyclic rings with N+O not provided for in C07D 261/00 -C07D 271/00 |
Only N + S:
1N+1S+3C; 1,2-thiazole, | |
1N+1S+3C; 1,3-thiazole, | |
1N+1S+4C; thiazine, | |
1N+1S+>4C, | |
Heterocyclic compounds according to more than one of groups C07D 275/00- C07D 281/00 | |
Heterocyclic compounds containing rings having N+S as the only ring heteroatoms, not provided for by groups C07D 275/00 - C07D 283/00 |
Only N + O + S:
N+O+S, |
N + Se/Te (+O)(+S):
N+Se/Te(+O)(+S) | |
Compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine, or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms |
Only O:
Preparation of oxirane | |
1O+2C; oxirane, | |
1O+3C; oxetane, | |
1O+4C; furan, | |
1O+5C; non-condensed pyran | |
1O+5C; condensed pyran | |
1O+>5C, | |
Heterocyclic compounds according to more than one of groups C07D 303/00- C07D 313/00 | |
2O+3C, | |
2O+4C, | |
Heterocyclic compounds containing rings with 2O not provided for by groups C07D 317/00 - C07D 319/00 | |
>2O, | |
Heterocyclic compounds according to more than one of groups C07D 317/00- C07D 321/00 |
Only O + S:
O+S, |
Only +Se/Te (+S):
O+Se/Te(+S), |
Only S:
1S+(2C or 3C); thiirane or thietane, | |
1S+4C; (tetrahydro)thiophene, | |
1S+5C; thiopyran, | |
1S+>5C, | |
2S, | |
>2S, |
Only S + Se/Te:
S+Se/Te, |
Only Se or Te:
Heterocyclic compounds containing rings with Se or Te as only ring hetero atoms |
Containing Halogen:
Heterocyclic compounds containing rings with halogen atoms |
NOTES to Table 1:
Unless otherwise stated the parent ring is non-condensed or condensed with a carbocyclic ring or ring system.
hydrogenated derivatives are together with the parent hetero ring, with the exception of C07D 211/00 and C07D 213/00.
The relative position of the hetero atoms in the hetero ring is given between brackets.
Table 2: Overview of main groups C07D 401/00 - C07D 421/00:
Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms (N) as the only ring hetero atom, at least one ring being a six-membered ring with only one nitrogen ring atom (C5N) | |
Heterocyclic compounds containing two or more hetero rings, having nitrogen atoms (N) as the only ring hetero atoms, not provided for by group C07D 401/00 | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen atoms (O) as the only ring hetero atom, and at least one ring with only nitrogen (N) as the only ring heteroatom | |
Heterocyclic compounds containing two or more hetero rings, having oxygen atoms (O) as the only ring hetero atoms, not provided for by group C07D 405/00 | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having sulphur atoms (S) as the only ring hetero atoms | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having oxygen and sulphur atoms (O+S) as the only ring hetero atoms | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and oxygen atoms (N+O) as the only ring hetero atoms | |
Heterocyclic compounds containing the thiamine skeleton | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen and sulphur atoms (N+S) not provided by group C07D 415/00 | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having nitrogen, oxygen, and sulphur atoms (N+O+S) as the only ring hetero atoms | |
Heterocyclic compounds containing two or more hetero rings, at least one ring having selenium, tellurium, or halogen atoms as the only ring hetero atoms (Se/Te; halogen; (+O) (+S) (+N)) |
Tables 3 and 4: Overview of main groups C07D 451/00 - C07D 521/00:
Table 3: Overview of specific condensed systems:
8-Azabicyclo[3.2.1]octane (tropane),) 6,7-Epoxy-8-azabicyclo[3.2.1]octane (scopolamine) and cyclic acetals; 9-Azabicyclo[3.3.1]nonane (granatane) | |
Quinuclidine or isoquinuclidine containing ring systems (e.g. quinine derivatives) | |
Quinolizine containing ring systems (e.g. berberine or emetine) | |
Indolo[4,3-f,g]quinoline, (e.g. Ergot alkaloids) | |
Benz[g] indolo[2,3-a]quinolizine (yohimbine) and lactones (reserpic acid lactone) | |
Indolo[3,2,1-d,e]pyrido[3,2,1-i,j] [1,5]]-naphthyridine ring systems, e.g. vincamine (dimeric indolo alkaloids C07D 519/04) | |
Carbacephalosporins | |
Purine | |
Pteridine | |
Thienamycins (Carbapenicillins) | |
4aH-8,9c-Iminoethanophenanthro[4,5-b,c,d]furan ring systems, (e.g. morphine) and 6,14-carbon bridged derivatives (oripavines) | |
Penicillins | |
Cephalosporins | |
Oxapenicillins | |
Oxacephalosporins | |
Condensed beta-lactam ring systems, not provided for by groups C07D 463/00, C07D 477/00 or C07D 499/00 - C07D 505/00 | |
Ergot and Vinca alkaloids containing two or more condensed systems in the molecule |
Table 4: Overview of other condensed systems:
(compounds of Table 3 take precedence)
The ring system contains as heteroatoms…
only nitrogen atoms, with at least one (hydro) pyridine, not provided for by Table 3 | |
only nitrogen atoms, not provided for by Table 3 and by C07D 471/00 | |
at least one ring containing only oxygen atoms and at least one ring containing only nitrogen atoms, not provided for by C07D 451/00, C07D 459/00, C07D 463/00, C07D 477/00 or C07D 489/00 | |
only oxygen atoms | |
at least one ring containing only sulphur atoms | |
at least one ring containing only oxygen and sulphur atoms | |
at least one ring containing only nitrogen and oxygen atoms | |
at least one ring containing only nitrogen and sulphur atoms (penicillin C07D 499/00; cephalosporin C07D 501/00) | |
at least one ring containing only nitrogen, oxygen and sulphur atoms | |
at least one ring containing selenium, tellurium or halogen atoms, with or without other hetero atoms | |
two or more condensed systems in the molecule not provided for by C07D 453/00 or C07D 455/00 | |
unspecified hetero rings |
In this place, the following terms or expressions are used with the meaning indicated:
Acyclic | The absence of a ring structure. Acyclic chains may be linear or branched. |
Bridged | Where two condensed rings share at least three adjacent ring members: |
Carbocyclic | Where all ring members in a ring are carbon atoms. |
Condensed | Where at least two rings share at least one ring member. |
Condensed ring system | A ring system in which all rings are condensed among themselves, i.e. a ring system wherein the scission of a single connection between two ring atoms cannot result in the division of the ring system into separate entities. Two or more hetero rings are considered part of the same condensed ring system if they are condensed among themselves or to a common carbocycle or carbocyclic system. |
Heterocyclic | Wherein at least one ring member in a molecule containing a ring of atoms is not a carbon atom. For the purposes of classification in this subclass, a narrower definition applies wherein heteroatoms may only be chosen from nitrogen, oxygen, sulphur, selenium, tellurium or halogen. |
Number of relevant rings | In a condensed ring system, this equals the minimum number of scissions necessary to convert the ring system into an acyclic chain, a scission being the disconnection of two bonded atoms, without regard for the bond order. |
Ortho-condensed | Where two condensed rings share two adjacent ring atoms in common. A ring system is deemed ortho-condensed if each ring shares only one face with any other ring, and no ring has two adjacent shared faces: |
Peri-condensed | Where three rings in a condensed ring system share a single ring atom in common: |
Relevant rings | These are the rings which account for all the bonds in a condensed system. In order to prevent ambiguity in classifying a condensed ring system, the rings which identify the ring system are determined according to the following hierarchy of criteria: the rings with the lowest number of members; the rings with the highest number of hetero atoms as ring members; the rings with the lowest number of members shared between rings; the rings with the last place in the classification scheme. |
Rings | Rings are considered as heterocycles only if they contain at least one atom selected from halogen, N, O, S, Se or Te as a ring member. Heterocyclic rings may be present as distinct entities or condensed, either with carbocycles or among themselves. |
Spiro-condensed | Where two condensed rings share only one atom in common:"free" "frozen" |
[x,y]-condensed | The letters in the square brackets refer to the sides around the heterocyclic ring with "a" for the side 1,2; "b" for the side 2,3; etc. For example: |
This place covers:
Separation or purification of unsubstituted lactams.
Attention is drawn to the following places, which may be of interest for search:
Separation of inorganic salts |
This place covers:
Heterocyclic compounds containing hydrogenated pyridine rings, not condensed with other rings, with only hydrogen or carbon atoms directly attached to the ring nitrogen atom, having no double bonds between ring members or between ring members and non-ring members, with hydrocarbon radicals substituted by singly bound oxygen or sulphur atoms attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The singly bound oxygen or sulphur atoms are bound to the same carbon atom |
This place covers:
Heterocyclic compounds containing six-membered rings, not condensed with other rings, with one nitrogen atom as the only ring hetero atom, having three double bonds between ring members or between ring members and non-ring members, having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen or carbon atoms directly attached to the ring nitrogen atom, with hydrocarbon radicals substituted by singly-bound oxygen or sulphur atoms attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The singly bound oxygen or sulphur atoms are bound to the same carbon atom |
This place covers:
Heterocyclic compounds containing quinoline or hydrogenated quinoline ring systems, having no bond between the ring nitrogen atom and a non-ring member or having only hydrogen atoms or carbon atoms directly attached to the ring nitrogen atom, with oxygen atoms directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
Quinophthalone dyes |
This place covers:
Heterocyclic compounds containing acridine or hydrogenated acridine ring systems, with nitrogen atoms directly attached to carbon atoms of the ring system.
Attention is drawn to the following places, which may be of interest for search:
Acridine dyes |
This place covers:
Aza-anthracenes other than acridines (dibenzo[b,e]pyridine).
Attention is drawn to the following places, which may be of interest for search:
Acridine |
This place covers:
Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings, having two double bonds between ring members or between ring members and non-ring members, with only hydrogen atoms or radicals containing only hydrogen and carbon atoms attached to ring carbon atoms, with triarylmethyl radicals attached to ring nitrogen atoms.
Attention is drawn to the following places, which may be of interest for search:
Triarylmethane dyes |
This place covers:
Heterocyclic compounds containing 1,3-diazole or hydrogenated 1,3-diazole rings, not condensed with other rings, having two double bonds between ring members or between ring members and non-ring members, with nitrogen atoms directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The nitrogen atom is part of a nitro radical |
This place covers:
Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings, not condensed with other rings, having three or more double bonds between ring members or between ring members and non-ring members, with one nitrogen atom directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
Benzenesulfonamido-pyrimidines |
This place covers:
Heterocyclic compounds containing 1,3-diazine or hydrogenated 1,3-diazine rings, not condensed with other rings, having three or more double bonds between ring members or between ring members and non-ring members, with two or more nitrogen atoms directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
Benzenesulfonamido-pyrimidines |
This place covers:
Heterocyclic compounds containing 1,3-thiazole or hydrogenated 1,3-thiazole rings, not condensed with other rings, having two or three double bonds between ring members or between ring members and non-ring members, with nitrogen atoms bound to hetero atoms, directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The nitrogen atom is part of a nitro radical |
This place covers:
Heterocyclic compounds containing polymethylene-imine rings with at least five ring members, 3-azabicyclo [3.2.2] nonane, piperazine, morpholine or thiomorpholine rings, having only hydrogen atoms directly attached to the ring carbon atoms, with hydrocarbon radicals substituted by doubly bound oxygen or sulphur atoms attached to ring nitrogen atoms.
Attention is drawn to the following places, which may be of interest for search:
Acylated ring nitrogen atoms |
This place covers:
Compounds containing oxirane rings, with hydrocarbon radicals, substituted by halogen atoms, nitro radicals or nitroso radicals, in which the oxirane rings are condensed with a carbocyclic ring system having three or more relevant rings.
Attention is drawn to the following places, which may be of interest for search:
Steroids |
This place covers:
Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings, having two or three double bonds between ring members or between ring members and non-ring members, with hydrocarbon radicals substituted by singly bound oxygen atoms attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The singly bound oxygen atoms are bound to the same carbon atom |
This place covers:
Heterocyclic compounds containing five-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings, having two or three double bonds between ring members or between ring members and non-ring members, with nitrogen atoms directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The nitrogen atom is part of a nitro radical |
This place covers:
Heterocyclic compounds containing six-membered rings having one oxygen atom as the only ring hetero atom, not condensed with other rings, having no double bonds between ring members or between ring members and non-ring members, with nitrogen atoms not forming part of a nitro radical directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The nitrogen atom is part of a nitro radical |
This place covers:
Benzo[b]pyrans, not hydrogenated in the carbocyclic ring, with oxygen or sulphur atoms directly attached in position 2, not hydrogenated in the hetero ring, substituted otherwise than in position 3 or 7.
Attention is drawn to the following places, which may be of interest for search:
The benzo[b]pyrans are substituted in position 4 by oxygen or sulphur |
This place covers:
Heterocyclic compounds containing five-membered rings having one sulphur atom as the only ring hetero atom, not condensed with other rings, not substituted on the ring sulphur, with nitrogen atoms directly attached to ring carbon atoms.
Attention is drawn to the following places, which may be of interest for search:
The nitrogen atom is part of a nitro or nitroso radical |
This place covers:
Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, at least one ring being a six-membered ring with one nitrogen atom, in which the condensed system contains two hetero rings and is ortho-condensed.
Attention is drawn to the following places, which may be of interest for search:
Carbacephalosporins |
This place covers:
Heterocyclic compounds containing nitrogen atoms as the only ring hetero atoms in the condensed system, in which the condensed system contains two hetero rings and is ortho-condensed.
Attention is drawn to the following places, which may be of interest for search:
Carbapenams, e.g. thienamycins |
This place covers:
Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, in which the condensed system contains three hetero rings and is ortho-condensed.
Attention is drawn to the following places, which may be of interest for search:
Alkylenedioxy derivatives of dibenzo[a, g]quinolizines, e.g. berberine |
This place covers:
Heterocyclic compounds containing in the condensed ring system both one or more rings having oxygen atoms as the only ring hetero atoms and one or more rings having nitrogen atoms as the only ring hetero atoms, in which the condensed system contains three hetero rings and is bridged.
Attention is drawn to the following places, which may be of interest for search:
3-Oxa-9-azatricyclo[3.3.1.0<2,4>]nonane ring systems, e.g. scopolamine |
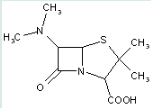
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and a carbon atom having three bonds to heteroatoms at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and a modified 2-carboxyl group at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
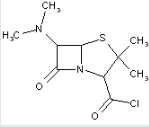
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and an acid anhydride at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
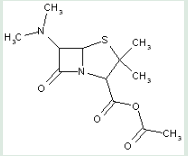
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and an ester at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
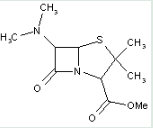
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and a thioacid (or ester thereof) at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
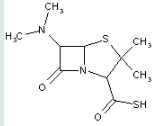
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and an O-ester of a thioacid at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
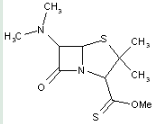
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and an S-ester of a thioacid at the 2-position.
The structure shown below is one example of a compound encompassed by this place
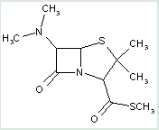
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with the following groups: a nitrogen atom at the 6-position; and an amide, hydrazide, or azide at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
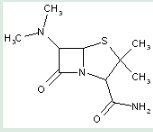
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with a free primary amino group at the 6-position.
The structure shown below is one example of a compound encompassed by this place.
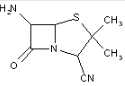
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by either an unsubstituted acyclic hydrocarbon group or an acyclic hydrocarbon group substituted by carbocyclic or heterocyclic rings.
The structure shown below is one example of a compound encompassed by this place.
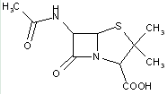
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by a carbon chain substituted by heteroatoms.
The structure shown below is one example of a compound encompassed by this place.
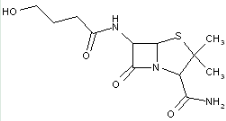
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by a carbon chain substituted by heteroatoms at the beta-position.
The structure shown below is one example of a compound encompassed by this place.
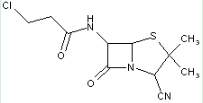
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by a carbon chain substituted by an oxygen or sulfur atom at the beta-position.
The structure shown below is one example of a compound encompassed by this place.
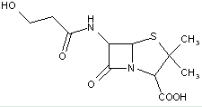
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by a carbon chain substituted by nitrogen atom(s) at the beta-position.
The structure shown below is one example of a compound encompassed by this place.
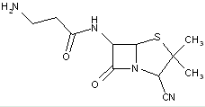
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by a carbon chain substituted by a carbon atom having three bonds to heteroatoms at the beta-position.
The structure shown below is one example of a compound encompassed by this place.
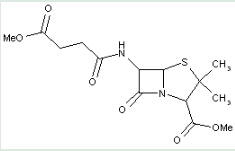
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position.
The structure shown below is one example of a compound encompassed by this place.
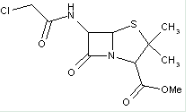
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by oxygen atom(s).
The structure shown below is one example of a compound encompassed by this place.
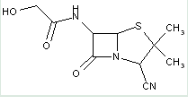
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by sulfur atom(s).
The structure shown below is one example of a compound encompassed by this place.
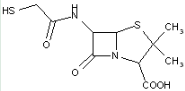
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by nitrogen atom(s).
The structure shown below is one example of a compound encompassed by this place.
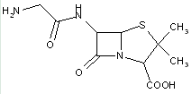
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by nitrogen atom(s) and alicyclic ring(s).
The structure shown below is one example of a compound encompassed by this place.
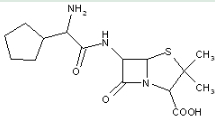
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by nitrogen atom(s) and aromatic ring(s).
The structure shown below is one example of a compound encompassed by this place.
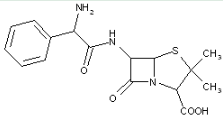
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted at the alpha-position by nitrogen atom(s) and heterocyclic ring(s).
The structure shown below is one example of a compound encompassed by this place.
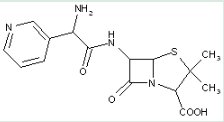
This place covers:
Heterocyclic compounds in which a 4-thia-1-aza-bicyclo[3.2.0] ring system is substituted at the 6-position with a carboxylic acid-acylated amino group, further substituted at the alpha-position of the carboxamide group by carbon atoms having three bonds to heteroatoms.
The structure shown below is one example of a compound encompassed by this place.
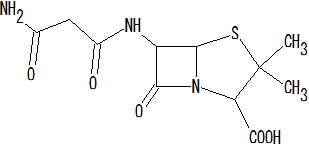
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by carbocyclic ring(s) directly attached to the carboxamide group.
The structure shown below is one example of a compound encompassed by this place.
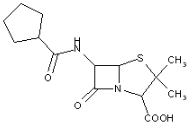
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted at the 6-position by a carboxylic acid-acylated amino group, further substituted by heterocyclic ring(s) directly attached to the carboxamide group.
The structure shown below is one example of a compound encompassed by this place.
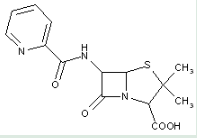
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with a carbonic acid-acylated amino group or a nitrogen or sulfur analogue thereof at the 6-position.
The structure shown below is one example of a compound encompassed by this place.
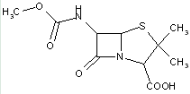
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with a nitrogen-containing ring attached with a ring nitrogen atom at the 6-position.
The structure shown below is one example of a compound encompassed by this place.
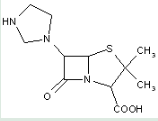
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with substituents other than nitrogen atoms directly attached to position 6 and a carbon atom having three bonds to heteroatom(s) at position 2.
The structure shown below is one example of a compound encompassed by this place.
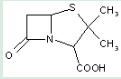
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with a hydrocarbon group or a substituted hydrocarbon group at position 6 and a carbon atom having three bonds to heteroatom(s) at position 2.
The structure shown below is one example of a compound encompassed by this place.
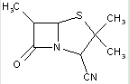
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with heteroatom(s) or carbon atoms having three bonds to heteroatom(s) at the 6-position.
The structure shown below is one example of a compound encompassed by this place.
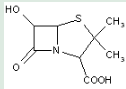
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system in position 3 is unsubstituted or substituted with groups other than 2 methyl groups and position 2 is substituted with a carbon atom having three bonds to heteroatom(s).
The structure shown below is one example of a compound encompassed by this place.
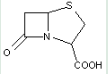
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system with a double bond is in between positions 2 and 3, in which position 2 is substituted with a carbon atom having three bonds to heteroatoms(s), and in which position 3 is substituted with a hydrogen atom or an unsubstituted hydrocarbon group.
The structure shown below is one example of a compound encompassed by this place.
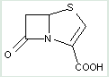
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system with a double bond is in between positions 2 and 3, in which position 2 is substituted with a carbon atom having three bonds to heteroatom(s), and in which position 3 is substituted with a substituted hydrocarbon group.
The structure shown below is one example of a compound encompassed by this place.
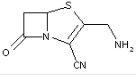
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system with a double bond is in between positions 2 and 3, in which position 2 is substituted with a carbon atom having three bonds to heteroatom(s), and in which position 3 is substituted with a heteroatom or a carbon atom having three bonds to heteroatom(s).
The structure shown below is one example of a compound encompassed by this place.
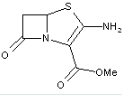
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system with a double bond is in between positions 2 and 3, in which position 2 is substituted with a carbon atom having three bonds to heteroatom(s), and in which position 3 is substituted with a heterocyclic ring or a condensed heterocyclic ring.
The structure shown below is one example of a compound encompassed by this place.
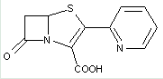
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is substituted with a group other than a carbon atom having three bonds to heteroatom(s) at the 2-position.
The structure shown below is one example of a compound encompassed by this place.
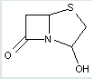
This place covers:
Heterocyclic compounds in which a 4-thia-1-azabicyclo[3.2.0]heptane ring system is further condensed with a carbocyclic ring or a ring system.
The structure shown below is one example of a compound encompassed by this place.
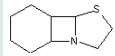
This group is only used for the classification of compounds the chemical structure of which is not specified, i.e. only in those cases where the compounds cannot be classified in any of groups: C07D 201/00 - C07D 519/00.