CPC Definition - Subclass C07C
This place covers:
Acyclic or carbocyclic (alicyclic) low molecular weight organic compounds
Processes for the preparation of acyclic or carbocyclic (alicyclic) low molecular weight organic compounds, whereby preparation also includes purification, separation, stabilisation or use of additives
The subclass C07C is divided into the following fields* (each field covering both compounds and processes):
Hydrocarbons (compounds containing exclusively carbon and hydrogen atoms) | |
Halogenated hydrocarbons (compounds containing exclusively carbon, hydrogen and halogen atoms) | |
Oxygen-containing compounds (may additionally contain halogen atoms) | |
Nitrogen-containing compounds (may additionally contain halogen and/or oxygen atoms) | |
Sulfur, Selenium and Tellurium-containing compounds (may additionally contain halogen, oxygen and/or nitrogen atoms) | |
Special compounds |
*) In the absence of an indication to the contrary, a product or process is classified in the last appropriate place (last place rule)
In class C07, in the absence of an indication to the contrary, a compound is classified in the last appropriate subclass. The compounds defined by the subclasses C07D, C07F, C07G, C07H, C07J and C07K and their preparation are not classified in C07C. For instance, acyclic peptides are classified in C07K and not in C07C.
General methods of organic chemistry:
In addition to the classification in C07C, a classification in C07B is generally assigned if a process is claimed broadly and the general applicability in different fields of C07 is shown by means of examples. All examples for C07C are still classified individually in the appropriate fields in C07C.
Compounds containing metals, e.g. metal salts and metal chelates:
The subclass C07F covers the following metal-containing compounds, and their preparation:
Compounds containing one or more metals whereby at least one metal is bound to carbon,
Compounds containing one or more metals, without metal-carbon bonds, that can be represented by the formula: (L1)n-Metal-(L2)m (n>0 and m>0); L1 and L2 are different metal-bound moieties, and their preparation (e.g. Mg(acac)OMe),
Zirconates and titanates.
Other metal salts (e.g. metal alcoholates, metal phenates, metal carboxylates, metal amides, such as lithium diisopropyl amide, or mercaptides) and metal chelates of acyclic or carbocyclic low molecular weight organic compounds, and their preparation, are classified in subclass C07C and not in subclass C07F.
Polymers (macromolecular compounds):
Oligomers (e.g. alkoxides, esters, amides) with up to 10 (ten) repeating units are classified in C07C as low molecular weight compounds. Compounds with 11 (eleven) or more repeating units are usually classified in C08 as macromolecular compounds.
MULTIPLE CLASSIFICATION
Subclass C07C relates to the compounds themselves and their preparation and does not cover the application or use of the compounds under the subclass definition.
Thus, in addition to the classification in C07C, a document should be assessed for potential classification in the places relating to the use or application of the compounds if such a use/application is claimed or specifically described (e.g. by means of examples). Likewise, documents disclosing apparatus features and catalysts used in processes should be assessed for potential classification in the appropriate places.
A non-exhaustive list of other places frequently encountered in association with compounds or processes classified in C07C is included in the Informative References below.
Biocidal, pest repellant, pest attractant, or plant growth regulatory activity of chemical compounds or preparations is further classified in A01P.
Therapeutic activity of chemical compounds classified as such in C07C is further classified in subclass A61P .Uses of cosmetics or similar toilet preparations are further classified in subclass A61Q.
Mixtures of compounds; preparation of mixtures:
There is no special place for mixtures of compounds in C07C. Accordingly, mixtures are not classified in C07C (see the relevant IPC classification rule concerning "Chemical Mixtures or Compositions"). Mixtures are classified according to their application/use (for a list of application related fields see the informative references below).
Similarly, the preparation of mixtures where the desired end product is the mixture and not a specific product is not generally classified in C07C (e.g. the preparation of a mixture of hydrocarbons for use as fuel is classified in C10G).
The only exceptions to this rule are:
Mixtures defined by a single component, e.g. the claim reads as follows: "A composition comprising a compound (I) of formula A." (Markush formula is given), which are considered as products and classified in the corresponding product group in C07C,
Mixtures which are used in the preparation of C07C compounds are classified in C07C (e.g. an azeotropic mixture of a halogenated hydrocarbon and HF, for use in a process for preparation or purification of a halogenated hydrocarbon).
Mixtures wherein one or more of the components are mere impurities present with the desired compound, e.g. of the form "A composition of compound A and less than 50 ppm of compound B" (wherein it is clear from the description that compound B is merely an undesired impurity of A). Such mixtures are effectively a definition of a certain compound in terms of a desired degree of chemical purity.
This place does not cover:
Inorganic compounds | |
Carbamic acid | |
Carbon, inorganic compounds thereof, e.g. fullerenes | |
Phosgene | |
Carbides | |
Hydrogen cyanide, cyanic and thiocyanic acid, isocyanic and isothiocyanic acid, cyanogen, cyanamide, and cyanogen halide | |
Heterocyclic compounds | |
Compounds containing elements other than carbon, hydrogen, halogen, oxygen, nitrogen, sulfur, selenium and tellurium | |
Compounds of unknown constitution | |
Sugars | |
Steroids | |
Peptides | |
Dyes and pigments | |
Liquid crystal compounds | |
Fermentative or enzymatic processes | |
Production of organic compounds by electrolysis or electrophoresis |
Attention is drawn to the following places, which may be of interest for search:
Pesticides, biocides, pest repellants, pest attractants, or plant growth regulatory compound/compositions | |
Biocidal, pest repellant, pest attractant or plant growth regulatory activity of chemical compounds or preparations | |
Foodstuffs | |
Preparations for medical, dental or toilet purposes | |
Cosmetics | |
Medicinal preparations containing active organic ingredients. | |
Therapeutic activity | |
Uses of cosmetics or similar toilet preparations | |
Separation in general, apparatuses therefor | |
Reactors | |
Catalysts | |
General methods of organic chemistry | |
Introduction of isotopes of elements into organic compounds | |
Organic macromolecular compounds | |
Use of organic substances as compounding ingredients for organic macromolecular compounds. | |
Dyes; Paints; Polishes; Natural resins; Adhesives | |
Organic luminescent materials | |
Lubricants | |
Essential oils and perfumes | |
Detergents, cleaning and washing compositions | |
Chemical libraries |
General rules
In the absence of an indication to the contrary, a product or process is classified in the last appropriate place (last place rule).
For the classification of compounds in groups C07C 1/00 - C07C 71/00 and C07C 401/00 - C07C 409/00:
a compound is classified considering the molecule as a whole (the "whole molecule approach")
a compound is considered to be saturated if it does not contain carbon atoms bound to each other by multiple bonds
a compound is considered to be unsaturated if it contains carbon atoms bound to each other by multiple bonds, including six-membered aromatic ring, unless otherwise specified or implicitly derivable from the subdivision (e.g. C07C 69/712).
For the classification of compounds in groups C07C 201/00 - C07C 395/00, i.e. after the functional group has been determined according to the "last place rule", a compound is classified according to the following principles:
compounds are classified in accordance with the nature of the carbon skeleton to which the functional group is attached
a carbon skeleton is a carbon atom, other than a carbon atom of a carboxyl group, or a chain of carbon atoms bound to each other, a carbon skeleton is considered to be terminated by every bond to an element other than carbon or to a carbon atom of a carboxyl group
when the molecule contains several functional groups, only functional groups linked to the same carbon skeleton as the one first determined are considered;
a carbon skeleton is considered to be saturated if it does not contain carbon atoms bound to each other by multiple bonds
a carbon skeleton is considered to be unsaturated if it contains carbon atoms bound to each other by multiple bonds, including a six-membered aromatic ring.
Examples of compounds classified according to the carbon skeleton:
Ph-NH-CH2-CH2-OH is classified in C07C 215/16 (not in C07C 215/68), while HO-CH2-Ph-NH-CH2-CH2-OH is classified in C07C 215/68
HO(O)C-CH2-CH2-C(O)-NH2 is classified in C07C 233/05 (not in C07C 235/74), while O=C(H)-CH2-CH2-C(O)-NH2 is classified in C07C 235/74.
Anhydrides and halides of carboxylic acids are classified as the relevant acids unless otherwise indicated.
Salts of a compound, unless specifically provided for, are classified as that compound, e.g. aniline hydrochloride is classified as aniline (in C07C 211/46), sodium malonate is classified as malonic acid (in C07C 55/08), and a mercaptide is classified as the mercaptan.
Metal chelates are dealt with in the same way.
Salts, adducts or complexes formed between two or more organic compounds are classified according to all compounds forming the salts, adducts or complexes.
If a group title is the name of a specific compound or a group of specific compounds (e.g. C07C 35/12 "Menthol") then only exclusively the compounds named are classified in this group (including isotopically labelled forms, and salts, if there is no special place for salts, but not including other derivatives thereof). For instance C07C 69/78 covers ester of benzoic acid, but not esters of chlorobenzoic acid (C07C 69/76).
On the other hand, when a compound is mentioned in the group title only as an example, such as in C07C 35/36 "the condensed ring system being a [4.4.0] system, e.g. naphthols"), the scope is not limited to this example.
Compounds claimed per se:
Which compounds are classified?
Real examples of claimed compounds, i.e. those which are prepared or for which physical data are given, and
compounds which are individually named or drawn in the claims.
Which compounds are generally not classified?
Long lists ("shopping lists") of prophetic compounds which fall within the scope of the claims but which have not actually been prepared and characterised or at least individually claimed.
Compounds disclosed in the description but which are not claimed as novel compounds (e.g. prior art compounds).
Markush enumeration of generic formulae to generate individual compounds, i.e. no attempt is made to classify all possible individual compounds falling within the scope of a Markush formula.
Compounds defined in terms of a process of preparation (product-by-process definition), e.g. "A compound prepared by the process of claim 1", are classified only if the compounds per se is claimed as novel.
How are compounds classified?
All examples are classified individually. Even if classification of the "fully identified" compounds would lead to the assigning of a large number of subgroups, no generalisation to the next hierarchically higher level is made.
Compounds having a covalent bond to a solid support are classified as the corresponding compound wherein the solid support has been replaced by a hydrogen atom. In addition an Indexing Code (Indexing Code) C07B 2200/11 ("Compounds covalently bound to a solid support") is assigned.
Protected compounds are classified according to their individual structure (note: a tetrahydropyranyl (THP) protected compound would be classified in C07D, a silyl protected compound in C07F).
Deuterated and other isotopically labelled (e.g. radiolabelled) compounds, regardless whether the non-labelled equivalents are already known or not, are classified as compounds in the same class as the corresponding non-labelled compounds. In addition the Indexing Code C07B 2200/05 is assigned and the document proposed for classification in C07B 59/00).
Polymorphic forms of known compounds are classified in the appropriate product class in same way as new compounds. In addition the Indexing Code C07B 2200/13 is assigned.
Processes for the preparation of (known) compounds:
Preparation also covers purification, separation, stabilisation or use of additives.
Which processes are classified?
Preparative examples of claimed processes, and
Processes for the preparation of products which are individually named or drawn in the claims.
In exceptional cases, when there are no preparative examples and the claims do not define any specific products (e.g. for industrial processes), the detailed embodiments in the description (often explained with reference to the figures) are classified. Occasionally, the product has to be deduced from the educt and the reaction.
Which processes are generally not classified?
The preparation of long lists ("shopping lists") of hypothetical products mentioned in the description which have not actually been made or at least individually claimed.
Processes disclosed in the description which are not claimed as novel processes (e.g. prior art processes).
The preparation of novel compounds is generally not classified. However, if the preparation appears to be of particular interest, the examiner may decide to classify such processes on a case by case basis. The rationale is to avoid filling the process groups with repeated standard methods used for making new compounds.
How is a process classified?
All examples are classified individually
A process is classified in a process group if a dedicated process group exists. Where there is no process group for making a particular compound (e.g. for ester of oxyacids of halogen C07C 71/00) a process is classified in the product group.
When a process is classified in a process group, combination sets are used to indicate the product of the process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups (which means that product groups as such (not in form of a combination set) are only used for classifying compounds which are claimed as novel).For example the combination set C07C 67/08, C07C 69/54 is used for the preparation of acrylic acid esters by esterification.
For multi-step processes (these can be industrial processes comprising multiple reaction and/or purification steps and multi-step syntheses of fine chemicals in a sequence of reaction steps) the following rules apply :
The last reaction step is always classified.
If the last step is purification/separation/recovery step, it is additionally classified if of interest (e.g. not a mere standard purification/recovery step such as "step f) of recovering the product").
All intermediate steps are generally also classified (especially those which are important for the invention) unless considered trivial and not useful for future searches.
A process for the preparation of a specific crystallographic form of a compound (polymorph) is generally classified as separation/purification by crystallisation. In addition the indexing term C07B 2200/13 is assigned.
Indexing of ring structures:
If a classified compound or the product of a classified processes contains a carbocyclic ring system other than phenyl and naphthyl, an Indexing Code for the ring structure (main groups C07C 2601/00 - C07C 2604/00) is assigned (e.g. C07C 2601/14 if a claimed or prepared compound contains a cyclohexyl ring). The following rules apply:
All the possible ring systems are to be indexed; this implies that if a compound comprises more than one ring system, each system is indexed.
Indexing Codes are assigned even if they provide no additional information about the ring system over the definition of the group in which the document is classified, e.g. C07C 2601/14 is used, if the document is classified in C07C 13/18.
The more general groups are only to be used for ring systems not specifically provided for.
For each ring or ring system, the last place rule applies. However, in determining the rings defining the indexation the following criteria have to be taken into account:
The number of rings in a condensed ring system equals the number of scissions necessary to convert the ring system into an acyclic chain.
The rings with the lowest possible number of ring members and the condensed systems with the lowest number of shared atoms are to be chosen.
Polycyclic compounds in which two rings have two, and only two, atoms in common are "ortho-condensed". Polycyclic compounds in which one ring contains two, and only two, atoms in common with each of two or more rings of a contiguous series of rings are "ortho- and peri-condensed".
Rings are spiro condensed if they contain two rings with only one common atom. The spiro system is free when there is only one union, direct or indirect, between the rings. Otherwise the spiro system is "not free".
Indexing of certain compound properties:
Certain properties (such as optical activity or presence of isotopes) of classified compounds or products of a classified processes are indexed using Indexing Codes (main group C07B 2200/00), e.g.
- C07B 2200/07 if the claimed or prepared compound is an optical isomer,
- C07B 2200/13 if the claimed or prepared compound is a crystalline form, e. g. a polymorphic form, which is characterised by the usual parameters, such a X-ray diffraction pattern, IR, differential scanning calorimetry (DSC). The Indexing Code C07B 2200/13 is not used when polymorphic forms of compounds are merely claimed without being actually prepared. The code should also not be used for standard crystallisation processes (e.g. for purification) leading to the usual crystalline forms of compounds.
In this place, the following terms or expressions are used with the meaning indicated:
Acyclic | Not containing any rings |
Carbocyclic | Containing a ring or ring system where all ring members are carbon atoms |
Mineral acid | Inorganic acids such as HF, HCl, HBr, HI, HNO3, H2SO3, H2SO4, H3PO3, B(OH)3 and H2CO3 |
Ester of a mineral acid | Ester of the above acids, including organic carbonates (e.g. ethylene carbonate) and R-Hal (e.g. CH3-Cl as ester of CH3-OH and HCl) |
Non-metals | H, B, C, Si, N, P, O, S, Se, Te, noble gases, halogens |
Metals | Elements other than non-metals |
Platinum group | Os, Ir, Pt, Ru, Rh, Pd |
Iron group | Fe, Co, Ni |
Bridged (rings) | Presence of at least one fusion other than ortho, peri or spiro, i.e. ring having more than two carbon atoms in common |
Condensed ring system | Two rings are "condensed" if they share at least one ring member, i.e. "spiro" and "bridged" are considered as condensed. |
"Number of rings" in a condensed ring system | Number of scissions necessary to convert the ring system into one acyclic chain |
Organic compound | A compound satisfying one of the following criteria:- at least two carbon atoms bonded to each other, or- one carbon atom bonded to at least one hydrogen atom or halogen atom, or- one carbon atom bonded to at least one nitrogen atom by a single or double bond.Exceptions to the above criteria are: compounds consisting of only carbon atoms (e.g., fullerenes, etc.), cyanogen, cyanogen halides, cyanamide, metal carbides, phosgene, thiophosgene, hydrocyanic acid, isocyanic acid, isothiocyanic acid, fulminic acid, unsubstituted carbamic acid, and salts of the previously mentioned acids; these exceptions are considered to be inorganic compounds for classification purposes |
Polycyclic | Containing two or more rings, condensed or isolated, e.g. a naphthyl ring or two isolated phenyl rings |
Preparation | Covers synthesis, purification, separation, stabilisation or use of additives, unless a separate place is provided in the classification scheme |
Quinones | Only compounds which can be considered oxidation products of aromatic compounds (hydroquinones) are encompassed (acenaphthenequinone |
This place covers:
Processes for the preparation of hydrocarbons, i.e. compounds containing only the elements of carbon and hydrogen, from compounds containing exclusively or additionally elements other than carbon and hydrogen.
Catalysts are classified in B01J 21/00 - B01J 49/90.
These classes or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used.
Reactors are classified in B01J 3/00 - B01J 19/32.
However, the reactor has to be described in terms of distinctive reactor features. If the reactor is merely described by features specifying the reaction to be carried out in the reactor, or the content of the reactor (for example the catalyst) rather than by technical reactor features, usually no class B01J for the reactor is given.
General separation/purification methods or a specific apparatus for the separation/purification are classified in B01D.
However, if the separation/purification relates to a standard method for specific C07C compounds, then no B01D class is given.
If the claims cover both the preparation of more specific hydrocarbon mixtures (i.e. singly identifiable compounds, e.g. lower olefins) and hydrocarbon mixtures of undefined composition, and the examples describe also the preparation of specific hydrocarbons, both C07C and C10G classes are given.
The description of the mixtures as fuel, diesel, or kerosene mixture or a definition by indicating a boiling range (20-200°C, or C5+ cut) is often an indicator that the process is not classified in C07C.
There is no clear line between a specific hydrocarbon and an undefined composition. While the expression "lower olefins" can be considered to describe a mixture of individually defined compounds and usually means ethylene and propylene, this is not the case for the expression "paraffins". In the "grey zone", both C07C and C10G classes are given.
This place does not cover:
Processes for the preparation of hydrocarbon mixtures where the mixture rather than the individual hydrocarbons is the desired product, e.g. Fischer Tropsch processes for the preparation of hydrocarbon mixtures. | |
Preparation of acetylene gas by wet methods (e.g. acetylene from CaC2) | |
Processes for the preparation of synthetic natural gas | |
Processes for the preparation of liquefied petroleum gas (LPG) |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts | |
Cracking of hydrocarbon oils; production of hydrocarbon mixtures; refining mixtures mainly consisting of hydrocarbons; reforming of naphtha; mineral waxes |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 1/24, C07C 11/04 for the preparation of ethylene from ethanol by elimination of water). A combination set consists of a process group (e.g. C07C 1/24), followed by and linked to the group of the product (e.g. C07C 11/04). The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Multi-step processes
Multi-step processes are classified as processes according to the specific rules of the subclass C07C, i.e. the last reaction step leading to the end product is always classified. Other reaction steps are also classified if they are not entirely trivial (such as the removal of a protecting group) or if they could be useful for future searches.
Please note that in the past, multi-step processes leading to hydrocarbon compounds had been classified in the corresponding product group for the final end product. These documents are continuously being reclassified. However, for the time being, a complete search should involve searching the product groups.
Indexing Codes for the catalysts
In addition to the Indexing Codes C07C 2601/00 - C07C 2604/00 used over the whole of C07C to describe rings or ring systems which might be present in the compounds (prepared or claimed per se), the Indexing Codes C07C 2521/00 - C07C 2531/38 are used to describe the catalysts used in preparation processes classified in C07C 1/00 - C07C 6/126.
These Indexing Codes are added irrespective of whether or not the document is also to be classified in the field of B01J for the catalysts.
More detailed rules for the use of these catalyst Indexing Codes is further explained in the Special rules of classification under C07C 2521/00.
C07C 1/02 - C07C 1/12: These classes are rarely used since e.g. Fischer-Tropsch processes wherein CO is reacted with hydrogen normally leads to the preparation of a hydrocarbon mixture rather than to specific hydrocarbon compounds. The process is for example classified in these classes if the process aims at the preparation of specific compounds or a small group of separately identifiable compounds (e.g. lower olefins).
C07C 1/08: preparation of iso-compounds (e.g. isoparaffins)
C07C 1/20: e.g. Oxygenate to olefin (OTO) and methanol to olefin (MTO) processes
C07C 1/321: non-metal atoms such as B or Si
Coupling reactions with boronic acid derivatives are classified in this class.
C07C 1/326: for example Grignard reactions
C07C 1/36: by splitting of esters other than carboxylic acid esters only (for example sulfuric acid esters)
Reactions involving the splitting of carboxylic acid esters are classified in C07C 1/213.
In patent documents, the following abbreviations are often used:
OTO process | Oxygenate to olefin process |
MTO process | Methanol to olefin process |
This place covers:
Processes for the preparation of hydrocarbons (i.e. compounds containing only the elements of carbon and hydrogen) from compounds at least one of which is a hydrocarbon. The reactions involve an increase in the number of carbon atoms in the skeleton. Processes involving the reaction between a hydrocarbon and a non-hydrocarbon are also classified under this group.
The following rules of thumb can help to decide whether a process is to be classified in C07C (dimerization and oligomerization) or C08F (polymerization):
Products are considered oligomers rather than polymers if:
- the molecular weight is smaller than 1000 g/mol;
- the number of repeating units is ≤ 10;
- the product is not solid.
The indication of the viscosity of the product is an indication that it might be rather polymeric in nature.
This place does not cover:
Polymerization reactions, for example ring-opening metathesis polymerization (ROMP) | |
Refining of hydrocarbon oils by reaction with hydrocarbons, i.e. by alkylation | |
Production of liquid hydrocarbon mixtures from lower carbon number hydrocarbons, e.g. by oligomerization |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Multi-step processes
Multi-step processes are classified as processes according to the specific rules of the subclass C07C, i.e. the last reaction step leading to the end product is always classified. Other reaction steps are also classified if they are not entirely trivial (such as the removal of a protecting group) or if they could be useful for future searches.
Please note that in the past, multi-step processes leading to hydrocarbon compounds had been classified in the corresponding product group for the final end product. These documents are continuously being reclassified. However, for the time being, a complete search should involve searching the product groups.
Indexing Codes for the catalysts used
The Indexing Codes C07C 2521/00 - C07C 2531/38 are used to describe the catalysts used in the preparation processes.
These Indexing Codes are added irrespective of whether or not the document is also to be classified in the field of B01J for the catalysts.
More detailed rules for the use of these catalyst Indexing Codes is further explained in the Special rules of classification under C07C 2521/00.
C07C 2/04: dimerization is also encompassed
C07C 2/30: processes wherein the olefin is added to the catalyst are classified in C07C 2/88 (growth and elimination reactions)
C07C 2/34: metal-hydrocarbon π-complexes only, for example metallocenes or complexes containing 1,5-cyclooctadiene (COD) ligands
C07C 2/62: reactions using protonic acids as the catalyst only
C07C 2/70: reactions using protonic acids as the catalyst only
C07C 2/76: for example reactions of the type
ethane/ethene -> benzene or
methane -> aromatic hydrocarbons such as benzene
Please note: Reactions of the type 2 CH4 + C4H10 -> C2H6 + C3H8
are classified in C07C 6/10.
C07C 2/78: for example reactions of the type CH4 + 3/2 O2 -> HC=CH + 3 H2O
C07C 2/80: for example condensation reactions with the aid of an electric arc / arc discharge
C07C 2/82: condensation reactions of hydrocarbons using an oxidant such as oxygen or ozone
C07C 2/88: the most common catalysts used for growth and elimination reactions are AlR3 and ZnR2
This place covers:
Processes for the preparation of hydrocarbons (i.e. compounds containing only the elements of carbon and hydrogen) from hydrocarbons involving a decrease in the number of carbon atoms in the skeleton.
This place does not cover:
Cracking of hydrocarbon mixtures such as hydrocarbon oils or waxes | C10G ( C10G 9/00, C10G 11/00, C10G 15/00, C10G 47/00, C10G 51/00) |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Multi-step processes
Multi-step processes are classified as processes according to the specific rules of the subclass C07C, i.e. the last reaction step leading to the end product is always classified. Other reaction steps are also classified if they are not entirely trivial (such as the removal of a protecting group) or if they could be useful for future searches.
Please note that in the past, multi-step processes leading to hydrocarbon compounds had been classified in the corresponding product group for the final end product. These documents are continuously being reclassified. However, for the time being, a complete search should involve searching the product groups.
Indexing Codes for the catalysts used
The Indexing Codes C07C 2521/00 - C07C 2531/38 are used to describe the catalysts used in the preparation processes.
These Indexing Codes are added irrespective of whether or not the document is also to be classified in the field of B01J for the catalysts.
More detailed rules for the use of these catalyst Indexing Codes is further explained in the Special rules of classification under C07C 2521/00.
C07C 4/025: The heat needed in the process is provided by "burning" part of the feed
This place covers:
Processes for the preparation of hydrocarbons (i.e. compounds containing only the elements of carbon and hydrogen) from hydrocarbons involving neither a decrease nor an increase of the number of carbon atoms in the skeleton.
This group covers hydrogenation and dehydrogenation reactions as well as isomerizations.
This group only covers processes aiming at the preparation of a single hydrocarbon or a mixture of individually defined hydrocarbons.
If a process is for example directed to the isomerization of a hydrocarbon mixture with the aim of obtaining a mixture (usually gasoline or fuel mixtures) having a higher octane number, said process is classified in C10G exclusively.
This place does not cover:
Hydrogenation of unsaturated compounds with the intention to eliminate them, e.g. the selective hydrogenation of butadiene or acetylene present in a hydrocarbon or mixture of hydrocarbons for purification purposes | |
Refining or hydrotreatment of hydrocarbon mixtures such as oils; reforming of naphtha | C10G, e.g. C10G 35/00, C10G 45/58 |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Multi-step processes
Multi-step processes are classified as processes according to the specific rules of the subclass C07C, i.e. the last reaction step leading to the end product is always classified. Other reaction steps are also classified if they are not entirely trivial (such as the removal of a protecting group) or if they could be useful for future searches.
Please note that in the past, multi-step processes leading to hydrocarbon compounds had been classified in the corresponding product group for the final end product. These documents are continuously being reclassified. However, for the time being, a complete search should involve searching the product groups.
Indexing Codes for the catalysts used
The Indexing Codes C07C 2521/00 - C07C 2531/38 are used to describe the catalysts used in the preparation processes.
These Indexing Codes are added irrespective of whether or not the document is also to be classified in the field of B01J for the catalysts.
More detailed rules for the use of these catalyst Indexing Codes is further explained in the Special rules of classification under C07C 2521/00.
Hydrogenation:
C07C 5/03: for example styrene -> ethylbenzene
C07C 5/10: for example styrene -> ethylcyclohexane
C07C 5/11: covers all hydrogenation reactions in which at least one of the aromatic bonds of a compound is not hydrogenated
Examples:
benzene -> cyclohexene;
biphenyl -> cyclohexylbenzene
C07C 5/22: This subgroup covers hydrogenation reactions wherein the carbon skeleton is modified during the reaction.
Examples:
cyclohexane -> n-hexane
n-butene -> isobutane
Isomerization:
If it is not entirely clear what happens during an isomerisation reaction, the process is classified in C07C 5/2206 and subgroups thereof.
Cis-trans-isomerization reactions are classified in C07C 5/22 or C07C 5/2206 and subgroups thereof, depending on the catalyst used.
Isomerization reactions of saturated compounds (e.g. linear alkanes) are classified in C07C 5/2702 or subgroups thereof since they involve both changing the branching point of an open chain and changing the number of side-chains.
Isomerization reactions involving the conversion of a mixture of C8 alkylaromatic compounds to another mixture (usually for the preparation of xylenes) are classified in C07C 5/2702 or subgroups thereof since they involve both changing the point of substitution on a ring and changing the number of side-chains.
Isomerization reactions with simultaneous hydrogenation are classified in C07C 5/13.
Isomerization reactions with simultaneous dehydrogenation are classified in C07C 5/373 and subgroups thereof.
C07C 5/2702: This class (and subgroups thereof, depending on the catalyst used) covers
- catalytic isomerization processes involving the rearrangement of carbon atoms in the hydrocarbon skeleton not covered by C07C 5/2732 - C07C 5/31
- catalytic isomerization processes involving the rearrangement of carbon atoms in the hydrocarbon skeleton covered by both C07C 5/2732 or subgroups thereof and C07C 5/277 or subgroups thereof
C07C 5/2718: for example with BF3-ether complex
C07C 5/29: for example the isomerization of a ring system containing 3 condensed rings (trimethylenenorbornane) to adamantane
Dehydrogenation:
A distinction is made between dehydrogenation reactions with formation of free hydrogen (C07C 5/32 and subgroups thereof) and dehydrogenation reactions with a hydrogen acceptor (C07C 5/42 and subgroups thereof; often called oxidative dehydrogenation).
C07C 5/327, C07C 5/3337: for example preparation of alkenes, or styrene from ethylbenzene
C07C 5/56: Both oxygen and the halogen or halogen compound should react with the hydrogen. This is difficult to find out. In most of the documents classified in this subgroup, the role of the halogen or halogen compound is not clear.
This place covers:
Processes for the preparation of hydrocarbons from educts at least one of which is a hydrocarbon, wherein both an increase and a decrease in the number of carbon atoms occurs.
This group only covers processes aiming at the preparation of a single hydrocarbon or a mixture of individually defined hydrocarbons.
If a process is for example directed to the preparation of a hydrocarbon mixture with the aim of obtaining a mixture rather than individual hydrocarbon compounds (e.g. for use as gasoline, kerosine or fuel), said process is classified in C10G exclusively.
This place does not cover:
Cracking of hydrocarbons; Refining or hydrotreatment of hydrocarbon mixtures such as oils; | C10G, e.g. C10G 9/00, C10G 11/00, C10G 15/00, C10G 47/00 |
Reforming of naphtha |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Multi-step processes
Multi-step processes are classified as processes according to the specific rules of the subclass C07C, i.e. the last reaction step leading to the end product is always classified. Other reaction steps are also classified if they are not entirely trivial (such as the removal of a protecting group) or if they could be useful for future searches.
Please note that in the past, multi-step processes leading to hydrocarbon compounds had been classified in the corresponding product group for the final end product. These documents are continuously being reclassified. However, for the time being, a complete search should involve searching the product groups.
Indexing Codes for the catalysts used
The Indexing Codes C07C 2521/00 - C07C 2531/38 are used to describe the catalysts used in the preparation processes.
These Indexing Codes are added irrespective of whether or not the document is also to be classified in the field of B01J for the catalysts.
More detailed rules for the use of these catalyst Indexing Codes is further explained in the Special rules of classification under C07C 2521/00.
C07C 6/04: Most documents in this class relate to the preparation of olefins either by self-metathesis of only one alkene, or cross-metathesis of two or more alkenes.
The metathesis reaction between an olefin (i.e. a hydrocarbon) and a non-hydrocarbon having an olefinic double-bond is also classified in this subgroup. Example: Ethenolysis of unsaturated fatty acids/esters.
C07C 6/10: This reaction is also called disproportionation or metathesis of alkanes. Examples:
methane + butane -> ethane + propane
isobutane + isobutane -> 2,3-dimethylbutane + ethane
C07C 6/123: for example disproportionation of toluene to benzene and xylene; also called dismutation
C07C 6/126: for example preparation of xylenes by transalkylation between benzene and C9 aromatics
In patent documents, the following words/expressions are often used as synonyms:
- "metathesis", "disproportionation" and "transalkylation"
This place covers:
Processes for the purification, separation and stabilization of compounds containing the elements carbon and hydrogen exclusively.
This group only covers processes aiming at the purification, separation or stabilization of a single hydrocarbon or a mixture of individually defined hydrocarbons.
If a process is for example directed to the working-up of a hydrocarbon mixture with the aim of obtaining a mixture rather than individual hydrocarbon compounds, said process is classified in C10G.
This place does not cover:
Working-up (separation, purification) of hydrocarbon mixtures obtained by cracking of mixtures of undefined composition (e.g. petroleum, naphtha) | C10G, e.g. C10G 70/00 |
Working-up natural gas or synthetic natural gas |
Attention is drawn to the following places, which may be of interest for search:
General methods for purification/separation | |
Reactors | |
Catalysts |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 9/00 - C07C 15/00.
Please note: No catalyst Indexing Codes C07C 2521/00 - C07C 2531/38 are used in combination with processes for the purification, separation or stabilization classified in C07C 7/00 and subgroups.
Multi-step purification/separation processes
Multi-step processes are classified in C07C 7/005. In addition, the individual steps can be classified separately if of interest.
C07C 7/13: for example by selective adsorption on zeolites
C07C 7/148: in the Note to this subgroup, the expression "contact masses" means catalyst supports etc.
C07C 7/14833: This subgroup does not cover processes involving metals as catalysts, but reacting metals only.
C07C 7/163, C07C 7/167: processes for the purification of hydrocarbons by selective hydrogenation of compounds containing double or triple bonds are sometimes formulated as hydrogenation reactions. In order to decide whether the reaction is a hydrogenation reaction to be classified under C07C 5/02 and subgroups thereof or a purification by hydrogenation, it should be checked whether the aim of the process is the elimination of the compound to be hydrogenated, or the hydrogenation product is the desired compound.
Example:
Combination set C07C 7/167, C07C 11/06 purification of propene by selective hydrogenation of acetylenic impurities (the aim is the removal of compounds containing a triple bond)
C07C 7/20: covers also the addition of polymerization inhibitors
This place covers:
Non-cyclic saturated compounds containing the elements of carbon and hydrogen exclusively.
Hydrocarbon mixtures where the mixture rather than the individual hydrocarbons is the desired product, or hydrocarbon mixtures of undefined composition wherein the components cannot be singly identified, are classified in C10G.
This place does not cover:
Preparation of methane by biological treatment of sludge/sewage | |
Natural gas or synthetic natural gas | |
Liquefied petroleum gas (LPG) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 1/00 - C07C 7/00.
C07C 9/21: 2,2,4-Trimethylpentane, also called isooctane, is an important component of gasoline and is used as an anti-knock agent. It is usually prepared by dimerization of isobutylene followed by hydrogenation of the obtained mixture of iso-octenes.
In patent documents, the following abbreviations are often used:
LPG | Liquefied Petroleum Gas |
LNG | Liquefied Natural Gas |
NGL | Natural Gas Liquids |
This place covers:
Non-cyclic unsaturated compounds containing the elements of carbon and hydrogen exclusively.
Hydrocarbon mixtures where the mixture rather than the individual hydrocarbons is the desired product, or hydrocarbon mixtures of undefined composition wherein the components cannot be singly identified, are classified in C10G.
The expression "lower olefins" is considered to be sufficiently specific (it usually means mainly ethylene and propylene) and is classified at least additionally in C07C.
This place does not cover:
Preparation of acetylene gas by wet methods (e.g. acetylene from CaC2) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 1/00 - C07C 7/00.
C07C 11/30: butenyne is also called vinylacetylene
This place covers:
Hydrocarbons (i.e. compounds containing the elements carbon and hydrogen exclusively) containing at least one non-aromatic ring or ring system.
Hydrocarbon mixtures where the mixture rather than the individual hydrocarbons is the desired product, or hydrocarbon mixtures of undefined composition wherein the components cannot be singly identified, are classified in C10G.
This place does not cover:
Cyclic hydrocarbons containing only aromatic rings or ring systems | |
Liquid crystalline compounds |
Attention is drawn to the following places, which may be of interest for search:
Organic luminescent materials | |
Organic light-emitting diodes (OLEDs) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 1/00 - C07C 7/00.
Indexing Codes for the ring system(s) C07C 2601/00 - C07C 2604/00 are given.
Compounds containing an aromatic ring or ring system in addition to a non-aromatic ring or ring system are classified in C07C 13/28 and subgroups thereof since such compounds necessarily contain two or more rings or ring systems, i.e. they are polycyclic.
However, it is the nature of the non-aromatic ring or ring system exclusively which determines the classification in these subgroups.
C07C 13/28: The wording "or acyclic derivatives thereof" in the title of this subgroup is also used in the title of the corresponding IPC group, but is meaningless and should be disregarded.
Compounds containing two or more rings, at least one of which is a non-condensed non-aromatic ring, are classified in this group C07C 13/28.
Examples: cyclohexyl-CH2CH2-cyclohexyl or cyclohexyl-CH2-phenyl
All compounds containing at least one condensed ring wherein at least one of the condensed rings in the ring system is not a 6-membered aromatic ring are classified in C07C 13/32-C07C 13/72.
This place covers:
Cyclic hydrocarbons (i.e. cyclic compounds containing the elements carbon and hydrogen exclusively) containing only aromatic rings or ring systems. The compounds can contain one or more rings which can be condensed or non-condensed.
Hydrocarbon mixtures where the mixture rather than the individual hydrocarbons is the desired product, or hydrocarbon mixtures of undefined composition wherein the components cannot be singly identified, are classified in C10G.
The expression "aromatics", for example, is usually not considered as describing a mixture of individually defined or identifiable components.
This place does not cover:
Cyclic hydrocarbons containing one or more non-aromatic rings or ring systems | |
Liquid crystalline compounds |
Attention is drawn to the following places, which may be of interest for search:
Organic luminescent materials | |
Organic light-emitting diodes (OLEDs) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 1/00 - C07C 7/00.
Indexing Codes for the ring system(s) C07C 2603/00 - C07C 2603/92 are given for aromatic rings or ring systems other than benzene, naphthalene and biphenyl.
C07C 15/20: Polycyclic aromatic condensed hydrocarbons with at least one ring system containing 5 or more rings condensed together are classified in this group (compounds containing 2, 3 or 4 condensed rings are classified in C07C 15/24-C07C 15/38).
Exception: polycyclic aromatic condensed hydrocarbons substituted by one or more unsaturated hydrocarbon radicals are classified in C07C 15/56 and subgroups thereof.
C07C 15/40: In this subgroup, "unsaturated carbon radicals" are hydrocarbon chains containing one or more double and/or triple bonds. Unsaturated non-aromatic cyclic moieties would be classified in C07C 13/00 and subgroups thereof. Aromatic moieties, even though normally considered unsaturated hydrocarbon groups, are not considered to be unsaturated carbon radicals in this context.
C07C 15/58: containing two rings in the condensed ring system
C07C 15/60: containing three rings in the condensed ring system
C07C 15/62: containing four rings in the condensed ring system
This place covers:
Processes for the preparation of compounds containing the elements of carbon, hydrogen and halogen exclusively.
General methods for the halogenation of organic compounds are additionally classified in C07B 39/00.
If a process relates to the separation of halogenated compounds from hydrogen halides such as HF, classification in C01B 7/00 and subgroups thereof should also be considered (e.g. C01B 7/196 for the separation/purification of HF by distillation).
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process. A combination set consists of a process group, followed by and linked to the group of the product. The products are selected from the corresponding product groups C07C 19/00 - C07C 25/00.
Example:
Combination set C07C 17/25, C07C 21/06 preparation of vinyl chloride by splitting off HCl from 1,2-dichloroethane
C07C 17/093: Examples for reactions classified in C07C 17/093:
R-COOH -> RCF3;
F2C=S -> CF4
C07C 17/18: replacement of oxygen atoms of carbonyl groups of aldehydes or ketones (the replacement of oxygen atoms in carboxy groups is classified in C07C 17/093)
C07C 17/156: of compounds having double and/or triple bonds including aromatic compounds.
Oxychlorination reactions of the type
CH2=CH2 + Cl2 -> ClCH2-CH2-Cl (in the presence of oxygen)
are also encompassed even though an addition rather than a replacement by halogen takes place.
C07C 17/21: Example: CCl2=CHCl + HF -> CF3-CH2Cl
C07C 17/263: A condensation reaction is a chemical reaction in which two molecules having functional groups combine to form one single molecule, together with the loss of a small molecule.
Examples: Condensation reactions with organozinc compounds and coupling reactions with boronic acid derivatives are classified in this class.
Please note: Reactions involving the introduction of halogenated alkyl groups into ring compounds are classified in C07C 17/32.
C07C 17/272: An addition reaction is a chemical reaction in which two molecules combine to form one single molecule. In contrast to a condensation reaction, the addition reaction does not lead to the loss of a small molecule.
Example: Addition or insertion of carbenes
C07C 17/32: for example C6H6 + CCl4 (+HF) -> C6H5-CF3
C07C 17/35: for example dehydration reactions or reactions involving the elimination of functional groups (-OH -> H; -NH2 -> H; SO3H -> H; RSO2Cl -> RCl) are classified in this class.
C07C 17/361: for example CH2F-OCH2F -> CH2F2
C07C 17/363: for example R-O(C=O)-Cl -> RCl; R-O(C=O)-Cl -> RF
C07C 17/37: i.e. the transhalogenation with only one reactant; this reaction is also called dismutation
C07C 17/383: encompasses also vaporisation
C07C 17/386: encompasses also reactive distillation
In patent documents, the following abbreviations are often used:
CFCs | Chlorofluorocarbons |
HCFCs | Hydrochlorofluorocarbons |
HFCs | Hydrofluorocarbons |
A list of abbreviations for specific halogenated hydrocarbons can be found under the corresponding product subclasses.
This place covers:
Non-cyclic saturated compounds containing the elements of carbon, hydrogen and halogen exclusively
Compositions defined as containing at least two components are normally not classified in C07C, but in the corresponding use fields only.
Example: Azeotrope or azeotrope-like compositions containing a halogenated hydrocarbon and one or more further compounds (for example a second halogenated hydrocarbon or HF) used as refrigerants are classified in C09K 5/00 and subgroups thereof.
Exception: If the only use of an azeotrope composition is the separation/purification of halogenated hydrocarbons, said azeotrope is classified in C07C.
This place does not cover:
Blowing agents (for polymers) containing organic halogen compounds | |
Materials for aerosols, propellants | |
Heat transfer agents such as refrigerants comprising halogenated organic compounds | |
Organic fireproofing materials containing halogen | |
Lubricating compositions | |
Cleaning or de-greasing metallic material using organic solvents containing halogenated hydrocarbons |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 17/00.
C07C 19/03: Chloromethanes containing 1 or 2 Cl atoms, i.e. CH3Cl or CH2Cl2
In patent documents, the following abbreviations are often used:
CFCs | ChloroFluorCarbons |
HCFCs | HydroChloroFluoroCarbons |
HFCs | HydroFluoroCarbons |
for saturated halogenated hydrocarbons:
Abbreviation | Structure; Name |
HFC-32 | CF2H2; difluoromethane |
CFC-11 (or Freon-11, R-11) | CCl3F; trichlorofluoromethane |
CFC-12 (or Freon-12, R-12) | CCl2F2; dichlorodifluoromethane |
CFC-13 (or Freon-13, R-13) | CClF3; chlorotrifluoromethane |
HCFC-22 (or R-22) | CHClF2; chlorodifluoromethane |
HCFC-21 (or R-21) | CHCl2F; dichlorofluoromethane |
HCFC-31 (or Freon 31, R-31) | CH2ClF; chlorofluoromethane |
H-1211 (or BCF, Halon 1211, Freon 12B1) | CBrClF2; bromochlorodifluoro-methane |
HFC-152a | CHF2CH3; 1,1-difluoroethane |
HFC-143a | CF3CH3; 1,1,1-trifluoroethane |
HFC-134 | CHF2CHF2; 1,1,2,2-tetrafluoroethane |
HFC-134a (or R-134a) | CF3CH2F; 1,1,1,2-tetrafluoroethane |
HFC-125 | CF3CHF2; 1,1,1,2,2-pentafluoroethane |
CFC-111 | CCl3CCl2F; pentachlorofluoroethane |
CFC-112 (or Freon 112, R-112) | CCl2FCCl2F; tetrachloro-1,2-difluoroethane |
CFC-112a (or Freon 112a, R-112a) | CClF2CCl3; tetrachloro-1,1-difluoroethane |
CFC-113 (or Freon 113, R-113) | Cl2FC-CClF2; 1,1,2-trichloro-1,2,2-trifluoroethane |
CFC-113a (or Freon 113a, R-113a) | Cl3C-CF3; 1,1,1-trichloro-2,2,2-trifluoroethane |
CFC-114 (or Freon 114, R-114) | ClF2C-CClF2; 1,2-dichloro-1,1,2,2-tetrafluoroethane |
CFC-114a | CF3CCl2F; 2,2-dichloro-1,1,1,2-tetrafluoroethane |
CFC-115 (or Freon 115, R-115) | ClF2C-CF3; 1-chloro-1,1,2,2,2-pentafluoroethane |
HCFC-121 (or Freon 121, R-121) | CCl2FCHCl2; 1,1,2,2-tetrachloro-1-fluoroethane |
HCFC-122 (or Freon 122, R-122) | CClF2CHCl2; 1,2,2-trichloro-1,1-difluoroethane |
HCFC-123 (or Freon 123, R-123) | CF3CHCl2; 2,2-dichloro-1,1,1-trifluoroethane |
HCFC-123a | CClF2CHClF; 1,2-dichloro-1,1,2-trifluoroethane |
HCFC-124 (or Freon 124, R-124) | CHFClCF3; 2-chloro-1,1,1,2-tetrafluoroethane |
HCFC-124a | CClF2CHF2; 1-chloro-1,1,2,2-tetrafluoroethane |
HCFC-131 | CHCl2CHClF; 1,1,2-trichloro-2-fluoroethane |
HCFC-133 | CClF2CH2F; 1-chloro-1,1,2-trifluoroethane |
HCFC-133a | CF3CH2Cl; 1,1,1-trifluoro-2-chloroethane |
HCFC-141b (or Freon 141b, R-141b) | Cl2FC-CH3; 1,1-dichloro-1-fluoroethane |
HCFC-142b (or Freon 142b, R-142b) | ClF2C-CH3; 1-chloro-1,1-difluoroethane |
HCFC-151 | CH2ClCH2F; 1-chloro-2-fluoroethane |
Halon 2311a | CHClFCBrF2; 1-bromo-2-chloro-1,1,2-trifluoroethane |
Halon 2311 | CF3CHBrCl; 2-bromo-2-chloro-1,1,1-trifluoroethane |
HCC-240fa | CCl3CH2CHCl2; 1,1,1,3,3-pentachloropropane |
HFC-245cb | CF3CF2CH3; 1,1,1,2,2-pentafluoropropane |
HFC-245eb | CF3CHFCH2F; 1,1,1,2,3-pentafluoropropane |
HFC-245fa | CF3CH2CHF2; 1,1,1,3,3-pentafluoropropane |
HFC-236ea | CF3CHFCHF2; 1,1,1,2,3,3-hexafluoropropane |
HFC-236fa | CF3CH2CF3; 1,1,1,3,3,3-hexafluoropropane |
HCFC-225ca (or R-225ca) | CF3CF2CHCl2; 1,1-dichloro-2,2,3,3,3-pentafluoropropane |
HCFC-225cb (or R-225cb) | CClF2CF2CHClF; 1,3-dichloro-1,2,2,3,3-pentafluoropropane |
HCFC-243 | CHCl2CH2CF3; 1,1-dichloro-3,3,3-trifluoropropane |
HCFC-244bb | CF3CClFCH3; 2-chloro-1,1,1,2-tetrafluoropropane |
HCFC-235cb | CF3CF2CH2Cl; 3-chloro-1,1,1,2,2-pentafluoropropane |
HCFC-235fa | CF3CHFCHClF; 1-chloro-1,2,3,3,3-pentafluoropropane |
HCFC-261 | CH2ClCClFCH3; 1,2-dichloro-2-fluoropropane |
HCFC-271 | CHClFCH2CH3; 1-chloro-1-fluoropropane |
HFC-365mfc | CF3CH2CF2CH3; 1,1,1,3,3-pentafluorobutane |
This place covers:
Non-cyclic unsaturated compounds containing the elements of carbon, hydrogen and halogen exclusively.
Compositions defined as containing at least two components are normally not classified in C07C, but in the corresponding use fields only.
Example: Azeotrope or azeotrope-like compositions containing a halogenated hydrocarbon and one or more further compounds (for example a second halogenated hydrocarbon or HF) used as refrigerants are classified in C09K 5/00 and subgroups thereof.
Exception: If the only use of an azeotrope composition is the separation/purification of halogenated hydrocarbons, said azeotrope is classified in C07C.
This place does not cover:
Blowing agents (for polymers) containing organic halogen compounds | |
Materials for aerosols, propellants | |
Heat transfer agents such as refrigerants comprising halogenated organic compounds | |
Organic fireproofing materials containing halogen | |
Lubricating compositions | |
Cleaning or de-greasing metallic material using organic solvents containing halogenated hydrocarbons |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 17/00.
C07C 21/08: vinylidene chloride is also called dichloroethylene
In patent documents, the following abbreviations are often used:
CFCs | chlorofluorocarbons |
HCFCs | hydrochlorofluorocarbons |
HFCs | hydrofluorocarbons |
F1123 | CF2=CHF; trifluoroethylene |
HFC-1114 (TFE) | CF2=CF2; tetrafluoroethylene |
CFC-1113 | CF2=CClF; chlorotrifluoroethylene |
HFC-1216 (HFP) | CF3CF=CF2; hexafluoropropene |
HFC-1225ye | CF3CF=CHF; 1,2,3,3,3-pentafluoro-1-propene |
HFC-1225zc | CF3CH=CF2; 1,1,3,3,3-pentafluoro-1-propene |
HFC-1225yc | CHF2CF=CF2; 1,1,2,3,3-pentafluoro-1-propene |
HFC-1234ye | CHF2CF=CHF; 1,2,3,3-tetrafluoro-1-propene |
HFC-1234yf | CF3CF=CH2; 2,3,3,3-tetrafluoro-1-propene |
HFC-1234ze | CF3CH=CHF; 1,3,3,3-tetrafluoro-1-propene |
HFC-1234yc | CH2FCF=CF2; 1,1,2,3-tetrafluoro-1-propene |
HFC-1234zc | CHF2CH=CF2; 1,1,3,3-tetrafluoro-1-propene |
HFC-1243yf | CHF2CF=CH2; 2,3,3-trifluoro-1-propene |
HFC-1243zf | CF3CH=CH2; 3,3,3-trifluoro-1-propene |
HFC-1243yc | CH3CF=CF2; 1,1,2-trifluoro-1-propene |
HFC-1243zc | CH2FCH=CF2; 1,1,3-trifluoro-1-propene |
HFC-1243ye | CH2FCF=CHF; 1,2,3-trifluoro-1-propene |
HFC-1243ze | CHF2CH=CHF; 1,3,3-trifluoro-1-propene |
HCFC-1233xf | CF3CCl=CH2; 2-chloro-3,3,3-trifluoro-1-propene |
HCFC-1233zd | CF3CH=CHCl; 1-chloro-3,3,3-trifluoro-1-propene |
FC-1318my | CF3CF=CFCF3; 1,1,1,2,3,4,4,4-octafluoro-2-butene |
FC-1318cy | CF3CF2CF=CF2; 1,1,2,3,3,4,4,4-octafluoro-1-butene |
HFC-1327my | CF3CF=CHCF3; 1,1,1,2,4,4,4-heptafluoro-2-butene |
HFC-1327ye | CHF=CFCF2CF3; 1,2,3,3,4,4,4-heptafluoro-1-butene |
HFC-1327py | CHF2CF=CFCF3; 1,1,1,2,3,4,4-heptafluoro-2-butene |
HFC-1327et | (CF3)2C=CHF; 1,3,3,3-tetrafluoro-2-(trifluoromethyl)-1-propene |
HFC-1327cz | CF2CH=CF2CF3; 1,1,3,3,4,4,4-heptafluoro-1-butene |
HFC-1327cye | CF2=CFCHFCF3; 1,1,2,3,4,4,4-heptafluoro-1-butene |
HFC-1327cyc | CF2=CFCF2CHF2; 1,1,2,3,3,4,4-heptafluoro-1-butene |
HFC-1336yf | CF3CF2CF=CH2; 2,3,3,4,4,4-hexafluoro-1-butene |
HFC-1336ze | CHF=CHCF2CF3; 1,3,3,4,4,4-hexafluoro-1-butene |
HFC-1336eye | CHF=CFCHFCF3; 1,2,3,4,4,4-hexafluoro-1-butene |
HFC-1336eyc | CHF=CFCF2CHF2; 1,2,3,3,4,4-hexafluoro-1-butene |
HFC-1336pyy | CHF2CF=CFCHF2; 1,1,2,3,4,4-hexafluoro-2-butene |
HFC-1336qy | CH2FCF=CFCF3; 1,1,1,2,3,4-hexafluoro-2-butene |
HFC-1336pz | CHF2CH=CFCF3; 1,1,1,2,4,4-hexafluoro-2-butene |
HFC-1336mzy | CF3CH=CFCHF2; 1,1,1,3,4,4-hexafluoro-2-butene |
HFC-1336qc | CF2=CFCF2CH2F; 1,1,2,3,3,4- hexafluoro-1-butene |
HFC-1336pe | CF2=CFCHFCHF2; 1,1,2,3,4,4- hexafluoro-1-butene |
HFC-1336ft | CH2=C(CF3)2; 3,3,3-trifluoro-2-(trifluoro-methyl)-1-propene |
HFC-1345qz | CH2FCH=CFCF3; 1,1,1,2,4-pentafluoro-2-butene |
HFC-1345mzy | CF3CH=CFCH2F; 1,1,1,3,4-pentafluoro-2-butene |
HFC-1345fz | CF3CF2CH=CH2; 3,3,4,4,4-pentafluoro-1-butene |
HFC-1345mzz | CHF2CH=CHCF3; 1,1,1,4,4-pentafluoro-2-butene |
HFC-1345sy | CH3CF=CFCF3; 1,1,1,2,3-pentafluoro-2-butene |
HFC-1345fyc | CH2=CFCF2CHF2; 2,3,3,4,4-pentafluoro-1-butene |
HFC-1345pyz | CHF2CF=CHCHF2; 1,1,2,4,4-pentafluoro-2-butene |
HFC-1345cyc | CH3CF2CF=CF2; 1,1,2,3,3-pentafluoro-1-butene |
HFC-1345pyy | CH2FCF=CFCHF2; 1,1,2,3,4-pentafluoro-2-butene |
HFC-1345eyc | CH2FCF2CF=CF2; 1,2,3,3,4-pentafluoro-1-butene |
HFC-1345ctm | CF2=C(CF3)(CH3); 1,1,3,3,3-pentafluoro-2-methyl-1-propene |
HFC-1345ftp | CH2=C(CHF2)(CF3); 2-(difluoromethyl)-3,3,3-trifluoro-1-propene |
HFC-1345fye | CH2=CFCHFCF3; 2,3,4,4,4-pentafluoro-1-butene |
HFC-1345eyf | CHF=CFCH2CF3; 1,2,4,4,4-pentafluoro-1-butene |
HFC-1345eze | CHF=CHCHFCF3; 1,3,4,4,4-pentafluoro-1-butene |
HFC-1345ezc | CHF=CHCF2CHF2; 1,3,3,4,4-pentafluoro-1-butene |
HFC-1345eye | CHF=CFCHFCHF2; 1,2,3,4,4-pentafluoro-1-butene |
HFC-1354fzc | CH2=CHCF2CHF2; 3,3,4,4-tetrafluoro-1-butene |
HFC-1354ctp | CF2=C(CHF2)(CH3); 1,1,3,3-tetrafluoro-2-methyl-1-propene |
HFC-1354etm | CHF=C(CF3)(CH3); 1,3,3,3-tetrafluoro-2-methyl-1-propene |
HFC-1354tfp | CH2=C(CHF2)2; 2-(difluoromethyl)-3,3-difluoro-1-propene |
HFC-1354my | CF3CF=CHCH3; 1,1,1,2-tetrafluoro-2-butene |
HFC-1354mzy | CH3CF=CHCF3; 1,1,1,3-tetrafluoro-2-butene |
FC-141-10myy | CF3CF=CFCF2CF3; 1,1,1,2,3,4,4,5,5,5-decafluoro-2-pentene |
FC-141-10cy | CF2=CFCF2CF2CF3; 1,1,2,3,3,4,4,5,5,5-decafluoro-1-pentene |
HFC-1429mzt | (CF3)2C=CHCF3; 1,1,1,4,4,4-hexafluoro-2-(trifluoromethyl-2-butene |
HFC-1429myz | CF3CF=CHCF2CF3; 1,1,1,2,4,4,5,5,5-nonafluoro-2-pentene |
HFC-1429mzy | CF3CH=CFCF2CF3; 1,1,1,3,4,4,5,5,5-nonafluoro-2-pentene |
HFC-1429eyc | CHF=CFCF2CF2CF3; 1,2,3,3,4,4,5,5,5-nonafluoro-1-pentene |
HFC-1429czc | CF2=CHCF2CF2CF3; 1,1,3,3,4,4,5,5,5-nonafluoro-1-pentene |
HFC-1429cycc | CF2=CFCF2CF2CHF2; 1,1,2,3,3,4,4,5,5-nonafluoro-1-pentene |
HFC-1429pyy | CHF2CF=CFCF2CF3; 1,1,2,3,4,4,5,5,5-nonafluoro-2-pentene |
HFC-1429myyc | CF3CF=CFCF2CHF2; 1,1,1,2,3,4,4,5,5-nonafluoro-2-pentene |
HFC-1429myye | CF3CF=CFCHFCF3; 1,1,1,2,3,4,5,5,5-nonafluoro-2-pentene |
HFC-1429eyym | CHF=CFCF(CF3)2; 1,2,3,4,4,4-hexafluoro-3-(trifluoromethyl)-1-butene |
HFC-1429cyzm | CF2=CFCH(CF3)2; 1,1,2,4,4,4-hexafluoro-3-(trifluoromethyl)-1-butene |
HFC-1429mzt | CF3CH=C(CF3)2; 1,1,1,4,4,4-hexafluoro-2-(trifluoromethyl)-2-butene |
HFC-1429czym | CF2=CHCF(CF3)2; 1,1,2,3,3,3-hexafluoro-3-(trifluoromethyl)-1-butene |
HFC-1438fy | CH2=CFCF2CF2CF3; 2,3,3,4,4,5,5,5-octafluoro-1-pentene |
HFC-1438eycc | CHF=CFCF2CF2CHF2; 1,2,3,3,4,4,5,5-octafluoro-1-pentene |
HFC-1438ftmc | CH2=C(CF3)CF2CF3; 3,3,4,4,4-pentafluoro-2-(trifluoromethyl)-1-butene |
HFC-1438czzm | CF2=CHCH(CF3)2; 1,1,4,4,4-pentafluoro-3-(trifluoromethyl)-1-butene |
HFC-1438ezym | CHF=CHCF(CF3)2; 1,3,4,4,4-pentafluoro-3-(trifluoromethyl)-1-butene |
HFC-1438ctmf | CF2=C(CF3)CH2CF3; 1,1,4,4,4-pentafluoro-2-(trifluoromethyl)-1-butene |
HFC-1447fzy | (CF3)2CFCH=CH2; 3,4,4,4-tetrafluoro-3-(trifluoromethyl)-1-butene |
HFC-1447fz | CF3CF2CF2CH=CH2; 3,3,4,4,5,5,5-heptafluoro-1-pentene |
HFC-1447fycc | CH2=CFCF2CF2CHF2; 2,3,3,4,4,5,5-heptafluoro-1-pentene |
HFC-1447czcf | CF2=CHCF2CH2CF3; 1,1,3,3,5,5,5-heptafluoro-1-pentene |
HFC-1447mytm | CF3CF=C(CF3)(CH3); 1,1,1,2,4,4,4-heptafluoro-3-methyl-2-butene |
HFC-1447fyz | CH2=CFCH(CF3)2; 2,4,4,4-tetrafluoro-3-(trifluoromethyl)-1-butene |
HFC-1447ezz | CHF=CHCH(CF3)2; 1,4,4,4-tetrafluoro-3-(trifluoromethyl)-1-butene |
HFC-1447qzt | CH2FCH=C(CF3)2; 1,4,4,4-tetrafluoro-2-(trifluoromethyl)-2-butene |
HFC-1447syt | CH3CF=C(CF3)2; 2,4,4,4-tetrafluoro-2-(trifluoromethyl)-2-butene |
HFC-1456szt | (CF3)2C=CHCH3; 3-(trifluoromethyl)-4,4,4-trifluoro-2-butene |
HFC-1456szy | CF3CF2CF=CHCH3; 3,4,4,5,5,5-hexafluoro-2-pentene |
HFC-1456mstz | CF3C(CH3)=CHCF3; 1,1,1,4,4,4-hexafluoro-2-methyl-2-butene |
HFC-1456fzce | CH2=CHCF2CHFCF3; 3,3,4,5,5,5-hexafluoro-1-pentene |
HFC-1456ftmf | CH2=C(CF3)CH2CF3; 4,4,4-trifluoro-2-(trifluoromethyl)-1-butene |
FC-151-12c | CF3(CF2)3CF=CF2; 1,1,2,3,3,4,4,5,5,6,6,6-dodecafluoro-1-hexene (or perfluoro-1-hexene) |
FC-151-12mcy | CF3CF2CF=CFCF2CF3; 1,1,1,2,2,3,4,5,5,6,6,6-dodecafluoro-3-hexene (or perfluoro-3-hexene) |
FC-151-12mmtt | (CF3)2C=C(CF3)2; 1,1,1,4,4,4-hexafluoro-2,3-bis(trifluoromethyl)-2-butene |
FC-151-12mmzz | (CF3)2CFCF=CFCF3; 1,1,1,2,3,4,5,5,5-nona-fluoro-4-(trifluoromethyl)-2-pentene |
HFC-152-11mmtz | (CF3)2C=CHC2F5; 1,1,1,4,4,5,5,5-octafluoro-2-(trifluoromethyl)-2-pentene |
HFC-152-11mmyyz | (CF3)2CFCF=CHCF3; 1,1,1,3,4,5,5,5-octafluoro-4-(trifluoromethyl)-2-pentene |
HFC-1549fz (or PFBE) | CF3CF2CF2CF2CH=CH2; 3,3,4,4,5,5,6,6,6-nonafluoro-1-hexene (or perfluorobutylethylene) |
HFC-1549fztmm | CH2=CHC(CF3)3; 4,4,4-trifluoro-3,3-bis(trifluoromethyl)-1-butene |
HFC-1549mmtts | (CF3)2C=C(CF3)(CH3); 1,1,1,4,4,4-hexafluoro-3-methyl-2-(trifluoromethyl)-2-butene |
HFC-1549fycz | CH2=CFCF2CH(CF3)2; 2,3,3,5,5,5-hexafluoro-4-(trifluoromethyl)-1-pentene |
HFC-1549myts | CF3CF=C(CH3)CF2CF3; 1,1,1,2,4,4,5,5,5-nonafluoro-3-methyl-2-pentene |
HFC-1549mzzz | CF3CH=CHCH(CF3)2; 1,1,1,5,5,5-hexafluoro-4-(trifluoromethyl)-2-pentene |
HFC-1558szy | CF3CF2CF2CF=CHCH3; 3,4,4,5,5,6,6,6-octafluoro-2-hexene |
HFC-1558fzccc | CH2=CHCF2CF2CF2CHF2; 3,3,4,4,5,5,6,6-octafluoro-2-hexene |
HFC-1558mmtzc | (CF3)2C=CHCF2CH3; 1,1,1,4,4-pentafluoro-2-(trifluoromethyl)-2-pentene |
HFC-1558ftmf | CH2=C(CF3)CH2C2F5; 4,4,5,5,5-pentafluoro-2-(trifluoromethyl)-1-pentene |
HFC-1567fts | CF3CF2CF2C(CH3)=CH2; 3,3,4,4,5,5,5-heptafluoro-2-methyl-1-pentene |
HFC-1567szz | CF3CF2CF2CH=CHCH3; 4,4,5,5,6,6,6-heptafluoro-2-hexene |
HFC-1567fzfc | CH2=CHCH2CF2C2F5; 4,4,5,5,6,6,6-heptafluoro-1-hexene |
HFC-1567sfyy | CF3CF2CF=CFC2H5; 1,1,1,2,2,3,4-heptafluoro-3-hexene |
HFC-1567fzfy | CH2=CHCH2CF(CF3)2; 4,5,5,5-tetrafluoro-4-(trifluoromethyl)-1-pentene |
HFC-1567myzzm | CF3CF=CHCH(CF3)(CH3); 1,1,1,2,5,5,5-heptafluoro-4-methyl-2-pentene |
HFC-1567mmtyf | (CF3)2C=CFC2H5; 1,1,1,3-tetrafluoro-2-(tri-fluoromethyl)-2-pentene |
FC-161-14myy | CF3CF=CFCF2CF2C2F5; 1,1,1,2,3,4,4,5,5,6,6,7,7,7-tetradecafluoro-2-heptene |
FC-161-14mcyy | CF3CF2CF=CFCF2C2F5; 1,1,1,2,2,3,4,5,5,6,6,7,7,7-tetradecafluoro-2-heptene |
HFC-162-13mzy | CF3CH=CFCF2CF2C2F5; 1,1,1,3,4,4,5,5,6,6,7,7,7-tridecafluoro-2-heptene |
HFC-162-13myz | CF3CF=CHCF2CF2C2F5; 1,1,1,2,4,4,5,5,6,6,7,7,7-tridecafluoro-2-heptene |
HFC-162-13mczy | CF3CF2CH=CFCF2C2F5; 1,1,1,2,2,4,5,5,6,6,7,7,7-tridecafluoro-3-heptene |
HFC-162-13mcyz | CF3CF2CF=CHCF2C2F5; 1,1,1,2,2,3,5,5,6,6,7,7,7-tridecafluoro-3-heptene |
This place covers:
Compounds having at least one unsubstituted aromatic or non-aromatic ring and containing the elements of carbon, hydrogen and halogen exclusively.
Compositions defined as containing at least two components are not classified in C07C, but in the corresponding use fields only.
This place does not cover:
Liquid crystalline compounds |
Attention is drawn to the following places, which may be of interest for search:
Organic luminescent materials | |
Organic light-emitting diodes (OLEDs) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 17/00.
Indexing Codes C07C 2601/00 - C07C 2604/00 are given for rings or ring systems other than benzene, naphthalene and biphenyl.
This place covers:
Cyclic compounds having at least one halogen-substituted non-aromatic ring or ring system and containing the elements of carbon, hydrogen and halogen exclusively. The compounds may also have one or more unsubstituted aromatic rings or ring systems in addition to the halogen-substituted non-aromatic ring(s).
Compositions defined as containing at least two components are normally not classified in C07C, but in the corresponding use fields only.
This place does not cover:
Liquid crystalline compounds |
Attention is drawn to the following places, which may be of interest for search:
Organic luminescent materials | |
Organic light-emitting diodes (OLEDs) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 17/00.
Indexing Codes C07C 2601/00 - C07C 2604/00 are given for rings or ring systems other than benzene, naphthalene and biphenyl.
C07C 23/18: Compounds with two or more non-condensed rings at least one of which is a halogen-substituted non-aromatic ring are classified in this class.
In patent documents, the following abbreviations are often used:
FC-C1316cc | cyclo-CF2CF2CF=CF-; 1,2,3,3,4,4-hexafluorocyclobutene |
HFC-C1334cc | cyclo- CF2CF2CH=CH-; 3,3,4,4-tetrafluorocyclobutene |
HFC-C1436 | cyclo- CF2CF2CF2CH=CH-; 3,3,4,4,5,5-hexafluorocyclopentene |
FC-C1418y | cyclo-CF2CF=CFCF2CF2-; 1,2,3,3,4,4,5,5-octafluorocyclopentene |
FC-C151-10y | cyclo- CF2CF=CFCF2CF2CF2-; 1,2,3,3,4,4,5,5,6,6-decafluorocyclohexene |
This place covers:
Aromatic compounds having at least one halogen substituent on the aromatic ring or ring system. The compounds may also have one or more non-aromatic substituted or unsubstituted rings in addition to the substituted aromatic ring(s).
Compositions defined as containing at least two components are normally not classified in C07C, but in the corresponding use fields only.
This place does not cover:
Liquid crystalline compounds |
Attention is drawn to the following places, which may be of interest for search:
Organic luminescent materials | |
Organic light-emitting diodes (OLEDs) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 17/00.
Indexing Codes C07C 2601/00 - C07C 2604/00 are given for rings or ring systems other than benzene, naphthalene and biphenyl.
C07C 25/06, C07C 25/08, C07C 25/10: Only compounds containing no further substituents on the benzene ring are classified in these groups.
Mono-, di- or trichlorobenzenes with further substituents on the benzene ring (for example Br or alkyl) are classified in C07C 25/02.
C07C 25/24: The unsaturated side-chain can also be cyclic (even though the expression "side-chain" appears to suggest non-cyclic moieties only); however, aromatic rings are not considered unsaturated side-chains.
This place covers:
The preparation of product mixtures of two or more classes of oxygen-containing compounds which are structurally not fully identified.
This place does not cover:
Preparation of specific oxygen-containing compounds | C07C 29/00, C07C 37/00, C07C 41/00, C07C 45/00, C07C 46/00, C07C 51/00, C07C 67/00, C07C 68/00, C07C 71/00 |
This group is rarely used. Document are classified in this group only when the products are not disclosed specifically enough to be classified in any of the main groups listed as references above.
Examples where this group is not used:
Co-production of cyclohexanol and cyclohexanone is classified under C07C 29/00 as a production of an alcohol (e.g. combination set C07C 29/50, C07C 35/08) and under C07C 45/00 as a production of a ketone (e.g. combination set C07C 45/33, C07C 49/403).
Reaction of ethylene carbonate and methanol to yield dimethyl carbonate and ethylene glycol is as a production of dimethyl carbonate under C07C 68/00 (e.g. combination set C07C 68/065, C07C 69/96) and - if of interest - as a production of ethylene glycol under C07C 29/00 (combination set C07C 29/128, C07C 31/202).
Hydrolysis of esters of carboxylic acids is classified as production of the resulting carboxylic acid and/or alcohol (depending on which product is of interest)
This place covers:
Preparation and purification/stabilisation of alcohols and alcoholates
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 29/60, C07C 31/205 for the preparation of propylene glycol from glycerol by hydrogenolysis). A combination set consists of a process group (e.g. C07C 29/60), followed by and linked to the group of the product (e.g. C07C 31/205). The products are selected from the corresponding product groups C07C 31/00, C07C 33/00 and C07C 35/00.
Please note: Not all documents in this main group are already classified using combination-sets. The remaining documents (which are continuously being reclassified) have to be searched using only the process group (no indication of the product).
Concerning certain (sub)groups :
C07C 29/00 (the main group itself) contains processes which do not fit in any of the subgroups, e.g. reductive hydrolysis of nitriles, or hydrogenolysis of glycerol to ethylene glycol or of sorbitol to glycerol (propylene glycol from glycerol is classified in C07C 29/60)
C07C 29/03 : addition of hydroxy groups "with the aid of H2O2" refers to hydroboration reactions and the like, which are overall an addition of water to an unsaturated C-C bond and not an oxidation (oxidative dihydroxylation using H2O2 is classified in C07C 29/48)
C07C 29/09 : e.g. hydrolysis of silyl ethers such as TMS or TBS protective groups
C07C 29/10 : e.g. hydrolysis of acyclic ethers (hydrolysis of silyl ethers such as TMS or TBS protective groups is classified in C07C 29/09); also elimination reaction of the type R-O-C-C -> R-OH + C=C are classified here, though this reaction is not a hydrolysis
C07C 29/103 : e.g. hydrolysis of THP protective groups
C07C 29/106 : often EO + H2O → EG;
but also vinylogous hydrolysis ![]()
C07C 29/12 : e.g. saponification of alkyl halides; or hydrolysis of cyclic carbonates to glycols
C07C 29/128 : the coproduction of alkylene glycol in processes for the preparation of dialkyl carbonate from alkylene carbonate, e.g. preparation of dimethyl carbonate and ethylene glycol from ethylene carbonate and methanol, is not systematically classified here (for search see the Combination-set C07C 68/065, C07C 69/96)
C07C 29/132 : e.g. reduction of cyclic ethers (epoxides) ![]()
C07C 29/14, C07C 29/143, C07C 29/147 : e.g. reduction with LiAlH4 or NaBH4
C07C 29/149 : e.g. adipic acid (esters) → 1,6-hexanediol (reduction of unsaturated acid (ester) to saturated alcohol is classified in C07C 29/177)
C07C 29/151 : typically syngas → MeOH (or EtOH)
C07C 29/1518 : i.e. making syngas, then alcohol
C07C 29/152 : characterised by the reactor used for making the alcohol
C07C 29/153 : characterised by the catalyst used for making the alcohol
C07C 29/16 : simultaneous oxo-reaction and reduction in one reactor (two separate step are classified in C07C 45/50 and C07C 29/141)
C07C 29/17 : e.g. 2-butyne-1,4-diol → 2-butene-1,4-diol
C07C 29/172 : e.g. 2-butyne-1,4-diol → 1,4-butanediol
C07C 29/175 : e.g. acrolein → propanol
C07C 29/177 : e.g. oleic acid → octadecanol
C07C 29/19 : e.g. naphthol → decalinol, or terephthalic (esters) → 1,4-cyclohexane dimethanol
C07C 29/20 : e.g. phenol → cyclohexanol
C07C 29/32 : e.g. homologation of methanol to ethanol, MeOH + CO/H2 → EtOH
C07C 29/34 : often 2 EtOH → nBuOH, or 2 nBuOH → 2-ethylhexanol
C07C 29/38 : e.g. TMP directly from butyraldehyde and >3eq CH2O (TMP by hydrogenation of the intermediate aldehyde is classified in C07C 29/141)
C07C 29/40 : e.g. Grignard + carbonyl, then hydrolysis
C07C 29/42 : often acetylene + 2 CH2O → 2-butyne-1,4-diol
C07C 29/48 : e.g. olefin + H2O2 → 1,2-diol
C07C 29/50 : often cyclohexane → cyclohexanol
C07C 29/54 : e.g. oxidation of trialkylaluminium compounds
C07C 29/56 : reactions not changing the molecular formula, i. e. the molecular formula of the educt and the product is the same, e. g.
![]() or
or 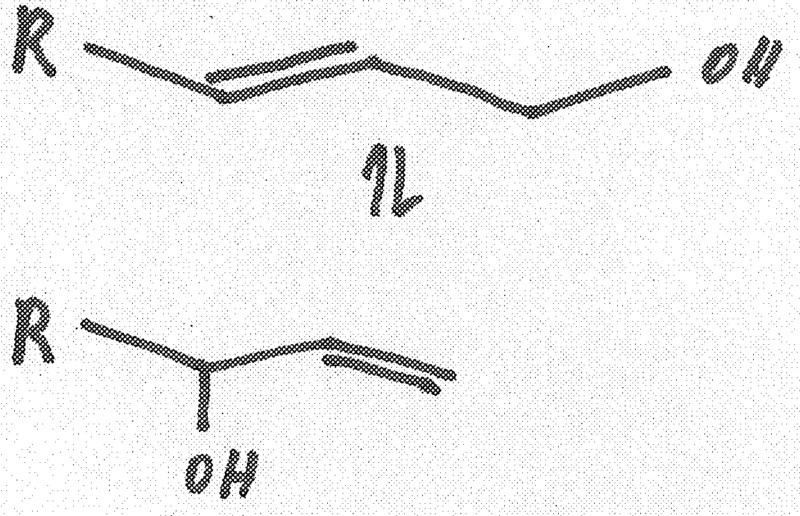
C07C 29/60 : beta elimination (dehydration); but also hydro-de-hydroxylation (hydrogenolysis), e. g. propylene glycol from glycerol (ethylene glycol from glycerol is classified in C07C 29/00)
C07C 29/68 : only used if the aim is the production of an alcoholate (typical Grignard reactions with intermediate alcoholate formation followed by hydrolysis are classified in C07C 29/40).
C07C 29/76 : e.g. use of membranes for separation; chemisorption
C07C 29/92 : e.g. the sequence esterification, purification, hydrolysis
In patent documents, the following abbreviations are often used:
EG | Ethylene Glycol |
EO | Ethylene Oxide |
PG | Propylene Glycol |
PO | Propylene Oxide |
BD | ButaneDiol |
TMP | TriMethylolPropane |
This place covers:
Saturated acyclic alcohols, such as methanol (C07C 31/04), ethanol (C07C 31/08) and stearyl alcohol (C07C 31/125).
Saturated alcohols containing carbocyclic rings having the hydroxy groups bound to acyclic carbon atoms, e.g. 1,4-cyclohexane dimethanol (C07C 31/276).
Metal alcoholates, e.g. sodium methanolate (C07C 31/30).
Halogenated alcohols such as 2,2,2-trifluoro ethanol (C07C 31/38).
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 29/00.
Concerning certain subgroups :
C07C 31/125 : often detergent range alcohols, e.g. C12-C15 alkyl alcohols
C07C 31/13 : this groups covers monohydroxylic alcohols containing saturated rings which do not fit into any of its subgroups C07C 31/133 - C07C 31/135, e.g. polycyclic alcohols containing of two or more isolated (non-condensed) rings
C07C 31/133 : e.g. monocyclic with seven or more-membered rings
C07C 31/135 : e.g. naphthenic alcohols such as ![]()
C07C 31/18 : e.g. pentahydroxylic alcohols
C07C 31/20 : e.g. nepentyl glycol, 1,6-hexanediol
C07C 31/22 : e.g. trimethylolpropane CH3-C(CH2OH)3
C07C 31/245 : pentaerythritol ![]()
C07C 31/26 : e.g. sorbitol
C07C 31/27 : e.g. this groups covers polyhydroxylic alcohols containing saturated rings which do not fit into any of its subgroups C07C 31/272 - C07C 31/278, e.g. polycyclic alcohols containing of two or more isolated (non-condensed) rings
C07C 31/276 : e.g. 1,4-cyclohexane dimethanol ![]() (1,4 -bis(hydroxymethyl) cyclohexane)
(1,4 -bis(hydroxymethyl) cyclohexane)
C07C 31/28 : this group and its subgroups refer to metal alcoholates (titanates and zirconates are classified under C07F 7/00; the rare cases of non-metal alcoholates, e.g. quarternary ammonium alcoholates, are classified as the corresponding alcohol)
C07C 31/34 - C07C 31/44 : due to the last place rule, these groups cover both halogenated alcohols and their alcoholates
C07C 31/34 : e.g. containing fluorine and other halogen, e. g. ClF2C-CH2OH
C07C 31/40 : perhalogenated means that all hydrogen atoms bound to carbon are replaced by halogen, e.g. (CF3)3COH, CCl3OH
In patent documents, the following abbreviations are often used:
EG | Ethylene glycol |
EO | Ethylene oxide |
PG | Propylene glycol |
PO | Propylene oxide |
BD | Butanediol |
TMP | Trimethylol propane |
This place covers:
Unsaturated acyclic alcohols and alcoholates, such as allyl alcohol (C07C 33/03) and 1,4-butynediol (C07C 33/044).
Unsaturated alcohols containing carbocyclic rings (saturated, unsaturated, aromatic) having the hydroxy or O-metal groups bound to acyclic carbon atoms, e.g. benzyl alcohol (C07C 33/22).
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 29/00.
The main group C07C 33/00 has no subgroups for metal alcoholates. Accordingly, alcoholates are classified as the parent alcohol. e. g. NaO-CH2-CH=CH2 is classified as allyl alcohol (C07C 33/03).
Concerning certain subgroups:
C07C 33/02 : mono- and polyhydroxylic alcohols with more than one double bond; also used when the number of double bonds is not clear, e.g. for undefined unsaturated fatty alcohols
C07C 33/04 : mono- and polyhydroxylic alcohols with more than one triple bond
C07C 33/05 - C07C 33/16 : mono- and polyhydroxylic alcohols, the rings can be isolated or in condensed ring systems
C07C 33/22 : benzylalcohol, i.e. Ph-CH2OH and phenethyl alcohol, i.e. Ph-CH2CH2OH or Ph-CH(OH)CH3
C07C 33/28 : mono- and polyhydroxylic polycyclic alcohols containing only six-membered aromatic rings as cyclic part with unsaturation outside aromatic rings
C07C 33/42 : halogenated unsaturated alcohols containing both, double and triple bonds
In patent documents, the following abbreviations are often used:
EG | Ethylene glycol |
EO | Ethylene oxide |
PG | Propylene glycol |
PO | Propylene oxide |
BD | Butanediol |
TMP | Trimethylol propane |
This place covers:
Saturated and unsaturated monocyclic and polycyclic alcohols having at least one hydroxy or O-metal group bound to a carbon atom of a ring other than a six-membered aromatic ring, e.g. cyclohexanol (C07C 35/08).
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 29/00.
All alcohols classified in this main group contain carbocyclic ring systems which are indexed using the corresponding codes from C07C 2601/00 - C07C 2604/00. These Indexing Codes are used even if the information is redundant in view of the subgroup title, e.g. azulenols are classified in C07C 35/34 and C07C 2602/30.
Concerning certain subgroups :
 C07C 35/08 : e.g. cyclohexanol
C07C 35/08 : e.g. cyclohexanol
C07C 35/12 : menthol (all stereoisomers)
C07C 35/18 : e. g. cyclohexenol
C07C 35/24 : the literal meaning of "condensed ring system having two rings and containing five carbon atoms" covers only alcohols having the hydroxy group bound to a bicyclo[2.1.0] system (![]() ). Please note: In the past also condensed ring systems having two or more rings and containing a five-membered ring might have been classified here. This group has to be consulted for search, while the documents are continuously being reclassified.
). Please note: In the past also condensed ring systems having two or more rings and containing a five-membered ring might have been classified here. This group has to be consulted for search, while the documents are continuously being reclassified.
C07C 35/26 : due to its position in the scheme as a subgroup of C07C 35/24 and the last place rule, this group should, in principle, be empty. Nevertheless, alcohols derived from cyclopentadiene dimers are classified here. These alcohols have hydroxy groups attached to a tricyclo[5.2.1.02,6]decyl ring system ![]() .
.
C07C 35/31 : e.g. alcohols having the OH group bound to a pentalene system ![]()
C07C 35/37 : e.g. a hydroxy group on a bridged system having three rings (alcohols derived from cyclopentadiene dimers are classified in C07C 35/26)
C07C 35/46 : this subgroup refers to metal alcoholates (the rare cases of non-metal alcoholates, e.g. quarternary ammonium alcoholates, are classified as the corresponding alcohol)
C07C 35/48 - C07C 35/52 : due to the last place rule, these groups cover both halogenated alcohols and their alcoholates
In patent documents, the following abbreviations are often used:
EG | Ethylene glycol |
EO | Ethylene oxide |
PG | Propylene glycol |
PO | Propylene oxide |
BD | Butanediol |
TMP | Trimethylol propane |
This place covers:
Preparation and purification/stabilisation of phenols and phenolates.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 37/08, C07C 39/04 for the preparation of phenol by decomposition of cumene hydroperoxide). A combination set consists of a process group (e.g. C07C 37/08), followed by and linked to the group of the product (e.g. C07C 39/04). The products are selected from the corresponding product group C07C 39/00.
Please note: Not all documents in this main group are already classified using combination-sets. The remaining documents (which are continuously being reclassified) have to be searched using only the process group (no indication of the product).
Concerning certain (sub)groups:
C07C 37/001, C07C 37/002, C07C 37/003 : modification in a side chain not leading to an increase or decrease of the number of carbon atoms.
C07C 37/20 : e.g. condensation of acetone and phenol to bisphenol A
In patent documents, the following abbreviations are often used:
CHP | Cumenehydroperoxide |
This place covers:
Phenols and phenolates.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 37/00.
Concerning certain subgroups :
C07C 39/04 : Ph-OH only.
C07C 39/235 : metal derivatives of non-halogenated phenols (the rare cases of non-metal phenolates, e.g. quarternary ammonium phenolates, are classified as the corresponding phenol)
C07C 39/44 : metal derivatives of a halogenated phenols (the rare cases of non-metal phenolates, e.g. quarternary ammonium phenolates, are classified as the corresponding halogenated phenol)
In patent documents, the following abbreviations are often used:
CHP | Cumenehydroperoxide |
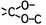 groups,
groups,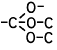 groups or
groups or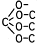 groups
groupsThis place covers:
Preparation and purification/stabilisation of acyclic or carbocylic
- ethers (C07C 41/01 - C07C 41/46)
- (hemi)acetals (C07C 41/48 - C07C 41/58)
- ortho esters and ortho carbonates (C07C 41/60)
This place does not cover:
Preparation of cyclic ethers wherein the ether oxygen is part of the ring |
Attention is drawn to the following places, which may be of interest for search:
Polyethers | C08G, e.g. C08G 65/00 |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 41/09, C07C 43/043 for the preparation of dimethyl ether from methanol by dehydration). A combination set consists of a process group (e.g. C07C 43/04), followed by and linked to the group of the product (e.g. C07C 43/043). The products are selected from the corresponding product group C07C 43/00 according to the following table:
Process : Product selected from
Please note: Not all documents in this main group are already classified using combination-sets. The remaining documents (which are continuously being reclassified) have to be searched using only the process group (no systematic indication of the product).
Concerning certain subgroups :
Preparation of ethers
C07C 41/01 : reactions which do not fit into any of the hierarchically lower subgroups; e.g. DME from syngas
C07C 41/02 : e.g. inserting oxiranes into existing ether bonds, DME + n EO → MeO(CH2CH2O)nMe or oligomerisation of oxiranes
C07C 41/05 : e.g. H2O + olefin → [alcohol + olefin] → ether
C07C 41/06 : e.g. alcohol + olefin, often preparation of MTBE
C07C 41/08 : e.g. preparation of vinyl ethers
C07C 41/09 : e.g. 2 MeOH -> DME or oligomerisation of glycols
C07C 41/16 : e.g. alcohol or phenol + alkyl halide or dialkyl sulfate
C07C 41/18 : e.g. elimination of water ![]() , orreactions decreasing the number of carbon atoms
, orreactions decreasing the number of carbon atoms
C07C 41/24 : also hydrodehalogenation R-O-R-CH2X → R-O-R-CH3
C07C 41/30 : increasing the number of carbon atoms of an existing ether compound; oligomerisation only in so far as not forming an ether bond (oligomerisation of glycols is classified in C07C 41/09, oligomerisation of alkylene oxides is classified in C07C 41/02)
Preparation of (semi)acetals
C07C 41/48 : e.g. reactions not involving the generation of a new (hemi)acetal function
C07C 41/54 : e.g. R-O-CH=CHR' + R''-OH -> R-O-CH(OR'')-CH2R'
C07C 41/56 : next to a condensation of aldehydes, paraformaldehyde, or ketones, also a condensation of 1,3,5-Trioxane - the cyclic formaldehyde trimer - is covered.
In this place, the following terms or expressions are used with the meaning indicated:
Esters of mineral acids | Esters of inorganic acids (see Glossary of C07C), including organic halides, e.g. CH3I or Ph-Br (which are esters of hydrohalic acids) or carbonates, e.g. CH3O-C(=O)OCH3 |
In patent documents, the following abbreviations are often used:
DME | Dimethylether |
MTBE | Methyltert-butyl ether |
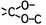 groups,
groups, groups or
groups or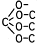 groups
groupsThis place covers:
Ethers, (Hemi)acetals, Ortho esters and ortho carbonates (C07C 43/02 -C07C 43/295, C07C 43/30 -C07C 43/317, C07C 43/32)
This place does not cover:
Cyclic ethers wherein the ether oxygen is part of the ring |
Attention is drawn to the following places, which may be of interest for search:
Polyethers | C08G ( C08G 65/00) |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 41/00.
Concerning certain subgroups :
Ethers
C07C 43/11 : containing oligomeric chains (formally) derived from alkylene oxide or alkylene glycol, e.g. containing -[CH2-CH2-O-]n and/or -[CH(CH3)-CH2-O-]n units.
C07C 43/13 - C07C 43/137 : the group C07C 43/11 takes precedence also over the subgroups of C07C 43/13, e.g. a halogen containing oligo ethylene glycol CF3-(O-CH2-CH2-)3-9OH is classified in C07C 43/11
C07C 43/17 : unsaturated ethers containing halogen and not containing rings
C07C 43/178 : unsaturated ethers containing hydroxy or O-metal groups and not containing rings
C07C 43/257 : e.g. ![]() , containing condensed six-membered aromatic rings and ether oxygen atoms not bound to carbon atoms of six-membered aromatic rings
, containing condensed six-membered aromatic rings and ether oxygen atoms not bound to carbon atoms of six-membered aromatic rings
C07C 43/263 : e.g. Ph-O-Ph-O-CH2CH2-O-CH3
C07C 43/267 : e.g. ![]()
C07C 43/275 : e.g. Ph-O-Ph
(Semi)acetals
C07C 43/30 : e.g. formals ![]() , the acetal carbon being -CH2-
, the acetal carbon being -CH2-
C07C 43/303 - C07C 43/307 are defined by the substituent bound to the acetal carbon C-O-C-O-C
C07C 43/313 : containing halogen bound to acetal and/or non-acetal carbon atoms
C07C 43/317 : e.g. hemiacetals and hemiketals, for search see also the corresponding aldehydes (C07C 47/00) and ketones (C07C 49/00).
In patent documents, the following abbreviations are often used:
DME | Dimethylether |
MTBE | Methyltert-butyl ether |
This place covers:
The preparation, separation, purification and stabilisation of acyclic or carbocyclic aldehydes or ketones or its chelates, including ketenes or dimeric ketenes.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 45/53, C07C 49/403). A combination set consists of a process group (e.g. C07C 45/53), followed by and linked to the group of the product (e.g. C07C 49/403). The products are selected from the corresponding product groups C07C 47/00 - C07C 49/00.
Concerning certain subgroups:
C07C 45/28 : e.g. oxidation of propene to acetone in the absence of oxygen, C07C 45/28, C07C 49/08
C07C 45/73 : e.g. condensation of 2 acetone to methyl iso-butyl ketone in the presence of hydrogen (combination set C07C 45/73, C07C 49/04)
This place covers:
The preparation, separation, purification and stabilisation of carbocyclic quinones.
Quinones include 1,2- and 1,4-benzoquinone, naphthoquinones, anthraquinones and higher conjugated aromatic quinones and also substituted quinones.
This place does not cover:
Preparation of quinone methides |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 46/02, C07C 50/08). A combination set consists of a process group (e.g. C07C 46/02), followed by and linked to the group of the product (e.g. C07C 50/08). The products are selected from the corresponding product group C07C 50/00.
This place covers:
Saturated and unsaturated acyclic and carbocyclic compounds having at least one aldehyde group.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 45/00.
In patent documents, the following words/expressions are often used as synonyms:
- "Chlora" and "trichloroacetaldehyde"
This place covers:
Saturated and unsaturated acyclic and carbocyclic compounds having at least one ketone, ketonic chelate, ketene or dimeric ketene group.
This place does not cover:
Heterocyclic compounds | |
Beta-Lactones | |
Sugars |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 45/00.
Concerning certain subgroups:
C07C 49/755 : e.g. ninhydrin
In patent documents, the following words/expressions are often used as synonyms:
- "Acetylacetone" and "2,4-pentanedione"
- " Methyl vinyl ketone (MVK)" and "butenone"
This place covers:
Unsaturated carbocyclic compounds having at least two CH-moieties in the aromatic skeleton exchanged for a C=O-moiety.
The keto groups can be in the 1,2 or 1,4 or 9,10, etc. positions.
Attention is drawn to the following places, which may be of interest for search:
Quinone methides |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 46/00.
In patent documents, the following words/expressions are often used as synonyms:
- "Chloranil" and "2,3,5,6-Tetrachlorocyclohexa-2,5-diene-1,4-dione"
This place covers:
The preparation, separation, purification and stabilisation of acyclic or carbocyclic carboxylic acids or their salts, carboxylic acid anhydrides or carboxylic acid halides.
This place does not cover:
Preparation of amino acids | |
Preparation of cyclic anhydrides | |
Heterocyclic carboxylic acid (e.g. ascorbic acid) | e.g. C07D 307/62 |
Preparation of steroid acids | |
Recovery of fatty acids from waste materials | |
Preparation of fatty acids from fats, fatty oils or waxes; Refining the fatty acids | |
Fatty acids by chemical modification of fats, oils or fatty acids obtained therefrom | |
Preparation of soaps | |
Preparation of acids via enzymes or microorganisms | |
Electrochemical preparation of carboxylic acids |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 51/09, C07C 59/84). A combination set consists of a process group (e.g. C07C 51/09), followed by and linked to the group of the product (e.g. C07C 59/84). The products are selected from the corresponding product groups C07C 53/00 - C07C 66/00.
Preparation of mixtures of dicarboxylic acids and cyclic anhydrides (e.g. maleic acid and maleic anhydride) are classified in both C07C and C07D
Concerning certain (sub)groups:
C07C 51/00 (the main group itself): processes which do not fit in any or the subgroups are classified here. e.g. hydrolysis of ketenes: -C=C=O --> -CO2H
Also the ring contraction of alpha-Cn-cycloketones to Cn-1-cycloalkyl carboxylic acid (Favorski rearrangement)
C07C 51/083 : includes also hydrogenolysis
C07C 51/09 : no separate class is given for the acidification. Group is also used for the hydrolysis of thioesters. Also the Friedel-Crafts reaction of a lactone with an aromatic compound.
C07C 51/093 : group is also used for the hydrolysis of C=CX2 --> C-COOH
C07C 51/10 - C07C 51/145 : use these groups only for preparation of acids (NOT anhydrides) via carbonylation.
C07C 51/16 : e.g. for the preparation of carboxylic acids AND carboxylic acid anhydrides via oxidation. The oxidation using oxidants like CrO3 or KMnO4 is also classified here (these last examples are NOT classified in C07C 51/285).
C07C 51/225 : oxidation usually leads to undefined mixtures with an acid number
C07C 51/295 : e.g. preparation via e.g. alkali fusion, dehydrogenation or Ag2O oxidation
C07C 51/347 : "reactions not involving formation of carboxyl groups" should be interpreted as reactions not changing the nature of the carboxyl group. An anhydride turning into an carboxylic acid does NOT belong here
C07C 51/353 : racemisation is also included in this group
C07C 51/363 - C07C 51/373 : "introduction" also means the additional introduction. e.g. when a halogen atom is already present, but an extra halogen atom is introduced, the C07C 51/363 is used. Also the hydrolysis of a lactone to a hydroxyacid is classified using C07C 51/367
C07C 51/41 : preparation of salts via process steps belonging to C07C 51/093 - C07C 51/34 takes precedence. The rearrangement reaction to lactic acid starting from glycerol or dihydroxyacetone in the presence of base should be classified in C07C 51/41
C07C 51/416 : e.g. rearrangement or disproportionation of carboxylate salt groups linked to a six-membered ring in the absence/presence of CO or CO2 e.g. K-benzoate + K2-ortho-phthalate --> benzene + K2-terephthalate (Henkel's reaction)
C07C 51/487 : resolution is included in this chemical modification; e.g. separation on a chiral column, but also resolution via a chiral reagent (e.g. an amine) etc.
C07C 51/54 : preparation of carboxylic acid anhydrides via oxidation are classified in C07C 51/16. Processes which do not fit in any of the groups C07C 51/56-C07C 51/573 are classified here, e.g. preparation of a cyclic anhydride via transimidation with a imide (e.g. phthalic anhydride from a N-alkyl phthalimide).
C07C 51/56 : preparation of carboxylic acid anhydrides from organic acids, their salts, their esters, or their halides (e.g. by carboxylation) or their amides. e.g. 2 R RCO2H + CO --> R-CO-O-CO-R. The preparation via transanhydrisation (e.g. 2 RC(=O)OH + CH3C(=O)OC(=O)CH3 --> 2 CH3C(=O)OH + RC(=O)OC(=O)R is also classified here. The carbonylation of a carboxylic acid via the reaction RCOOH + R'CH=CH2 + CO --> RC(=O)-O-C(=O)CH2CH2R' is also classified here
C07C 51/58 : e.g. ArCCl3 --> ArC(=O)Cl
C07C 51/60 : preparation by conversion of compounds having the same carboxyl-group. For example of carboxylic acids, the carboxylic acid anhydrides, esters, lactones, carboxylic acid salts, acid chlorides
C07C 51/62 : e.g. FC(=O)F + CH2=CF2 --> F3CC(=O)F
This place covers:
Saturated acyclic and alicyclic compounds having one carboxylic acid group, a carboxylic anhydride group or a carboxylic acid halide group to an acyclic carbon atom or to hydrogen.
This place does not cover:
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
Concerning certain subgroups :
C07C 53/128 : e.g. valproic acid
In patent documents, the following words/expressions are often used as synonyms:
- "Formic acid" and "Methanoic acid"
- "Acetic acid" and "Ethanoic acid"
- "Propionic acid" and "Propanoic acid"
This place covers:
Saturated acyclic or alicyclic carboxylic acids, acid anhydrides and acid halides having more than one carboxyl group bound to acyclic carbon atoms.
This place does not cover:
Succinic acid anhydride |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
Concerning certain subgroups:
C07C 55/02 : e.g. suberic acid (C-8) or octanedioic acid, which does not have a special subgroup
In patent documents, the following words/expressions are often used as synonyms:
- "Oxalic acid" and "Ethanedioic acid"
- "Malonic acid" and "Propanedioic acid"
- "Succinic acid" and "Butanedioic acid"
- "Glutaric acid" and "Pentanedioic acid"
- "Adipic acid" and "Hexanedioic acid"
- "Pimelic acid" and "Heptanedioic acid"
- "Azelaic acid" and "Nonanedioic acid"
- "Sebacic acid" and "Decanedioic acid"
This place covers:
Unsaturated cyclic and alicyclic carboxylic acids, acid anhydrides and acid halides bound to acyclic carbon atoms.
Aromatic groups (not bound to a carboxylic acid) are also counted as unsaturation. e.g. acrylic acid C07C 57/04; phenylacetic acid C07C 57/32; cyclohexylphenylacetic acid C07C 57/46.
This place does not cover:
Maleic anhydride |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
Concerning certain subgroups :
C07C 57/30 : e.g. Ibuprofen
In patent documents, the following words/expressions are often used as synonyms:
- "Acrylic acid" and "prop-2-enoic acid"
- "Methacrylic acid" and "2-methylpropenoic acid"
- "Crotonic acid" and " (E)-but-2-enoic acid"
- "Sorbic acid" and " (2E,4E)-hexa-2,4-dienoic acid"
- "Maleic acid" and " (Z)-butenedioic acid"
- "Fumaric acid" and " (E)-butenedioic acid"
- "Citraconic acid" and " (2Z)-2-methylbut-2-enedioic acid"
- "Muconic acid" and " (2E,4E)-hexa-2,4-dienedioic acid"
- "Propiolic acid" and "2-propynoic acid"
- "Acetylene dicarboxylic acid" and "but-2-ynedioic acid"
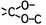 groups,
groups, groups, or
groups, or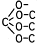 groups
groupsThis place covers:
Saturated and unsaturated acyclic and alicyclic carboxylic acids, carboxylic acid anhydrides and carboxylic acid halides bound to acyclic carbon atoms and containing any of the groups OH, O-metal, -CHO, keto, ether, hemiacetal, acetal, orthoester or orthocarbonate.
E.g. levulinic acid (4-oxo-pentanoic acid) C07C 59/185; citric acid C07C 59/265
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
Concerning certain subgroups :
C07C 59/105 : e.g. gluconic acid
C07C 59/185 : e.g. levulinic acid
C07C 59/245 : e.g. malic acid
C07C 59/68 : the oxygen atom of the ether group can be bound to any (NOT "the") non-condensed six-membered aromatic ring
In patent documents, the following words/expressions are often used as synonyms:
- "Glycolic acid" and "2-hydroxyethanoic acid"
- "Lactic acid" and "2-hydroxypropanoic acid"
- "Glyoxylic acid" and "oxoethanoic acid"
- "Pyruvic acid" and "2-oxopropanoic acid"
- "Tartaric acid" and "2,3-dihydroxybutanedioic acid"
- "Citric acid" and "2-hydroxypropane-1,2,3-tricarboxylic acid"
- "Ricinoleic acid" and " (9Z,12R)-12-hydroxyoctadec-9-enoic acid"
- "Mandelic acid" and "2-hydroxy-2-phenylacetic acid"
This place covers:
Saturated and unsaturated carboxylic acids, carboxylic acid anhydride or a carboxylic acid halide group bound to carbon atoms of carbocyclic ring(s) other than a six-membered aromatic ring(s).
The unsaturated compounds from C07C 61/16 onwards include unsaturation from aromatics.
E.g. cyclohexanecarboxylic acid C07C 61/08; chrysanthemumic acid C07C 61/37.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
In patent documents, the following words/expressions are often used as synonyms:
- "Chrysanthemumic acid" and "2,2-dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylic acid "
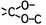 groups,
groups, groups, or
groups, or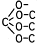 groups
groupsThis place covers:
Saturated and unsaturated alicyclic carboxylic acids, carboxylic acid anhydrides and carboxylic acid halides, bound to carbon atoms of rings other than six-membered aromatic rings and containing any of the groups OH, O-metal, -CHO, keto, ether, hemiacetal, acetal, orthoester or orthocarbonate ester
The unsaturated compounds from C07C 62/30 onwards include unsaturation from aromatics.
E.g. 3-methoxy-cyclopentanecarboxylic acid C07C 62/08
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
All carboxylic acids classified in this main group contain carbocyclic ring systems which are indexed using the corresponding codes from C07C 2601/00 - C07C 2604/00. These Indexing Codes are used even if the information is redundant in view of the subgroup title.
Concerning certain subgroups :
C07C 62/32 : e.g. shikimic acid
This place covers:
Unsaturated carbocyclic carboxylic acids, carboxylic acid anhydrides and carboxylic acid halides bound to carbon atom of a carbocyclic six-membered aromatic ring.
E.g. terephthalic acid C07C 63/26; benzoic acid C07C 63/06
This place does not cover:
Phthalic anhydride | |
Trimellitic anhydride | |
Naphthalenic anhydride |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
Concerning certain subgroups :
C07C 63/10, C07C 63/22 and C07C 63/30 : the halides refers to aroyl halides only. Compounds with a halogen atom substitution on the carbon skeleton are classified in C07C 63/68-C07C 63/74.
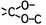 groups,
groups,  groups, or
groups, or 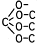 groups (cyclic anhydrides C07D)
groups (cyclic anhydrides C07D)This place covers:
Unsaturated carbocyclic carboxylic acids, carboxylic acid anhydrides and carboxylic acid halides bound to carbon atoms of carbocyclic six-membered aromatic rings and containing any of the groups OH, O-metal, -CHO, keto, ether, hemiacetal, acetal, orthoester or orthocarbonate.
E.g. 3-hydroxy-benzoic acid C07C 65/03; 3-acetyl-naphthoic acid C07C 65/34
This place does not cover:
Cyclic anhydrides |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
In patent documents, the following words/expressions are often used as synonyms:
- "Salicylic acid" and "2-hydroxybenzoic acid"
This place covers:
Carbocyclic quinone carboxylic including 1,2- and 1,4-benzoquinone, naphthoquinones, anthraquinones and higher conjugated aromatic quinones and also substituted quinones.
Carboxylic acids of quinone methides should be classified in the relevant group of a carboxylic acid with a ketone in the ring and not in this group.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 51/00.
This place covers:
Preparation and purification/stabilisation of acyclic or carbocylic carboxylic acid esters.
This place does not cover:
Preparation of lactones |
Attention is drawn to the following places, which may be of interest for search:
Polyesters | C08G, e.g. C08G 63/00 |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 67/08, C07C 69/54 for the preparation of (meth)acrylic acid esters by direct esterification). A combination set consists of a process group (e.g. C07C 67/08), followed by and linked to the group of the product (e.g. C07C 69/54). The products are selected from the corresponding product groups C07C 69/00 - C07C 69/95.
Concerning certain (sub)groups :
C07C 67/00 (the main group itself) : processes which do not fit in any of the subgroups are classified here, e. g.
the preparation of enol esters from aldehydes or ketones and carboxylic acid anhydrides,
hydrogenation of carboxylic acids leading directly to esters,
preparation of methyl esters using diazomethane (CH2N2)
C07C 67/02 : the term "transesterification" is somewhat misleading. This group covers interesterification (ester interchange) of the type ester1 + ester2, e.g.
R1-C(O)O-R2 + R3-C(O)O-R4 → R1-C(O)O-R4 + R3-C(O)O-R2. One ester can also be a mineral ester.
C07C 67/03 : this group covers reactions of the type ester1 + alcohol2, i. e. transesterification : R1-C(O)O-R2 + R3-OH → R1-C(O)O-R3 + R2-OH, e.g. reaction of methyl (meth)acrylate and 2-ethyl hexanol to 2-ethyl hexyl (meth)acrylate. When used as a starting material, also cyclic esters (lactones) are considered as esters, e.g. in the ring opening of γ-butyrolactone with methanol to methyl 4-hydroxy butyrate.
C07C 67/035 : also reactions like the preparation benzyl acetate from toluene and acetic acid under oxidative conditions, wherein the saturated methyl group reacts, are classified here, though toluene as a whole is not a saturated hydrocarbon.
C07C 67/04 : e.g. preparation of ethyl acetate from acetic acid and ethylene or preparation of vinyl acetate from acetic acid and acetylene
C07C 67/05, C07C 67/055 : often preparation of vinyl acetate from acetic acid and ethylene
C07C 67/10 : this group covers reactions of the type R1-C(O)O-R2 + R3-C(O)OH → R3-C(O)O-R2 + R1-C(O)OH, e.g. the preparation of a vinyl ester by reacting a carboxylic acid with vinyl acetate (the reaction of carboxylic acids with mineral acid esters such as carbon halogen bonds, e.g. alkyl halides, or dialkyl carbonates is classified in C07C 67/11)
C07C 67/11 : e.g. reaction of carboxylic acids with dialkyl sulfate, dialkyl carbonate or alkyl halides
C07C 67/12 : acyclic unsymmetrical anhydrides have two different acyl groups, e.g. PivOAc; cyclic anhydrides are unsymmetrical if two different - including stereochemically different - products are obtained depending on which of the two acyl groups react with the alcohol. For example, succinic anhydride is symmetrical (to be classified in C07C 67/08), 2-methyl succinic anhydride is unsymmetrical.
C07C 67/28 - C07C 67/297 : the limitation to modifications "not being an introduction of an ester group" is construed such as to exclude reactions merely forming new ester groups which were not already present in the educt(s) (e.g. the further esterification of hydroxy groups of a polyol partial ester is classified as esterification in C07C 67/08)
C07C 67/30 - C07C 67/347 : the limitation to modifications "not being an introduction of an ester group" is construed such as to exclude reactions merely forming new ester groups which were not already present in the educt(s) (e.g. the esterification of an existing carboxyl group of a polycarboxylic acid partial ester is classified as esterification in C07C 67/08)
C07C 67/30 : this group covers reactions which do not fit into any of its subgroups C07C 67/303 - C07C 67/347. An example is the partial hydrolysis of esters of polyacids, e. g. making sodium methyl succinate from dimethyl succinate.
C07C 67/31 : e.g. deprotection of an OH group in the acid moiety or ring opening of epoxide in the acid moiety (the transesterification/ring opening of lactones to hydroxy carboxylic acid esters is classified in C07C 67/03).
C07C 67/313 : e.g. oxidation of a hydroxy group to a carbonyl group (the introduction of a formyl group by hydroformylation of a double bond in the acid moiety, which increases the size of the carbon skeleton, is classified under C07C 67/347; an ozonolysis leading to an introduction of a carbonyl group while decreasing the number of carbon atoms of the carbon skeleton is classified under C07C 67/333)
C07C 67/327 : the term elimination is construed as beta-elimination leading to a double (or triple) bond.
C07C 67/333 : next to isomerisation, also reactions leading to a decrease of the number of carbon atoms of the carbon skeleton are classified here, e.g. ethenolysis of methyl oleate to methyl decenoate, or ozonolysis of maleic acid esters to glyoxylic acid esters
C07C 67/347 : e.g. introduction of a formyl group by hydroformylation of a double bond in the acid moiety, which increases the size of the carbon skeleton
C07C 67/36 - C07C 67/38 : these groups cover reaction leading to new carboxylic ester groups by reaction with carbon monoxide or formates (a mere introduction of a formyl group by hydroformylation of a double bond in an existing ester is classified under C07C 67/293 or C07C 67/347)
C07C 67/36 : e.g. carbonylation of methanol to methyl acetate
C07C 67/37 : e.g. carbonylation of dimethyl ether to methyl acetate; alkoxycarbonylation (i.e. reaction with CO and R-OH) of alkylene oxides to hydroxy carboxylic acid esters
C07C 67/38 : alkoxycarbonylation of olefins, e.g. preparation of methyl propionate by methoxycarbonylation of ethylene
C07C 67/465, C07C 67/47 : these groups are not used systematically. They can be used in addition to a classification of the reaction underlying the oligomerisation/telomerisation.
C07C 67/475 : e.g. metathesis reactions involving two unsaturated esters leading to one product containing more ester groups and another product containing less/no ester groups, such as the preparation of long-chain unsaturated α,ω-dicarboxylic acid esters by homometathesis (self metathesis) of unsaturated fatty acid esters (cross metathesis of unsaturated ester and unsaturated hydrocarbons are classified in C07C 67/293, C07C 67/333 or C07C 67/343)
C07C 67/60 : chemical modification can be the modification of an impurity or the temporary modification of the desired target compound
In this place, the following terms or expressions are used with the meaning indicated:
Mineral ester groups | Esters of inorganic acids (see glossary of C07C), including organic halides, e.g. CH3I or Ph-Br (which are esters of hydrohalic acids) or carbonates, e.g. CH3O-C(=O)OCH3 |
Platinum group metals | Os, Ir, Pt, Ru, Rh, Pd |
This place covers:
Preparation or purification/stabilisation of acyclic or carbocylic organic carbonates or haloformates.
Attention is drawn to the following places, which may be of interest for search:
Preparation of cyclic carbonates, e.g. ethylene carbonate | |
Polycarbonates |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 68/08, C07C 69/96). A combination set consists of a process group (e.g. C07C 68/08), followed by and linked to the group of the product (i.e. C07C 69/96). Presently, there is only one product, namely C07C 69/96.
Concerning certain (sub)groups :
C07C 68/00 : processes which do not fit in any of the subgroups, e.g.
- dimethyl carbonate from urea or carbamate
- decarbonylation of diphenyl oxalate to diphenyl carbonate
- oxidative carbonylation using other oxidants than oxygen (rare)
C07C 68/01 : e.g. oxidative carbonylation of phenol in the presence of oxygen, 2 Ph-OH + CO + ½O2 → O=C(OPh)2 + H2O
C07C 68/06 : often preparation of diaryl carbonate from dialkyl carbonate and aryl alcohol, e.g. diphenyl carbonate from DMC and phenol; also also reactions leading from one carbonate to another carbonate not involving the carbonate group
C07C 68/065 : often preparation of DMC from ethylene carbonate and methanol.
In patent documents, the following abbreviations are often used:
DMC | dimethyl carbonate |
This place covers:
Esters of acyclic or carbocylic carboxylic acids; Acyclic or carbocylic carbonates or haloformates.
This place does not cover:
Ortho esters | |
Lactones or alkylene carbonates | |
Esters of sugars | |
Fats, oils |
Attention is drawn to the following places, which may be of interest for search:
Polyesters | C08G, e.g. C08G 63/00 |
Polycarbonates | C08G, e.g. C08G 64/00 |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 67/00 or C07C 68/00.
There is no distinction between full and partial esters of polycarboxylic acids. E. g. Dimethyl succinate and monomethyl succinate as well as salts and acid halides of the half ester are all classified in C07C 69/40.
The following IPC groups are not used: C07C69/025, C07C69/03, C07C69/035, C07C69/347, C07C69/353, C07C69/527, C07C69/767, C07C69/773, C07C69/83.
Concerning certain subgroups :
C07C 69/003 - C07C 69/017 : "esters having a variably-specified acid moiety, i.e. covered by more than one of groups C07C 69/02, C07C 69/34, C07C 69/52, C07C 69/608, C07C 69/612, C07C 69/62, C07C 69/66, C07C 69/74, C07C 69/76, C07C 69/95, C07C 69/96" are classified in the groups C07C 69/003 - C07C 69/017 according to their hydroxylic moiety (IPC). This IPC rule is construed in the following way: an ester is always classified (at least) according to the carboxylic acid moiety or carbonic acid moiety. In addition, the ester is classified in the groups C07C 69/003 - C07C 69/017 according the hydroxylic (alcohol) moiety if the claim defines the alcohol moiety in a specific way and the carboxylic or carbonic acid moiety in a generic way, falling into more than one of the aforementioned groups C07C 69/02 - C07C 69/96.
Example: a claim relates to the preparation of vinyl esters of the formula CH2=CH-O-C(O)-R from ethylene and the corresponding carboxylic acid under oxidative condition in the presence of a Pd catalyst. The claim defines a specific alcohol moiety (CH2=CH-O-) and a generic acid mioety (-C(O)-R). The examples show the preparation of vinyl acetate and vinyl benzoate. The reaction is classified as leading to vinyl esters (C07C 69/01), to vinyl acetate (C07C 69/15) and to vinyl benzoate (C07C 69/78), i.e. as combination-sets (C07C 67/055, C07C 69/01), (C07C 67/055, C07C 69/15), (C07C 67/055, C07C 69/78).
C07C 69/02 : this group is only used if the acyclic saturated monocarboxylic acid is not disclosed specifically, e.g. only as "lower alkyl carboxylic acid", and cannot be classified in any of the subgroups of C07C 69/02
C07C 69/34 : esters of acyclic saturated polycarboxylic acids other than those named in C07C 69/36 - C07C 69/50, e.g. suberic acid esters or methyl malonic acid esters.
C07C 69/36 - C07C 69/50 : only esters of the acids named in the titles are classified here (C-alkylated derivatives, e.g. methyl malonic acid esters, are classified in C07C 69/34)
C07C 69/52 : esters acyclic unsaturated carboxylic acids with an unknown or mixed degree of unsaturation, e.g. esters of fatty acids from vegetable oils, are classified here.
C07C 69/56 : esters of CH3-CH=CH-COOH and CH2=CH-CH2-COOH
C07C 69/58 : often oleic acid esters
C07C 69/587 : often esters of linolic or linoleic acid or PUFAs
C07C 69/606 : this group covers esters of acids having only carbon-to-carbon triple bonds as well as esters of acids having carbon-to-carbon double bonds and additionally carbon-to-carbon triple bonds in the carboxylic acid moiety
C07C 69/616 : the term "polycyclic" covers condensed rings, e.g. a naphthyl or indanyl ring, or two or more uncondensed rings, e.g. two phenyl rings or a phenyl and a cyclohexyl ring
C07C 69/618 : e.g. cinnamic acid esters
C07C 69/62 - C07C 69/657 : the halogen(s) can be bound to the acid moiety and/or the alcohol moiety (acid halides of polycarboxylic acid partial esters are classified as the corresponding polycarboxylic acid partial esters)
C07C 69/716, C07C 69/738 : esters of carboxylic acids having keto and/or aldehyde (-CHO) groups in the acid moiety, i.e. esters of keto-carboxylic acids or aldehydo-carboxylic acids are classified here.
C07C 69/76 : esters of carboxylic acids having an esterified carboxyl group bound to a carbon atom of a six-membered aromatic ring which do not fit into any of the subgroups C07C 69/78 - C07C 69/94, e.g. esters chlorobenzoic acid, toluene carboxylic acid, or naphthalene carboxylic acid.
C07C 69/78 : esters of benzoic acid only (derivatives such esters chlorobenzoic acid or toluene carboxylic acid are classified under C07C 69/76)
In this place, the following terms or expressions are used with the meaning indicated:
Polycyclic | containing two or more rings, condensed or isolated |
In patent documents, the following abbreviations are often used:
VA, VAM | vinyl acetate (monomer) |
PUFAs | polyunsaturated fatty acids |
This place covers:
Acyclic or alicyclic esters of oxyacids of halogens, i. e. compounds of the type R-O-X(O)n, n>=0, and their preparation/purification and stabilisation.
Examples are esters of hypochlorous acid R-O-Cl;
Also covered here are compounds of the type R-X(O)n, n>0, which are not exactly esters of oxyacids of halogens, but for which there is no better place under C07C.
This place covers:
The preparation, separation, purification and stabilization of
- esters of nitric acid (-C-O-NO2)
- esters of nitrous acid (-C-O-NO)
- nitro compounds (-C-NO2)
- nitroso compounds (-C-NO)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 201/16, C07C 205/06 for the purification of nitrobenzene). A combination set consists of a process group (e.g. C07C 201/16), followed by and linked to the group of the product (e.g. C07C 205/06). The products are selected from the corresponding product groups C07C 203/00 - C07C 207/00.
Please note: Not all documents in this main group are already classified using combination sets. Some documents concerning the preparation of esters of nitric or nitrous acid or of compounds containing nitro or nitroso groups are presently only classified in the corresponding product groups. These documents (which are continuously being reclassified) have to be searched using only the product group.
In patent documents, the following abbreviations are often used:
DNT | dinitrotoluene |
TNT | trinitrotoluene |
This place covers:
- Esters of nitric acid (O2N-O-C-)
- Esters of nitrous acid (ON-O-C-)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 201/00.
This place covers:
Organic nitro compounds (-C-NO2)
Attention is drawn to the following places, which may be of interest for search:
Explosives |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 201/00.
Concerning certain (sub)groups:
C07C 205/01: -C-NO2 (C: acyclic carbon)
C07C 205/05: Cy- NO2 (Cy: cycloalkyl, cycloalkenyl)
C07C 205/06: Ar-NO2 (Ar: aromatic ring)
C07C 205/07 - C07C 205/12: -NO2 + halo on the carbon skeleton
C07C 205/13 - C07C 205/26: -NO2 + -OH on the carbon skeleton
C07C 205/27 - C07C 205/38: -NO2 + -OR on the carbon skeleton
C07C 205/39 - C07C 205/43: -NO2 + -O-C(O)-R on the carbon skeleton
C07C 205/44 -NO2 + -CHO on the carbon skeleton
C07C 205/45 - C07C 205/48: -NO2 + a keto group on the carbon skeleton
C07C 205/49 - C07C 205/61: -NO2 + -COOH or -NO2 + -COOR on the carbon skeleton.
In patent documents, the following abbreviations are often used:
DNT | dinitrotoluene |
TNT | trinitrotoluene |
In patent documents, the following words/expressions are often used as synonyms:
- "Styphnic acid" and "2,4,6-trinitrobenzene-1,3-diol"
This place covers:
Organic nitroso compounds (-C-NO)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 201/00.
This place covers:
The preparation, purification, separation and stabilization of organic amines (-C-NR2).
This place does not cover:
Preparation of amines protected by an acyl group, e.g. amines protected by an acetyl group (Ac) or a benzoyl group (bz) | |
Preparation of amines protected by a Cbz, Boc or Fmoc group | |
Preparation of cyclic amines wherein the nitrogen atom is part of a ring, e.g. piperidine, morpholine |
Attention is drawn to the following places, which may be of interest for search:
For some subgroups e.g. C07C 209/36 and C07C 209/48 reduction- and/or hydrogenation catalysts |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 209/84, C07C 211/46 for the purification of aniline). A combination set consists of a process group (e.g. C07C 209/84), followed by and linked to the group of the product (e.g. C07C 211/46). The products are selected from the corresponding product group C07C 211/00.
Concerning certain (sub)groups:
C07C 209/02: e.g. direct amination of hydrocarbons; substitution reactions like CH3-Br + CH3-NH2 → CH3-NH-CH3 are to be classified in C07C 209/08 (substitution of halogen) due to the last place rule.
C07C 209/24: e.g. NH3 or RNH2 + R-C(O)-R'
- Hofmann: R-C(O)-NH2 + NaOBr → RNH2
- Curtius: R-C(O)-N3 → R-N=C=O → RNH2
- Schmidt: R-COOH + HN3 → RNH2
- Lossen: R-C(O)-NH-OH → R-N=C=O → RNH2
C07C 209/78: CH2O + C6H5NH2 → H2N-C6H4-CH2-C6H4-NH2
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
In patent documents, the following words/expressions are often used as synonyms:
- "Aniline" and "aminobenzene"
- "Toluidine" and "'o'-, 'm'-, or 'p'-methylaniline"
- "Phenylenediamines" and "1,2- or 1,3- or 1,4-diaminobenzene"
- "Hexamethylenediamine" and "1,6-diaminohexane"
This place covers:
Acyclic, cyclic and aromatic organic amines (-C-NR2)
This place does not cover:
Amines protected by an acyl group, e.g. amines protected by an acetyl group (Ac) or a benzoyl group (bz) | |
Amines protected by a Cbz, Boc or Fmoc group | |
Cyclic amines wherein the nitrogen atom is part of a ring, e.g. piperidine, morpholine |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 209/00.
Concerning certain (sub)groups:
C07C 211/01 - C07C 211/32: -C-NR2, C = acyclic carbon
C07C 211/33 - C07C 211/42: Cy-NR2, Cy = cycloalkyl, cycloalkenyl
C07C 211/43 - C07C 211/61: Ar-NR2, Ar = aromatic ring
C07C 211/57 - C07C 211/61: Ar-NR2, Ar = aromatic ring and part of a
condensed ring system
C07C 211/47: for example H2N-C6H4-CH3
C07C 211/51: for example H2N-C6H4-NH2
C07C 211/55: for example C6H5-NH-C6H5
C07C 211/62 - C07C 211/64: quaternary ammonium compounds
C07C 211/65: metal complexes of amines
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
In patent documents, the following words/expressions are often used as synonyms:
- "Aniline" and "aminobenzene"
- "Toluidine" and "'o'-, 'm'-, or 'p'-methylaniline"
- "Phenylenediamines" and "1,2- or 1,3- or 1,4-diaminobenzene"
- "Hexamethylenediamine" and "1,6-diaminohexane"
This place covers:
The preparation, purification, separation and stabilization of:
- amino alcohols (R2N-A-OH, A represents a hydrocarbon residue)
- amino ethers (R2N-A-OR, A represents a hydrocarbon residue)
- compounds having an amino group and an esterified hydroxy-group (R2N-A-O-C(O)-R, A represents a hydrocarbon residue)
This place does not cover:
Preparation of amino acids and esters thereof |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 213/10, C07C 215/08 for the purification of aminoethanol). A combination set consists of a process group (e.g. C07C 213/10), followed by and linked to the group of the product (e.g. C07C 215/08). The products are selected from the corresponding product groups C07C 215/00, C07C 217/00 and C07C 219/00.
Concerning certain (sub)groups:
C07C 213/04: e.g. ethylenoxide or epichlorohydrin + NH3 or RNH2
C07C 213/08: this group covers also the deprotection of protected amino alcohols, amino ethers and amino esters.
This place covers:
Aminoalcohols (R2N-A-OH, A represents a hydrocarbon residue).
Attention is drawn to the following places, which may be of interest for search:
Detergent compositions |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 213/00.
Concerning certain (sub)groups:
C07C 215/02 - C07C 215/38: -OH and -NR2 are both bound to acyclic carbon atoms
C07C 215/40: the amino group is quaternized and the amino and the -OH group are both bound to acyclic carbon atoms
C07C 215/42 - C07C 215/44: -OH or -NR2 is bound to cycloalkyl or cycloalkenyl
C07C 215/46 - C07C 215/66: -OH bound to an aromatic ring and -NR2 not
C07C 215/68 - C07C 215/72: -NR2 bound to an aromatic ring and -OH not
C07C 215/74 - C07C 215/82: -NR2 and -OH are both bound to an aromatic ring
C07C 215/84 - C07C 215/88: -NR2 bound to an aromatic ring which is part of a condensed ring system
C07C 215/90: quaternary amino group and -OH are both bound to an aromatic ring.
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
This place covers:
Aminoethers (R2N-A-OR, A and R represent hydrocarbon residues).
Attention is drawn to the following places, which may be of interest for search:
Detergent compositions |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 213/00.
Concerning certain (sub)groups:
C07C 217/02 - C07C 217/50: -OR and -NR2 are both bound to acyclic carbon atoms
C07C 217/14 - C07C 217/24: -OR' and -NR2 are both bound to acyclic carbon atoms and R' is aromatic
C07C 217/20: only if the substituent is directly attached to the aromatic ring
C07C 217/52: -OR or -NR2 is bound to cycloalkyl or cycloalkenyl
C07C 217/54 - C07C 217/74: -OR bound to an aromatic ring and -NR2 not
C07C 217/76: -NR2 bound to an aromatic ring and -OR not
C07C 217/78 - C07C 217/94: -NR2 and -OH are both bound to an aromatic ring
C07C 217/94: -NR2 bound to an aromatic ring which is part of a condensed ring system
This place covers:
Compounds containing an amino group and an esterified hydroxy group (R2N-A-O-C(O)-R, A represents a hydrocarbon residue).
This place does not cover:
Esters of amino acids(R2N-A-C(O)-O-R) |
Attention is drawn to the following places, which may be of interest for search:
Detergent compositions |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 213/00.
Concerning certain (sub)groups:
C07C 219/02 - C07C 219/22: -O-C(O)-R and -NR2 are both bound to acyclic carbon atoms
C07C 219/24: -O-C(O)-R and/or -NR2 is bound to cycloalkyl or cycloalkenyl
C07C 219/26 - C07C 219/30: -O-C(O)-R bound to an aromatic ring and -NR2 not
C07C 219/32: -NR2 bound to an aromatic ring and -O-C(O)-R not
C07C 219/34: -NR2 and -O-C(O)-R are both bound to an aromatic ring.
This place covers:
The preparation, purification, separation and stabilisation of
- aminoketones (R2N-A-C(O)-C, A represents a hydrocarbon residue)
- aminoaldehydes (R2N-A-CHO, A represents a hydrocarbon residue)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 221/00, C07C 225/34 for the preparation of aminoanthraquinone). A combination set consists of a process group (e.g. C07C 221/00), followed by and linked to the group of the product (e.g. C07C 225/34). The products are selected from the corresponding product groups C07C 223/00 and C07C 225/00.
This place covers:
Amino aldehydes (R2N-A-CHO, A represents a hydrocarbon residue).
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 221/00.
This place covers:
Aminoketones R2N-A-C(O)-C, A represents a hydrocarbon residue)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 221/00.
Concerning certain (sub)groups:
C07C 225/02 - C07C 225/18: -NR2 is bound to an acyclic carbon atom
C07C 225/20: -NR2 is bound to cycloalkyl or cycloalkenyl
C07C 225/22: -NR2 is bound to an aromatic ring
C07C 225/24 - C07C 225/36: the carbon skeleton contains quinone rings
This place covers:
The preparation, purification, separation and stabilisation of
- amino acids (R2N-A-C(O)-OH, A represents a hydrocarbon residue)
- esters of amino acids (R2N-A-C(O)-OR, A and R represent hydrocarbon residues).
This place does not cover:
Preparation of amino acids or esters of amino acids in which the amino group is acyl protected e.g. by an acetyl group (Ac) or a benzoyl group (bz) | |
Preparation of amino acids or esters of amino acids in which the amino group is protected by a Cbz, Boc or Fmoc group | |
Preparation of carboxylic acids or esters of amino acids by microorganisms or enzymes e.g. by fermentation |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 227/40, C07C 229/26 for the purification of lysine). A combination set consists of a process group (e.g. C07C 227/40), followed by and linked to the group of the product (e.g. C07C 229/26). The products are selected from the corresponding product groups C07C 229/00.
Concerning certain (sub)groups:
C07C 227/22: -C(R)-C=N-OH → -C-C(O)-NHR
C07C 227/26: carboxylic acid + amine + HCN→ aminonitrile intermediate formed in situ -> hydrolysis to an amino acid or from aminonitriles
This place covers:
- amino acids (R2N-A-C(O)-OH, A represents a hydrocarbon residue)
- esters of amino acids (R2N-A-C(O)-OR, A represents a hydrocarbon residue)
This place does not cover:
Amino acids or esters of amino acids in which the amino group is acyl protected e.g. by an acetyl group (Ac) or a benzoyl group (bz) | |
Amino acids or esters of amino acids in which the amino group is protected by a Cbz, Boc or Fmoc group | |
Amino acids containing guanidine groups e.g. arginine | |
Sulfur containing amino acids, e.g. methionine | |
Heterocyclic amino acids, e.g. Proline, histidine |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 227/00.
Concerning certain (sub)groups:
C07C 229/02 - C07C 229/36: -NR2 and -COOR both bound to acyclic carbon atoms
C07C 229/34: the carbon skeleton contains an aromatic ring
C07C 229/38: -NR2 bound to acyclic carbon atom and -COOR to an aromatic ring
C07C 229/40 - C07C 229/44: -COOR bound to an acyclic carbon atom and -NH2 bound to an aromatic ring
C07C 229/46 - C07C 229/50: -NR2 and/or -COOR bound to a cycloalkyl or cycloalkenyl ring
C07C 229/52 - C07C 229/66: -NR2 and -COOR both bound to an aromatic ring
C07C 229/68 - C07C 229/74: -NR2 and -COOR both bound to an aromatic ring which is part of condensed ring system
C07C 229/76: metal complexes of amino acids
This place covers:
The preparation, separation, purification and stabilisation of carboxylic acid amides R-C(O)-NR2
This place does not cover:
Preparation of peptides | |
Preparation of polyamides | |
Preparation of carboxylic acid amides by microorganism or enzymes e.g. by fermentation |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 231/24, C07C 233/05 for the purification of acetamide). A combination set consists of a process group (e.g. C07C 231/24 : purification of amides), followed by and linked to the group of the product (e.g. C07C 233/05 : acetamide). The products are selected from the corresponding product groups C07C 233/00, C07C 235/00, C07C 237/00.
This place covers:
Carboxylic acid amides of the following type: A-C(O)-NR2 (A represents hydrogen or a hydrocarbon residue); in the case that A represents a hydrocarbon residue this is either unsubstituted or only substituted by halogen, nitro or nitroso groups
This place does not cover:
Polyamides |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 231/00.
Concerning certain (sub)groups:
C07C 233/56: X-C(O)-C(O)-NR2, X = halo, -OR or -NR2
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
This place covers:
Carboxylic acid amides of the following type: A-C(O)-NR2 in which the carbon skeleton of the acid part (residue A) is substituted by singly-bound oxygen atoms (-OH, -OR, -O-C(O)-R) or doubly-bound oxygen atoms (a keto group or CHO).
This place does not cover:
Polyamides |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 231/00.
Attention: a carboxyl group is not considered as a doubly-bound oxygen atom because a carboxyl group terminates the carbon skeleton (see carbon skeleton approach under general rules applicable for the whole subclass as defined under C07C), thus -O-C(O)-A-C(O)-NR2 (A represents a hydrocarbon residue) has to be classified in a subgroup of C07C 233/00 because the carbon of the carboxyl group attached to A does not belong to the carbon skeleton
The compound -C(O)-O-A-C(O)-NR2 (A represents a hydrocarbon residue) is classified in a subgroup of C07C 235/00 because in this case an esterified hydroxy group is attached to A
Concerning certain (sub)groups:
C07C 235/70 - C07C 235/84: attention: only keto groups and aldehyde groups are considered as doubly-bound oxygen but not carboxyl groups (see C07C 233/56)
C07C 235/70: R-C(O)-C(O)-NR2
C07C 235/80: R-C(O)-CH2-C(O)-NR2
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
This place covers:
carboxylic acid amides of the following type: A-C(O)-NR2 in which the carbon skeleton of the acid part (residue A) is substituted by amino groups
Peptides C07K: peptides are compounds containing at least two amino acid units, which are bound through at least one normal peptide link, including oligopeptides, polypeptides and proteins; a normal peptide link is a link between an alpha amino group of an amino acid and the carboxyl group - in position 1 - of another alpha amino acid
For example the following compounds (I) to (III) are peptides:
(I) HOOC-CHR-NH-[C(O)-CHR-NH]n-C(O)-CHR-NH2 with n>=0
(II) ROOC-CHR-NH-[C(O)-CHR-NH]n-C(O)-CHR-NH2 with n>=0
(III) H2NOC-CHR-NH-[C(O)-CHR-NH]n-C(O)-CHR-NH2 with n>=0
(R represents a hydrocarbon residue)
This place does not cover:
Polypeptides | |
Polyamides |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 231/00.
Concerning certain (sub)groups:
C07C 237/52: -A-C(O)-N(R)-C(O)-, A is substituted by singly-bound nitrogen
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
This place covers:
- N-halogenated amines (-C-NR-halo, R represents a hydrocarbon residue)
- N-halogenated amides (-C(O)-NR-halo, R represents a hydrocarbon residue)
- hydroxylamino compounds (-C-N-OH)
- ethers of hydroxylamino compounds (-C-N-OR, R represents a hydrocarbon residue)
- esters of hydroxylamino compounds (-C-N-O-C(O)-R, R represents a hydrocarbon residue)
and the preparation, purification, separation and stabilisation of N-halogenated amines, N-halogenated amides, hydroxylamino compounds, ethers of hydroxylamino compounds and esters of hydroxylamino compounds
This place does not cover:
Oximes (-C=N-OH or -C=N-OR) or their preparation, purification,separation or stabilisation | |
Hydroxamic acids (-C-C(O)-NH-OH) or their preparation, purification,separation or stabilisation | |
Unsubstituted hydroxylamine and its preparation, purification,separation or stabilisation |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for N-halogenated amines, N-halogenated amides, hydroxylamino compounds or ethers or esters thereof. In case the application relates to the preparation, purification, separation or stabilisation of N-halogenated amines, N-halogenated amides, hydroxylamino compounds or ethers or esters thereof, the product which is prepared, purified separated or stabilised is classified in the appropriate product subgroup.
This place covers:
the preparation, purification, separation or stabilisation of
- hydrazines (R2N-NR2) R is hydrogen or a hydrocarbon residue and at least one R is hydrocarbon residue
- hydrazides [R2N-NR-C(O)-R] R is hydrogen or a hydrocarbon residue
- triazanes (R2N-NR-NR2) R is hydrogen or a hydrocarbon residue and at least one R is hydrocarbon residue
This place does not cover:
Preparation, purification, separation or stabilisation of unsubstituted hydrazine |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 241/02, C07C 243/22 for the preparation of phenylhydrazine). A combination set consists of a process group (e.g. C07C 241/02 : preparation of hydrazines), followed by and linked to the group of the product (e.g. C07C 243/22 : phenylhydrazine). The products are selected from the corresponding product group C07C 243/00.
This place covers:
- N-nitro compounds(R2N-NO2)
- N-nitroso compounds(R2N-NO)
- hydrazines (R2N-NR2) R is hydrogen or a hydrocarbon residue and at least one R is hydrocarbon residue
- hydrazides [R2N-NR-C(O)-R] R is hydrogen or a hydrocarbon residue
- triazanes (R2N-NR-NR2) R is hydrogen or a hydrocarbon residue and at least one R is hydrocarbon residue
This place does not cover:
Unsubstituted hydrazine |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 241/00.
In this place, the following terms or expressions are used with the meaning indicated:
Quaternary ammonium compounds | the nitrogen atom of the amino group is bound to 4 carbon atoms |
This place covers:
- azo compounds (R-N=N-R)
- diazo compounds (=N2),
- diazonium compounds (=N2+)
- compounds with the group R-N=N-NR2
and the preparation, purification, separation or stabilisation of azo-, diazo-, diazonium compounds and of compounds with the group R-N=N-NR2
This place does not cover:
Azoxy compounds | |
Azo dyes and the preparation of azo dyes |
Attention is drawn to the following places, which may be of interest for search:
Azo dyes |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for azo-, diazo- and diazonium compounds. In case the application relates to the preparation, separation, purification or stabilisation of an azo-, diazo- or diazonium compound, the product which is prepared, separated, purified or stabilised is classified in the appropriate product subgroup.
Concerning certain (sub)groups:
C07C 245/02 - C07C 245/10: symmetrically or unsymmetrically substituted azo compounds (R-N=N-R)
C07C 245/12 - C07C 245/18: diazo compounds (=N2)
C07C 245/20 : diazonium compounds (=N2+)
C07C 245/22 - C07C 245/24: compounds R-N=N-NR2
This place covers:
azido compounds: R-N3 (R-N=N+=N-) and the preparation, purification, separation or stabilisation of azido compounds
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for azido compounds. In case the application relates to the preparation, separation, purification or stabilisation of an azido compound, the product which is prepared, separated, purified or stabilised is classified in the appropriate product subgroup.
This place covers:
the preparation, purification, separation and stabilisation of for example
- imines (-C=NR)
- oximes (-C=N-OR)
- hydrazones (-C=N-NR2)
This place does not cover:
Preparation, purification, separation and stabilisation of diazo compounds |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 1/24, C07C 11/04 for the purification of benzaldehyde oxime). A combination set consists of a process group (e.g. C07C 249/14 : purification of oximes), followed by and linked to the group of the product (e.g. C07C 251/48 : benzaldehyde oxime). The products are selected from the corresponding product groups C07C 251/00.
This place covers:
for example:
- imines (-C=NR)
- oximes (-C=N-OR)
- hydrazones (-C=N-NR2)
This place does not cover:
Diazo compounds |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 249/00.
Concerning certain (sub)groups:
C07C 251/02 - C07C 251/30: imines -C=NR
C07C 251/32 - C07C 251/70: oximes -C=N-OR
C07C 251/64: -C=N-O-C(O)-R
C07C 251/70: metal complexes
C07C 251/72 - C07C 251/88: hydrazones -C=N-NR2
C07C 251/88: -C=N-N=C-
This place covers:
the preparation, separation, purification and stabilisation of organic nitriles (-C-CN)
This place does not cover:
Preparation of cyanogen compounds (e.g. HCN, metal cyanides, cyanic acid, cyanamides) |
Attention is drawn to the following places, which may be of interest for search:
Ammoxidation catalysts |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 253/10, C07C 255/07 for the preparation of 3-pentenenitrile by addition of HCN to a carbon double bond). A combination set consists of a process group (e.g. C07C 253/10), followed by and linked to the group of the product (e.g. C07C 255/07). The products are selected from the corresponding product group C07C 255/00.
Concerning certain (sub)groups:
C07C 253/24: hydrocarbon + O2 + NH3
C07C 253/26: for example CH2=CH-CH3 + O2 + NH3
In patent documents, the following words/expressions are often used as synonyms:
- "Acrylonitrile", "methacrylonitrile" and "CH2=CH-CN, CH2=C(CH3)-CN"
- "Adiponitrile" and "NC-CH2-CH2-CH2-CH2-CN"
- "Cyanohydrin" and " (RR')C(OH)-CN"
This place covers:
Organic nitriles (C-CN)
This place does not cover:
Cyanogen compounds (e.g. HCN, metal cyanides, cyanic acid, cyanamides) |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 253/00.
Concerning certain (sub)groups:
C07C 255/01 - C07C 255/44: -C-CN, C= acyclic carbon atom
C07C 255/25: R-N(R')-CH2-CN
C07C 255/45 - C07C 255/48: Cy-CN, Cy = cycloalkyl or cycloalkenyl
C07C 255/49 - C07C 255/60: Ar-CN, Ar = aromatic ring
C07C 255/61: -C=N-A-CN, A = hydrocarbon residue
C07C 255/62: -C=N-O-A-CN, A = hydrocarbon residue
C07C 255/64: for example NC-C-C-N-OCH3
In patent documents, the following words/expressions are often used as synonyms:
- "Acrylonitrile", "methacrylonitrile" and "CH2=CH-CN, CH2=C(CH3)-CN"
- "Cyanohydrin" and "(RR')C(OH)-CN"
This place covers:
for example
- iminohalides (-C(=NR)-halo),
- iminoethers (-C(=NR)-OR)
- amidines (-C(=NR)-NR2
and the preparation, purification, separation and stabilization of iminohalides, iminoetheres and amidines
This place does not cover:
Imines and oximes or the preparation of imines and oximes |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for iminohalides, iminoethers and amidines. In case the application relates to the preparation, separation, purification or stabilization of an iminohalide, an iminoether or an amidine, the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
Concerning certain (sub)groups:
C07C 257/02: iminohalides (-C(=NR)-halo)
C07C 257/04 - C07C 257/08: iminoethers (-C(=NR)-OR)
C07C 257/10 - C07C 257/22: amidines (-C(=NR)-NR2)
C07C 257/20: -C(=NR)-NR-C(O)-
C07C 257/22: -C(=NR)-NR-NR2
This place covers:
for example
- hydroximic acid halogenides (R-O-N=C(X)-, X=halo)
- hydroxamic acid and derivatives (-C(O)-NR-O-)
- N-hydroxyamidine derivatives (R2N-C(=N-OR)-)
and the preparation, purification, separation and stabilisation of hydroximic acid halogenides, hydroxamic acid and of N-hydroxyamidines and derivatives thereof.
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for hydroximic acid halogenides, hydroxamic acid and of N-hydroxyamidines. In case the application relates to the preparation, separation, purification or stabilisation of a hydroximic acid halogenide, a hydroxamic acid (derivative) or a N-hydroxyamidines (derivative), the product which is prepared, separated, purified or stabilised is classified in the appropriate product subgroup.
Concerning certain (sub)groups:
C07C 259/02: hydroximic acid halogenides (R-O-N=C(X)-)
C07C 259/04 - C07C 259/10: hydroxamic acid and derivatives (-C(O)-NR-O-)
C07C 259/12 - C07C 259/20: N-hydroxyamidines derivatives (H2N-C(=N-OR)-)
C07C 259/20: -N-NH-C(=N-OR)-
This place covers:
For example
- cyanates (-C-O-CN; esters of cyanic acid HO-CN)
- cyanamides (-C-N(R)-CN; amides of cyanic acid HO-CN)
and the preparation, separation, purification and stabilisation of cyanates and cyanamides.
This place does not cover:
Unsubstituted cyanamide (H2N-CN) |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for cyanates and cyanamides. In case the application relates to the preparation, separation, purification or stabilisation of a cyanate or cyanamide, the product which is prepared, separated, purified or stabilised is classified in the appropriate product subgroup.
This place covers:
The preparation, purification, separation and stabilisation of isocyanates (R-N=C=O)
This place does not cover:
Preparation of unsubstituted isocyanic acid |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 263/10, C07C 265/14 for the preparation of hexamethylene diisocyanate by reaction of an hexamethylenediamine with phosgene). A combination set consists of a process group (e.g. C07C 263/10), followed by and linked to the group of the product (e.g. C07C 265/14). The products are selected from the corresponding product group C07C 265/00.
In patent documents, the following abbreviations are often used:
HDI | hexamethylene diisocyanate |
TDI | toluene diisocyanate |
MDI | methylenediphenyl diisocyanate |
This place covers:
Isocyanates (R-N=C=O)
This place does not cover:
Unsubstituted isocyanic acid |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 263/00.
In patent documents, the following abbreviations are often used:
HDI | hexamethylene diisocyanate |
TDI | toluene diisocyanate |
MDI | methylenediphenyl diisocyanate |
This place covers:
Carbodiimides (R-N=C=N-R) and the preparation, purification, separation and stabilization of carbodiimides
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group for carbodiimides. In case the application relates to the preparation, separation, purification or stabilization of carbodiimides, the product which is prepared, separated, purified or stabilised is classified in the product.
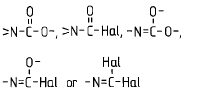 , the nitrogen atom not being part of nitro or nitroso groups
, the nitrogen atom not being part of nitro or nitroso groupsThis place covers:
The preparation, purification, separation and stabilisation of for example
- substituted carbamic acid and salts thereof
- carbamic acid halides
- carbamic acid esters (= urethanes)
This place does not cover:
Preparation of unsubstituted carbamic acid and salts thereof | |
Preparation of polyurethanes |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 269/04, C07C 271/22 for the preparation of tert.-butyloxycarbonyl (Boc) protected phenylglycine (Ph-CH(NH2)-COOH) from phenylglycine and di-tert.-butyl-dicarbonate). A combination set consists of a process group (e.g. C07C 269/04), followed by and linked to the group of the product (e.g. C07C 271/22). The products are selected from the corresponding product group C07C 271/00.
Cbz | carbobenzyloxy (Bn-O-C(O)-) |
BOC | tert.-butyloxycarbonyl |
FMOC | 9-fluorenylmethyloxycarbonyl |
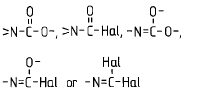 , the nitrogen atom not being part of nitro or nitroso groups
, the nitrogen atom not being part of nitro or nitroso groupsThis place covers:
For example
- substituted carbamic acid (R-N(R)-C(O)-OH and R-N=C(OR)-OH)
- carbamic acid halides (R-N(R)-C(O)-Hal, R-N=C(OR)-Hal and R-N=C(Hal)-Hal)
- carbamic acid esters (urethanes) (R-N(R)-C(O)-OR and R-N=C(OR)-OR)
This place does not cover:
Unsubstituted carbamic acid and salts thereof | |
Polyurethanes |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 269/00.
Concerning certain (sub)groups:
C07C 271/02: substituted carbamic acid (R-N(R)-C(O)-OH and R-N=C(OR)-OH)
C07C 271/04: carbamic acid halides (R-N(R)-C(O)-Hal, R-N=C(OR)-Hal and R-N=C(Hal)-Hal)
C07C 271/06 - C07C 271/58: carbamic acid esters (urethanes) (R'-N(R)-C(O)-OR and R'-N=C(OR)-OR)
C07C 271/08 - C07C 271/30: R'-N(R)-C(O)-O-C- and R'-N=C(OR)-O-C-, C = acyclic carbon atom
C07C 271/32 - C07C 271/38: R'-N(R)-C(O)-O-Cy and R'-N=C(OR)-O-Cy, Cy = cycloalkyl, cycloalkenyl)
C07C 271/40 - C07C 271/58: R'-N(R)-C(O)-O-Ar and R'-N=C(OR)-O-Ar, Ar = aromatic ring)
C07C 271/60: R'-N(R)-C(O)-O-N-
C07C 271/62 - C07C 271/66: compounds containing any of the groups: -O-C(O)-N(R)-C(=X)-Y, Hal-C(O)-N(R)-C(=X)-Y, -O-C(O)-N=C(-X)-Y, Hal-C(O)-N=C(-X)-Y with X = hetero atom and Y= any atom, e.g. N-acylcarbamates
C07C 271/68: compounds containing any of the groups -N=C(-OR)-O-, -N=C(-OR)-Hal, -N=C(Hal)-Hal
In patent documents, the following abbreviations are often used:
Cbz | carbobenzyloxy (Bn-O-C(O)-) |
BOC | tert.-butyloxycarbonyl |
FMOC | 9-fluorenylmethyloxycarbonyl |
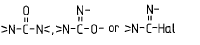 , the nitrogen atoms not being part of nitro or nitroso groups
, the nitrogen atoms not being part of nitro or nitroso groupsThis place covers:
The preparation, purification, separation and stabilisation of:
- urea and its salts, complexes and addition compounds
- substituted ureas
This place does not cover:
Preparation of polyureas |
Attention is drawn to the following places, which may be of interest for search:
Apparatuses |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 273/18, C07C 275/28 for the preparation of N,N'-diphenylurea). A combination set consists of a process group (e.g. C07C 273/18), followed by and linked to the group of the product (e.g. C07C 275/28). The products are selected from the corresponding product groups C07C 275/00. Note: There is not group under C07C 275/00 for urea itself (i.e. H2N-C(O)-NH2). The preparation, purification or stabilisation of urea itself is classified only in the corresponding process group.
Concerning certain (sub)groups:
C07C 273/02 - C07C 273/16: preparation, purification, separation and stabilisation of unsubstituted urea
C07C 273/08: ammonial liquor from the dry distillation of coke
C07C 273/18: preparation, purification, separation and stabilisation of
substituted ureas and isoureas
Melamine | 1,3,5-triazine-2,4,6-triamine |
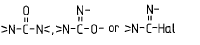 , the nitrogen atoms not being part of nitro or nitroso groups
, the nitrogen atoms not being part of nitro or nitroso groupsThis place covers:
For example
- salts, complexes and addition compounds of urea H2N-C(O)-NH2
- substituted ureas (R2N-C(O)-NR2), R represents hydrogen or a hydrocarbon residue and at least one R is a hydrocarbon residue
- acyl ureas [-N-C(O)-N-C(=X)-Y or -N-C(O)-N=C(XY)- with X being a hetero atom and Y being any atom]
- isoureas -N=C(NRR')-O- or a compound -N=C(NRR')-Hal
This place does not cover:
Polyureas |
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 273/00.
Concerning certain (sub)groups:
C07C 275/42: a nitrile group is also considered as a carboxyl group
C07C 275/70: isoureas -N=C(NRR')-O- or a compound -N=C(NRR')-Hal
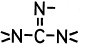 , the singly-bound nitrogen atoms not being part of nitro or nitroso groups
, the singly-bound nitrogen atoms not being part of nitro or nitroso groupsThis place covers:
The preparation, purification or separation of guanidine (H2N-C(=NH)-NH2) and substituted guanidines (R2N-C(=NR)-NR2)
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 277/08, C07C 279/04 for the preparation of methylguanidine hydrochloride). A combination set consists of a process group (e.g. C07C 277/08), followed by and linked to the group of the product (e.g. C07C 279/04). The products are selected from the corresponding product group C07C 279/00.
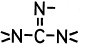 , the singly-bound nitrogen atoms not being part of nitro or nitroso groups
, the singly-bound nitrogen atoms not being part of nitro or nitroso groupsThis place covers:
- guanidine (H2N-C(=NH)-NH2) and salts, complexes or addition compounds of guanidine
- derivatives of guanidine (R2N-C(=NR)-NR2), for example:
- acylated guanidines (R2N-C(=NR)-NR-C(O)-R)
- biguanidines (R2N-C(=NR)-NR-C(=NR)-NR2)
- cyanoguanidines (R2N-C(=NR)-NR-CN)
- optionally substituted N-nitroso and N-nitroguanidines: R2N-C(=NR)-NR-NO and R2N-C(=NR)-NR-NO2
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 277/00.
Concerning certain (sub)groups:
C07C 279/02: guanidine (H2N-C(=N)-NH2) and salts, complexes or addition compounds of guanidine
C07C 279/04 - C07C 279/18: substituted guanidines (R2N-C(=NR)-NR2)
C07C 279/20 - C07C 279/26: compounds containing any of the groups: R2N-C(=NR)-NR-C(=X)-Y, R2N-C(=NR)-N=C(-X)-Y and R2N-C(-NR2)=N-C(=X)-Y, X = hetero atom and Y= any atom
C07C 279/22: acylated guanidines (R2N-C(=NR)-NR-C(O)-R)
C07C 279/26: biguanides (R2N-C(=NR)-NR-C(O)-R)
C07C 279/28: for example (R2N-C(=NR)-NR-CN)
C07C 279/30 - C07C 279/36: for example optionally substituted N-nitroso andN-nitroguanidines: R2N-C(=NR)-NR-NO and R2N-C(=NR)-NR-NO2
This place covers:
- compounds R2N-NR-C(O)-OR or R2N-N=C(OR)-OR, e.g.carbazates
- compounds R2N-NR-C(O)-NR2 or R2N-N=C(-OR)-NR2 or R2N-NR C(OR)=NR, e.g. semicarbazides
- compounds R2N-NR-C(=NR)-NR2 or R2N-N=C(-NR2)-NR2, e.g. aminoguanidine
- and the preparation, purification, separation and stabilization of these compounds
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. In case the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
Concerning certain (sub)groups:
C07C 281/02 - C07C 281/04: compounds R2N-NR-C(O)-OR or R2N-N=C(OR)- OR, e.g.carbazates
C07C 281/06 - C07C 281/14: compounds R2N-NR-C(O)-NR2 or R2N-N=C(-OR)-NR2 or R2N-NR-C(OR)=NR, e.g. semicarbazides
C07C 281/16 - C07C 281/18: compounds R2N-NR-C(=NR)-NR2 or R2N-N=C(-NR2)-NR2, e.g. aminoguanidine
C07C 281/20: e.g. azoformamide HN=N-C(=O)-NH2 and its derivatives
This place covers:
Various N-compounds and their preparation, purification or stabilisation.
The last place rule and the carbon skeleton approach applies. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. In case the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
Concerning certain (sub)groups:
C07C 291/02: e.g. nitrones R2C=NR'+-O-, R' does not represent hydrogen
C07C 291/04: e.g. R3N+-O-
C07C 291/06: R3C-CN+-O-
C07C 291/08: RN=N(->O)-R
C07C 291/10: R-N+-triple bond-C-
C07C 291/12: -O-N+-triple bond-C- (fulminate ion), this group is rarely used (empty group)
C07C 291/14: this group is rarely used (empty group)
This place covers:
Acyclic and carbocyclic organic mono- and diesters of sulfurous acid (RO−SO−OH and RO−SO−OR) as well as the preparation, purification, separation and stabilization of these compounds
This place does not cover:
Sulfurous acid and its preparation, purification, separation and stabilization | |
Cyclic esters |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Preparation, purification and stabilisation of esters and amides of sulfuric acid as well as sulfonic acids and their esters, halides, anhydrides and amides
This place does not cover:
The preparation, purification, separation and stabilization of sulfuric acid | |
The preparation, purification, separation and stabilization of sulfuric acid mono- and diamides | |
The preparation of "overbased" sulfonate derivatives |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 303/44, C07C 309/29 for the purification of benzenesulfonic acid). A combination set consists of a process group (e.g. C07C 303/44), followed by and linked to the group of the product (e.g. C07C 309/29). The products are selected from the corresponding product groups C07C 305/00 - C07C 311/00.
This place covers:
Acyclic and carbocyclic organic mono- and diesters of sulfuric acid (RO−SO2−OH and RO−SO2−OR)
This place does not cover:
Sulfuric acid | |
Cyclic esters |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 303/00.
This place covers:
Acyclic and carbocyclic organic mono- and diamides of sulfuric acid (RO−SO2−NR2 R2N−SO2−NR2 where each R is hydrogen or organic group, but not all hydrogen)
This place does not cover:
Sulfuric acid mono- and diamides |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 303/00.
This place covers:
Acyclic and carbocyclic organic sulfonic acids as well as their halides, esters and anhydrides (RO−SO−OH and RO−SO−OR)
This place does not cover:
"Overbased" sulfonate derivatives |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 303/00.
This place covers:
Acyclic and carbocyclic organic amides of sulfonic acids (RO−SO2−NR2 and R2N−SO2−NR2)
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 303/00.
This place covers:
Acyclic and carbocyclic organic sulfinic acids and sulfenic acids as well as their halides, esters, anhydrides and amides (RSO−OH, RSO−X, RSO−OR, RSO−NR2, RSO−O−SOR, RS−OH, RS−X, RS−OR, RS−O−SR and RS−NR2)
as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Preparation, purification and stabilisation of sulfones and sulfoxides
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 315/06, C07C 317/14 for the purification of diphenylsulfone). A combination set consists of a process group (e.g. C07C 315/06: purification of sulfones), followed by and linked to the group of the product (e.g. C07C 317/14 : e.g. diphenylsulfone). The products are selected from the corresponding product group C07C 317/00.
This place covers:
Acyclic and carbocyclic organic sulfones and sulfoxides (R−SO2−R and R−SO−R)
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 315/00.
This place covers:
Preparation, purification and stabilisation of thiols, sulfides, hydropolysulfides and polysulfides.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 319/28, C07C 321/30 for the purification of diphenylsulfide). A combination set consists of a process group (e.g. C07C 319/28 : purification of sulfides), followed by and linked to the group of the product (e.g. C07C 321/30 : e.g. diphenylsulfide). The products are selected from the corresponding product groups C07C 321/00 and C07C 323/00.
This place covers:
Acyclic and carbocyclic organic thiols, sulfides, hydropolysulfides and polysulfides (R−SH, R−S−R, R−Sn−H and R−Sn−R) not containing other heteroatoms
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 319/00.
This place covers:
Acyclic and carbocyclic organic thiols, sulfides, hydropolysulfides and polysulfides (R−SH, R−S−R, R−Sn−H and R−Sn−R) substituted by halogen, oxygen or nitrogen atoms or by other sulfur-containing groups
This place does not cover:
Thiols, sulfides, hydropolysulfides and polysulfides not containing other heteroatoms |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 319/00.
Concerning certain subgroups:
C07C 323/60: "the carbon atom of at least one of the carboxyl groups bound to a nitrogen atom" refers to groups such as –CONH2 and –CN.
This place covers:
Acyclic and carbocyclic organic thioaldehydes, thioketones and thioquinones as well as their oxides (R−CHS, R−CS−R, R−CHSOn and R−CSOn−R)
as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Acyclic and carbocyclic organic mono- and dithiocarboxylic acids as well as their esters and amides (RCO−SH, RCS−SH, RCO−SR, RCS−SR and RCS−NR2)
as well as the preparation, purification, separation and stabilization of these compounds
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Acyclic and carbocyclic organic mono-, di- and trithiocarbonic acids as well as their halides, esters and anhydrides,
as well as the preparation, purification, separation and stabilization of these compounds.
This place does not cover:
Mono-, di- and trithiocarbonic acid and their preparation, purification, separation and stabilization |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Acyclic and carbocyclic organic thiocyanic acid and isothiocyanic acid derivatives (R−S−CN and RN=C=S)
as well as the preparation, purification, separation and stabilization of these compounds.
This place does not cover:
Thiocyanic acid and isothiocyanic acid and their preparation, purification, separation and stabilization |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
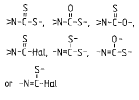 , the nitrogen atom not being part of nitro or nitroso groups
, the nitrogen atom not being part of nitro or nitroso groupsThis place covers:
Acyclic and carbocyclic organic mono- and dithiocarbamic acids well as their esters, as well as the preparation, purification, separation and stabilization of these compounds.
This place does not cover:
Mono- and dithiocarbamic acid and their preparation, purification, separation and stabilization |
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
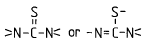 , the nitrogen atoms not being part of nitro or nitroso groups
, the nitrogen atoms not being part of nitro or nitroso groupsThis place covers:
Acyclic and carbocyclic organic thiourea and isothiourea derivatives
as well as the preparation, purification, separation and stabilization of these compounds
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Acyclic and carbocyclic organic thiocarbamic acid, thiourea and isothiourea derivatives in which at least one nitrogen atom is further bound to a nitrogen atom, particularly thiocarbazides, thiosemicarbazides and thiosemicarbazones as well as compounds in which the two nitrogen atoms are doubly bound to each other as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Other acyclic and carbocyclic organic compounds containing sulfur, particularly thiosulfates, thiosulfonates, compounds containing sulfur bound only to two nitrogen atoms, compounds containing sulfur doubly bound to nitrogen atoms, sulfonium compounds and compounds having a carbon atom having bonds only to heteroatoms with a double bond to a sulfur atom and at least one bond to a sulfur atom further doubly bound to oxygen atoms () as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
Concerning certain subgroups:
C07C 381/12: Compounds having a charged sulfonium ion and zwitterionic compounds are classified in this subgroup. Sulfur ylide compounds are classified in C07C 381/00 (>S+−-C<) or C07C 381/10 (>S+−N-−).
This place covers:
Acyclic and carbocyclic organic compounds containing selenium as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Acyclic and carbocyclic organic compounds containing tellurium as well as the preparation, purification, separation and stabilization of these compounds.
The last place rule and the carbon skeleton approach apply. General rules applicable for the whole subclass are defined under C07C.
There is no specific preparation group. Where the application relates to the preparation, separation, purification or stabilization of a compound as defined under the definition statement the product which is prepared, separated, purified or stabilized is classified in the appropriate product subgroup.
This place covers:
Vitamin D derivatives and the preparation thereof.
The compounds usually have a partially or completely hydrogenated indane ring which is connected to a cyclohexane ring via an unsaturated alkylene chain. Chemically, the various forms of vitamin D are secosteroids, i.e. steroids in which one of the bonds in the steroid rings is broken.
Example: Ergocalciferol (Vitamin D2)
This place does not cover:
Heterocyclic derivatives of vitamin D | |
Vitamin D derivatives containing elements other than C, H, Hal, O, N, S, Se and Te, e.g. Si, B or P | |
Vitamins of unknown constitution | |
Steroids |
Attention is drawn to the following places, which may be of interest for search:
Food or foodstuffs | |
Cosmetics | |
Compounds for use in medicine/therapy; pharmaceutical compositions |
The last place rule and the whole-molecule-approach apply. General rules applicable for the whole subclass are defined under C07C.
In addition to the compound group itself, Indexing Codes for the two ring systems are given, i.e. C07C 2601/14 for the cyclohexane ring and C07C 2602/24 for the completely or partially hydrogenated indane ring system. If the cyclohexane ring merely contains "exo" double bonds on the cyclohexane ring, the ring is considered saturated (a cyclohexene or cyclohexadiene ring having a double bond between two ring carbon atoms would get the Indexing Code C07C 2601/16).
The carbon atoms in the skeleton are numbered according to the parent steroid as follows:
In this place, the following terms or expressions are used with the meaning indicated:
Didehydro | an additional double bond |
Dihydro | reduction of a double bond |
Homo | an additional methylene group |
Nor | loss of a methylene |
In patent documents, the following words/expressions are often used as synonyms:
- "Ergocalciferol", "ercalciol" and "vitamin D2"
- "Cholecalciferol", "colecalciferol", "calciol" and "vitamin D3"
- "1,25-dihydroxycholecalciferol" and "Calcitriol"
This place covers:
Compounds having a non-aromatic 6-membered ring substituted by an unsaturated side-chain of at least 4 carbon atoms, and the preparation, purification or stabilisation thereof.
This place does not cover:
Vitamins of unknown constitution |
Attention is drawn to the following places, which may be of interest for search:
Compounds classified in C07C 403/24 are often additionally classified in the following fields: Food chemistry | |
Cosmetic preparations containing vitamins | |
Compounds for use in medicine/therapy; pharmaceutical compositions | |
Vitamins of unknown constitution | |
Ionones, damascones and damascenones, i.e. compounds having a keto functional group in the side chain which are classified in C07C 403/14, are often also classified in the following field: Perfumes |
Cyclohexane, cyclohexene or cyclohexadiene derivatives having a saturated side-chain, or having a side-chain with less than four carbon atoms are not classified here.
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
In addition to the classification in C07C 403/00, an Indexing Code for the ring is given:
C07C 2601/14 if the 6-membered ring is saturated;
C07C 2601/16 if the 6-membered ring is a cyclohexene or cyclohexadiene ring.
C07C 403/04 - C07C 403/22: substitution on the ring(s) is disregarded, i.e. compounds (or preparation thereof) substituted on the ring itself, but unsubstituted on the unsaturated side chain(s) are classified in C07C 403/02.
C07C 403/24: Compounds having two non-aromatic 6-membered rings connected via an unsaturated alkylene chain of at least 4 carbon atoms (usually 18 carbon atoms). The most common compounds classified in this group are: β-carotene, lutein, zeaxanthin, astaxanthin
This place covers:
Prostaglandins and preparation thereof.
Example:
Derivatives or analogues
- wherein the 5-membered ring is replaced by other rings or ring systems,
- wherein the carboxyl group in one of the side-chains is replaced by other functional groups, or
- wherein the oxygen attached to the ring in ortho-position of one of the side-chains is replaced by a different atom such as chlorine,
are also classified under this main group.
This place does not cover:
Prostaglandin derivatives containing a heterocyclic ring | |
Prostaglandin derivatives containing elements other than C, H, Hal, O, N, S, Se and Te, e.g. Si, B or P | |
Preparation of prostaglandins by microorganisms or enzymes |
Attention is drawn to the following places, which may be of interest for search:
Prostaglandin derivatives for use in therapy/ as a medicament, and pharmaceutical compositions |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
In addition to the classification in C07C 405/00, an Indexing Code for the ring is given, usually either C07C 2601/08 (if the 5-membered ring is saturated) or C07C 2601/10 (if the 5-membered ring is unsaturated).
If the 5-membered ring is replaced by another ring or ring system, the corresponding Indexing Code is given for said ring or ring system.
The formulation "analogues and derivatives thereof" renders the actual definition of this subclass somewhat vague. In cases of doubt, a "normal" C07C group is given in addition to the group C07C 405/00 and subgroups thereof.
A common example for such a "grey zone" is where a benzene ring interrupts the second side-chain, i.e. in said side chain, there is no oxygen atom attached in gamma-position to the ring.
This place covers:
The preparation, separation, purification and stabilization of organic compounds containing an oxygen-oxygen single bond.
This place does not cover:
Inorganic peroxides and preparation thereof |
Attention is drawn to the following places, which may be of interest for search:
Biocides containing peroxyacid derivatives | |
Polymerisation catalysts containing peroxides | |
Use of organic peroxides as compounding ingredients not provided for in specific use fields | |
Detergent compositions containing peroxides | |
Bleaching fibres, threads, yarns, fabrics, feathers, or made-up fibrous goods, leather, or furs using compounds which develop oxygen |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Combination sets are used to indicate the product of a preparation, purification or stabilisation process (e.g. C07C 407/00, C07C 409/10 for the preparation of cumene hydroperoxide). A combination set consists of a process group (e.g. C07C 407/00), followed by and linked to the group of the product (e.g. C07C 409/10). The products are selected from the corresponding product groups C07C 409/00.
Please note: Not all documents concerning the preparation of peroxy compounds are already classified using combination sets. Some documents relating to the preparation of peroxy compounds are classified in the product group C07C 409/00 and subgroups exclusively. While these documents are being reclassified, the search for a preparation of peroxy compounds has to be carried out in both the product group and the combination set.
This place covers:
Organic compounds containing an oxygen-oxygen single bond.
This place does not cover:
Inorganic peroxides, e.g. H2O2 |
Mixtures defined as containing an organic peroxide and at least one further component are classified in the use fields.
Attention is drawn to the following places, which may be of interest for search:
Biocides containing peroxyacid derivatives | |
Polymerisation catalysts containing peroxides | |
Use of organic peroxides as compounding ingredients not provided for in specific use fields | |
Detergent compositions containing peroxides | |
Bleaching fibres, threads, yarns, fabrics, feathers, or made-up fibrous goods, leather, or furs using compounds which develop oxygen |
The last place rule and the whole molecule approach apply. General rules applicable for the whole subclass are defined under C07C.
Only compounds claimed as such are classified in this group. Products of preparation, purification or stabilisation processes are classified in combination with the process as combination sets following the special rules of C07C 407/00. Please note: In the past, the preparation of peroxy compounds was classified in the product groups C07C 409/00 and subgroups exclusively. While these documents are continuously being reclassified into combination sets, the product groups cover both products claimed as novel and processes for the preparation of novel or known compounds.
C07C 409/32: In this subgroup, acylperoxycarbonates and peroxydicarbonates are classified, i.e. compounds having one or two <C=O groups belonging to a carbonic acid/ester.
R-C(=O)-O-O-C(=O)-OR' acylperoxycarbonate
RO-C(=O)-O-O-C(=O)-OR' peroxydicarbonate
C07C 409/34: In this subgroup, diacylperoxides are classified.
R-C(=O)-O-O-C(=O)-R' diacylperoxide
CH3-C(=O)-O-O-C(=O)-CH3 diacetyl peroxide
C07C 409/38: In this subgroup, monoperoxycarbonates and diperoxycarbonates are classified.
R-O-C(=O)-O-O-R' monoperoxycarbonate
R-O-O-C(=O)-O-O-R' diperoxycarbonate
This place covers:
Catalysts comprising Mg, B, Al, C, Si, Ti, Zr or Hf in the form of the element, oxide or hyroxide used in a process classified in C07C 1/00 - C07C 6/126.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
The Indexing Codes C07C 2521/00 - C07C 2531/38 are given to describe the catalysts used in processes classified in C07C 1/00 - C07C 6/126, i.e. they are used in connection with processes for the preparation of hydrocarbons exclusively.
Please note: No catalyst Indexing Codes C07C 2521/00 - C07C 2531/38 are used in combination with processes for the purification, separation or stabilization classified in C07C 7/00 and subgroups thereof.
The Indexing Codes for catalysts are an indexing system. This implies that each component receives a code. The concept "catalyst" covers the whole catalytic system (i.e. active component, support, activator, etc.).
Example: Palladium on carbon (Pd/C) receives the Indexing Codes C07C 2521/18 for the carbon support and C07C 2523/44 for the catalytically active component Pd.
Each code is used according to its meaning: if in a document the catalyst is defined as "a metal of group 6" without further specification, then the code C07C 2523/24 is given.
If one of the catalyst components can be selected from Cr, Mo or W, then the codes C07C 2523/26, C07C 2523/28 and C07C 2523/30 are given.
An unspecified metal of group 8 receives the codes C07C 2523/40 and C07C 2523/74.
Components like molybdates are indexed as the oxide of molybdenum and as the cation (e.g. sodium molybdate receives the codes C07C 2523/04 and C07C 2523/28).
However, salts provided for in C07C 2527/00 are only coded as the anion part of the salt, i.e. the metal cation does not receive a code.
Example: NaCl receives only the code C07C 2527/10 for the chloride part of the salt.
Acids are coded as the "hydrogen salt" unless there is an indication to the contrary.
Example: HBr receives the code C07C 2527/08.
Codes for combination of metals (e.g. C07C 2523/76) are only given if such a combination is always present in the catalyst, not if its presence results from the selection of each of the components from a list of possible components: if the catalyst comprises Fe and Mo then the code is C07C 2523/881, but if the catalyst comprises a first component selected from Mn, Ca, Fe and a second component selected from V, Th, Mo, then all the possible codes for the first component and all the possible codes for the second component are given.
Raney metals are covered by C07C 2525/00 (not: C07C 2523/00 and subgroups).
C07C 2521/04: this code covers also bauxite
C07C 2521/12: this code encompasses also silicoaluminates even if they are crystalline. Zeolites, however, are classified under C07C 2529/00 and subgroups thereof.
C07C 2521/14: this code covers also sepiolite
C07C 2521/16: for example bentonite, kaolin
C07C 2521/18: for example activated carbon, graphite
This place covers:
Catalysts comprising metals other than Mg, B, Al, C, Si, Ti, Zr or Hf in the form of the element, oxide or hydroxide used in a process classified in C07C 1/00 - C07C 6/126.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
General rules for the use of catalyst Indexing Codes are described under C07C 2521/00.
Please note: Raney-type catalysts are classified under C07C 2525/00.
C07C 2523/10: rare earths = Sc, Y, lanthanides (elements with atomic numbers 57 to 71 inclusive)
C07C 2523/12: actinides = elements with atomic numbers 89 to 103 inclusive
C07C 2523/54 - C07C 2523/68: These Indexing Codes are given for catalysts containing a noble metal in combination with at least one further metal (or oxide or hydroxide thereof) as defined under C07C 2523/02 - C07C 2523/36.
Additionally, the Indexing Codes for the single components in the catalyst composition are given.
C07C 2523/76 - C07C 2523/889: These Indexing Codes are given for catalysts containing an iron group metal or copper in combination with at least one further metal (or oxide or hydroxide thereof) as defined under C07C 2523/02 - C07C 2523/36.
Additionally, the Indexing Codes for the single components in the catalyst composition are given.
C07C 2523/89: This Indexing Code is given for catalysts containing an iron group metal or copper in combination with noble metals.
Additionally, the Indexing Codes for the single components in the catalyst composition are given.
This place covers:
Raney catalysts used in a process classified in C07C 1/00 - C07C 6/126.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
The most common example is Raney nickel.
These catalysts are sometimes also called sponge catalysts.
General rules for the use of catalyst Indexing Codes are described under C07C 2521/00.
This place covers:
Inorganic catalysts comprising non-metals other than B and Si or inorganic carbon compounds other than elemental carbon used in a process classified in C07C 1/00 - C07C 6/126.
Inorganic acids used as catalysts are also classified here.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
General rules for the use of catalyst Indexing Codes are described under C07C 2521/00.
Please note: Organic compounds, organo-metallic compounds, metal hydrides or coordination complexes used as catalysts are classified under C07C 2531/00 and subgroups thereof.
C07C 2527/03: for example HSO3Cl, NaSO3Cl, i.e. inorganic halosulfonic acids only
C07C 2527/06: "Compounds thereof" means inorganic halogen compounds only. The elements (e.g. chlorine) are also classified under this code.
C07C 2527/10: The element chlorine is classified in C07C 2527/06.
C07C 2527/12: for example AgBF4
C07C 2527/1213: BF3 only; the salts BF4- are classified in C07C 2527/12
C07C 2527/122: for example copper chlorate or CuCl2
C07C 2527/125: for example sodium chloroaluminate (NaAlCl4)
C07C 2527/126: AlCl3 only; the salts AlCl4- are classified in C07C 2527/125
C07C 2527/13: for example PtCl2
C07C 2527/14: "Compounds thereof" means inorganic phosphorus compounds only.
C07C 2527/18: Inorganic oxygen-containing phosphorus derivatives other than phosphates are classified under this code.
C07C 2527/182: compounds containing phosphorus and silicon in one compound
C07C 2527/185: compounds containing phosphorus and iron group metals or platinum group metals in one compound
C07C 2527/20: inorganic carbon compounds only; elemental carbon (e.g. graphite, activated carbon) is classified under the code C07C 2521/18
C07C 2527/236: e.g. M(OH)(CO3)
C07C 2527/24: inorganic nitrogen compounds only
C07C 2527/26: inorganic cyanides only
Attention is drawn to the following places, which may be of interest for search:
Phosphates or other compounds comprising the anion (PnO3n+1)(n+2)- |
This place covers:
Molecular sieve catalysts used in a process classified in C07C 1/00 - C07C 6/126. Zeolites are the most common example.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
Zeolites claimed per se are also classified in C01B 39/00 and subgroups thereof.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
General rules for the use of catalyst Indexing Codes are described under C07C 2521/00.
C07C 2529/06 - C07C 2529/076: Zeolite catalysts for which no specific structure is indicated are classified under these Indexing Codes.
C07C 2529/70 - C07C 2529/78: Zeolite catalysts for which a specific structure is indicated which does not fall within the specific structures in groups C07C 2529/08 - C07C 2529/69 are classified under these Indexing Codes.
Example: zeolite beta
C07C 2529/80: If a mixture of zeolites is used as the catalyst or catalyst system, the Indexing Code C07C 2529/80 is given in addition to the specific Indexing Codes for the components of the mixture.
C07C 2529/83: aluminophosphates (APO compounds) are also called ALPO compounds
This place covers:
Metal hydrides, inorganic or organic coordination complexes, organic compounds or organo-metallic compounds used as catalysts in a process classified in C07C 1/00 - C07C 6/126.
Catalysts claimed per se are also classified in B01J 21/00 - B01J 49/90.
These groups or corresponding Indexing Codes B01J are often given also if the catalyst itself is not claimed, but preparation examples for the catalyst are described, or if the process is mainly characterized by the specific catalyst used. Consequently, a document should be forwarded for classification in the field of B01J 21/00 - B01J 49/90 also if preparation examples for catalysts are disclosed, or if the catalyst used in the process is of particular importance.
General rules for the use of catalyst Indexing Codes are described under C07C 2521/00.
C07C 2531/025: sulfonic acid salts are also encompassed, for example CuOTf (TfOH = trifluorosulfonic acid)
C07C 2531/12: for example tetraalkyl tin; NaH
C07C 2531/14: for example trimethylaluminium; methylaluminoxane (MAO); triisobutylaluminoxane (TiBAO)
C07C 2531/18: Inorganic coordination complexes, for example metal complexes containing nitroso (NO) ligands, or complexes having ligands containing the elements As, Sb or P other than phosphanes are classified under this code.
C07C 2531/22: Schrock carbenes commonly used as metathesis catalysts are for example classified under this code provided that they do not contain phosphine ligands (phosphine complexes such as the Grubbs' catalysts get the Indexing Code C07C 2531/24).
This place covers:
Indexing Codes for carbocyclic non-condensed rings, e.g.
- cyclopropyl rings : C07C 2601/02
- cyclohexyl rings : C07C 2601/14
- cyclohexenyl rings : C07C 2601/16.
Attention is drawn to the following places, which may be of interest for search:
Carbocyclic ring systems containing two condensed rings | |
Carbocyclic ring systems containing at least three condensed rings | |
Fullerene ring systems |
Phenyl rings are not indexed.
Only carbocyclic ring systems of compounds classified in subclass C07C as claimed compounds or as products of claimed production / purification / stabilisation processes are indexed. See the special rules of classification within the subclass C07C.
Please note: The indexing of compounds classified in this subclass is not yet complete. Missing codes are continuously being added.
Exocyclic double bonds do not count as unsaturation of the ring. Rings having only exocyclic double bonds are indexed as saturated rings.
Concerning certain subgroups :
C07C 2601/08 : e.g. cyclopentyl rings, but also ![]()
C07C 2601/10 : e.g. cyclopentenyl rings, cyclopentadienyl rings
C07C 2601/14 : e.g. cyclohexyl rings, but also ![]()
C07C 2601/16 : e.g. cyclohexenyl rings, cyclohexadienyl rings (phenyl rings are not indexed)
This place covers:
Indexing Codes for carbocyclic ring systems containing two condensed rings, e.g.
- pentalene, i.e.
 : C07C 2602/22
: C07C 2602/22 - bicyclo[2.2.1] rings, i.e.
 , as in (nor)bornan or campher : C07C 2602/42.
, as in (nor)bornan or campher : C07C 2602/42.
Attention is drawn to the following places, which may be of interest for search:
Carbocyclic non-condensed rings | |
Carbocyclic ring systems containing at least three condensed rings | |
Fullerene ring systems |
Aromatic naphthyl rings are not indexed and are not classified in this group.
Only carbocyclic ring systems of compounds classified in subclass C07C as claimed compounds or as products of claimed production / purification / stabilisation processes are indexed. See the special rules of classification within the subclass C07C.
Please note: The indexing of compounds classified in this subclass is not yet complete. Missing codes are continuously being added.
Concerning certain subgroups:
C07C 2602/08 : e.g. ![]() indane (non-aromatic hydroindanes are indexed with C07C 2602/24)
indane (non-aromatic hydroindanes are indexed with C07C 2602/24)
C07C 2602/10 : e.g. ![]() tetraline (non-aromatic [4.4.0] rings are indexed with C07C 2602/28; naphthalene rings are not indexed)
tetraline (non-aromatic [4.4.0] rings are indexed with C07C 2602/28; naphthalene rings are not indexed)
C07C 2602/18 : ![]() or
or ![]()
C07C 2602/20 : ![]() or
or ![]()
C07C 2602/22 : e.g. ![]() pentalene
pentalene
C07C 2602/24 : e.g. ![]() perhydroindane (indane is indexed with C07C 2602/08)
perhydroindane (indane is indexed with C07C 2602/08)
C07C 2602/28 : e.g. ![]() (tetraline is indexed with C07C 2602/10)
(tetraline is indexed with C07C 2602/10)
C07C 2602/38 : ![]() bicyclo[1.1.1]
bicyclo[1.1.1]
C07C 2602/40 : ![]() bicyclo[2.1.1]
bicyclo[2.1.1]
C07C 2602/42 : e. g. ![]() bicyclo[2.2.1] as in (nor)bornan, campher
bicyclo[2.2.1] as in (nor)bornan, campher
C07C 2602/50 : e. g. ![]()
This place covers:
Indexing Codes for carbocyclic ring systems containing at least three condensed rings, e.g.
- (hydrogenated) anthracene rings, i.e.
 : C07C 2603/24
: C07C 2603/24 - (hydrogenated) dicyclopentadienes, i.e.
 : C07C 2603/68
: C07C 2603/68 - adamantyl rings, i.e.
 : C07C 2603/74.
: C07C 2603/74.
Attention is drawn to the following places, which may be of interest for search:
Carbocyclic non-condensed rings | |
Carbocyclic ring systems containing two condensed rings | |
Fullerene ring systems |
Only carbocyclic ring systems of compounds classified in subclass C07C as claimed compounds or as products of claimed production / purification / stabilisation processes are indexed. See the special rules of classification within the subclass C07C.
Please note: The indexing of compounds classified in this subclass is not yet complete. Missing codes are continuously being added.
Concerning certain subgroups :
C07C 2603/32 : e.g. ![]()
C07C 2603/34 : e. g. ![]()
C07C 2603/46 : e.g. tetracyclines ![]()
C07C 2603/68 : ![]() tricyclo[5.2.1.02,6]dec(en)yl-structure
tricyclo[5.2.1.02,6]dec(en)yl-structure
C07C 2603/72 : e. g.![]()
C07C 2603/74 : ![]() (diadamantanes are indexed with C07C 2603/90)
(diadamantanes are indexed with C07C 2603/90)
C07C 2603/82 : tricyclo[5.4.3.01,8] ring structure ![]() ≡
≡ ![]() , e.g. as in pleuromutiline
, e.g. as in pleuromutiline
C07C 2603/88 : e.g.![]()
C07C 2603/90 : e.g. diadamantanes ![]()
C07C 2603/92 : e.g. cyclophanes ![]()
C07C 2603/94 : e.g. ![]()
C07C 2603/97 : e.g. ![]()
This place covers:
Indexing Codes for fullerene ring systems, e.g. C60 (buckminsterfullerene) or C70, in organic compounds classified in C07C
This place does not cover:
Fullerenes as such (consisting only of carbon) |
Attention is drawn to the following places, which may be of interest for search:
Carbocyclic non-condensed rings | |
Carbocyclic ring systems containing two condensed rings | |
Carbocyclic ring systems containing at least three condensed rings |
Only carbocyclic ring systems of compounds classified in subclass C07C as claimed compounds or as products of claimed production / purification / stabilisation processes are indexed. See the special rules of classification within the subclass C07C.
Please note: The indexing of compounds classified in this subclass is not yet complete. Missing codes are continuously being added.