![[Search a list of Patent Appplications for class 520]](../as.gif) CLASS 520, CLASS 520, | SYNTHETIC RESINS OR NATURAL RUBBERS -- PART OF THE CLASS 520 SERIES |
| Click here for a printable version of this file | |
SECTION I - CLASS DEFINITION
Class 520, Synthetic Resins or Natural Rubbers -- Part of the Class 520 Series is to be considered as an integral part of Class 260 and retains all pertinent definitions and class lines of Class 260. Class 520, subclass 1 is the generic subclass for synthetic resins or natural rubbers. All the other classes of the 520 series (i.e., 521,522, 523, 524, 525, 526, 527, and 528) are indented thereunder.
Retrieval of information in the Class 520 series is identical to retrieval in any ordinary class that has subclasses rather than classes indented thereunder. Each of the classes indented (i.e., 521-528) etc., merely recites an art area that for convenience of numbering has been categorized as a class rather than a conventional subclass. Classes 521-528 are arranged in hierarchical order under Class 520, subclass 1, and each of Classes 521-528 operates as a normal class within this hierarchy. The 520 series of classes encompasses solid synthetic resins and natural rubbers, and the preparation and treatment thereof. The series also encompasses compositions not elsewhere provided for of solid synthetic resins and natural rubbers, as well as the preparation and treatment of such compositions.
In this class and in the indented subclasses are placed all patents which are directed to the preparation and treatment of the so-called synthetic resins or natural rubbers (e.g., complex organic compounds produced from ingredients which are generally nonresinous and which final products simulate the natural resins).
SOME OF THE GENERAL TYPES OF SUBJECT MATTER WITHIN THIS CLASS
A. Solid synthetic resins, per se, regardless of utility.
B. Processes of preparing a synthetic resin involving a chemical reaction including those chemical reactions which utilize energy.
C. Reactable compositions which form a product as in (a) above upon the addition of a catalyst or promoter, or which merely require the presence of heat and/or pressure and wherein all of the necessary reactants to form the final desired product are present, or processes of preparing.
D. Processes of purifying a solid synthetic resin or composition containing a synthetic resin by a chemical or physical process.
E. Process of reclaiming or recovering a solid synthetic resin.
F. Processes of treating a synthetic resin or specified intermediate condensation product (SICP, see the Glossary) with a reactant, or product thereof.
G. Blends of solid synthetic resins, processes of preparing or treating such blends.
H. Chemically reacted solid synthetic resins or processes of preparing.
I. Potentially reactable compositions which contain a solid synthetic resin or SICP and products of such a reaction, or processes of preparing.
J. Room vulcanizable potentially reactive compositions which merely require moisture and which are usually activated by heat and/or pressure to form a product proper herein, or processes of preparing.
K. Compositions of a nonreactant material and a solid synthetic resin or SICP, processes of preparing or treating.
L. Processes of using a solid synthetic resin or composition containing a solid synthetic resin.
M. Compositions containing a structurally defined material (e.g., coated web, or fiber having dimension, etc.) which is dispersed in a matrix which is not identified by overall dimension or some structure or processes of preparing.
N. Single-layered products containing a solid synthetic resin or composition thereof reciting no structure or dimension, or a fiber, filament, etc., which is no more than the material from which it is made. Nonstructured single-layered web or sheet is encompassed.
O. Particles or a powder, per se, of a solid synthetic resin or composition thereof for which particles or powder no dimensions are recited.
P. Reactable compositions which form a product proper for this class and contain a photoinitiator or photosensitizer which are activated by wave energy or processes of preparing.
It must be remembered that the list above is exemplary not exhaustive of the types of subject matter that may be found in this class. Not all subject matter relating to solid synthetic resins and natural rubbers is to be found in this class, since other classes provide for claims involving synthetic resins (e.g., utility classes, separation classes, etc.).
SECTION II - LINES WITH OTHER CLASSES AND WITHIN THIS CLASS
A. A GENERAL OUTLINE OF THE CLASS 520 SERIES IS AS FOLLOWS
Class 520, generic subclass 1.
Class 521 provides for ion-exchange polymers, processes of reclaiming a solid synthetic resin, and for cellular synthetic resins.
Class 522 provides for processes of preparing or treating a solid polymer utilizing wave energy and for compositions which contain a photosensitizer and which when reacted form a product proper for the 520 Series of Classes.
Classes 523 and 524 provide for solid synthetic resins or specified intermediate condensation products admixed with a nonreactant material.
Class 525 provides for certain combinations of polyesters and certain reactable materials, for blends of solid synthetic resins, and for chemically modified solid synthetic resins.
Class 526 provides for certain manipulative processes which are generic to both ethylenic polymers and to condensation polymers, and also provides for polymers derived from ethylenic monomers only.
Class 527 provides for solid synthetic resins derived from at least one saturated material and certain special reactants (e.g., carbohydrates, proteins, natural resins, lignin, tannin, bituminous material, etc.).
Class 528 provides for solid synthetic resins derived from at least one nonethylenic reactant, and also for processes of treating a polymer either derived from ethylenic or nonethylenic reactants wherein chemical bonds in the polymer are left unaffected.
This list above is merely to be taken as a shorthand method in approaching the Class 520 series. The areas above generally provide for processes of preparing the indicated products. Once a class in the series is identified as having subject matter in which one may be interested, it is best to consult the individual class schedule or to look at the one-dot subclasses indented under Class 520, subclass 1.
B. RULES FOR DETERMINING PLACEMENT BETWEEN THE 520 SERIES OF CLASSES AND THE MONOMER, ETC. AREAS OF CLASS 260
To be classified in the 520 Series of Classes, a patent must contain a claim to a solid synthetic resin, natural resin, preparation or treatment thereof, or compositions containing solid synthetic resins or natural rubbers.
When a patent (1) sets forth claims drawn to species that may or may not be solid synthetic resins as per disclosure (e.g., the patent may present claims to nonsolid polymers or to monomeric compounds), or a patent (2) contains only generic claims and the disclosure sets forth species, embraced by the claims, some of which are and some of which are not solid synthetic polymers, the patent is classified as an original with the nonresinous species and is cross-referenced to the appropriate Class 520 series area.
Where both claims and disclosure are devoid of any reference to a solid synthetic resin, the patent is classified in the appropriate Class 260 compound area (principally the Class 532 Series of Classes and Class 585) that provides for the monomer or liquid polymer. In the event that a composition is claimed when neither claims nor disclosure refer to a solid synthetic resin, the patent is classified in an appropriate composition class other than the Class 520 series.
Determination of whether a product is a solid synthetic resin proper for this area (the Class 520 series) or a compound proper for another Class 260 area (such as the Class 532 Series) is as follows:
1. In the absence of disclosure to the contrary:
a. A polymer derived from ethylenic reactants only will be considered to be a solid polymer, per se, and proper for Class 520.
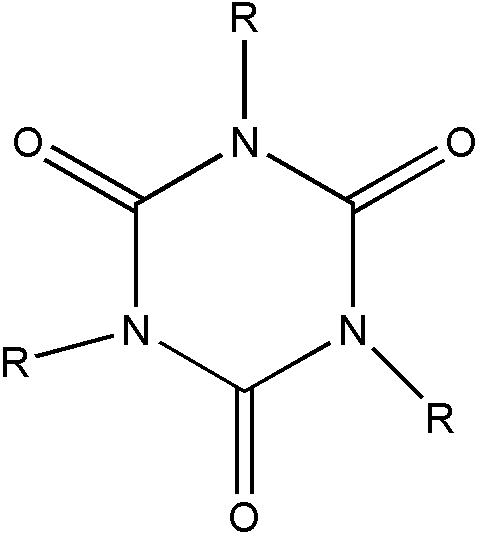
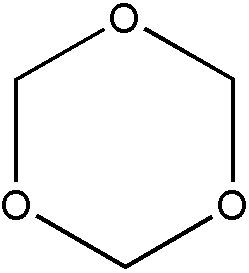
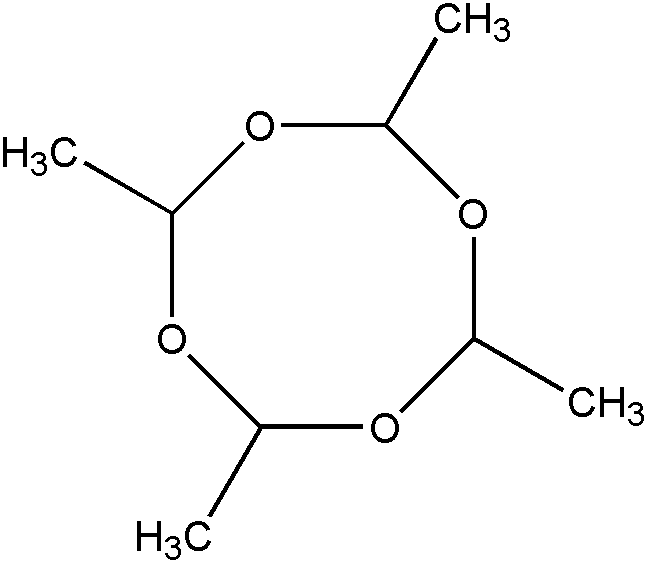
b. A polymer derived from at least one nonethylenic reactant will be considered to be a liquid and, therefore, properly classified in Class 260 as a compound. An exception to this rule pertains to certain low molecular weight polymers which despite being solids are nonetheless regarded as nonpolymeric and therefore excluded from Class 520. Consider, for example, the low molecular weight polymers of organic isocyanates (nRNCO), formaldehyde (mCH2O), and acetaldehyde (pCH3CHO), respectively. Organic isocyanates can be polymerized to produce isocyanurates (I), cyclic trimers where n=3. Formaldehyde can be polymerized to produce the cyclic trimer, trioxane (II) where m=3. Finally, acetaldehyde can be polymerized to produce the cyclic tetramer, metaldehyde (III), where p=4.
I
II
III
Despite being solids, these "polymers" are, in fact, compounds with sharp melting points, exhibiting no film-forming, elastomeric or other rheological properties and are therefore properly classified in the compound classes.
2. A polymer is a solid when so stated or when it is described in one or more of the following terms. This list is not to be taken as limiting a solid to the enumerated terms. Other terms in patents (not noted in the following list) may be interpreted as being solid when proper description is given therein: coagulated, brittle, ductile, Durran m.p, elastic, elastomer, fiber-forming, friable, fusible, gum, meltable, melting point, millable, molten, pliable, powder, rubber, rubbery, thermoplastic, and thermoset. A wax is not considered to be a solid even if defined by one or more of the above terms.
Determining whether a product derived from a natural rubber, protein, natural resin, natural gum, bituminous material, nonvolatile residue of distillation processes, naturally occurring fatty acids, fatty oils, fats, waxes and modified forms thereof, carbohydrates or derivatives, or tall oil or fatty acids derived from tall oil is proper subject matter for this 520 Series of Classes or for a compound area of Class 260 follow.
C. RULES PERTAINING TO NATURALLY OCCURRING FATTY ACIDS, FATS, FATTY OILS, OR TALL OIL OR FATTY ACIDS DERIVED FROM TALL OIL
In the absence of disclosure to the contrary, the following rules apply:
1. The reaction product of fatty acid or oil + SICP (or SICP ingredients) is presumed to be solid and is classified in Class 525 or 528.
2. The reaction product of a fatty acid or oil + polyol + polycarboxylic acid or derivative is presumed to be a liquid and is classified in Class 554, Organic Compounds, subclasses 1+.
3. The reaction product of a fatty acid or oil + SPFI (other than SICP ingredients) is presumed to be a liquid and is classified in Class 554, Organic Compounds, subclasses 1+.
4. The reaction product of an ethylenic reactant preformed reaction product of fatty acid + polyol + polycarboxylic acid or derivative is classified in class 525, subclass 7, when the ethylenic reactant is not solely a fatty acid glycerol ester, a fatty acid derived from a naturally occurring glyceride, tall oil, or a tall oil fatty acid.
It is to be pointed out that the ethylenic reactant can be a natural resin, e.g., rosin, etc. the rule in Class 525, subclass 7, regarding the role of the ethylene reactant is to be contrasted with situations elsewhere in Classes 526, 528, and also in class 525, where it often is appropriate to view a drying oil or drying oil acid as an ethylenic reactant so long as there is present a 520 series system in the absence of the fatty oil or acid.
Example: The reaction product of solid polyethylene glycol terephthalate + linseed oil is classified in Class 525, subclasses 10+.
But: If the polyester is not specifically disclosed as a solid or the reaction product is not disclosed as a solid prior to oxidative cross-linking (i.e., air drying), then the system is classified in Class 554, Organic Compounds, subclasses 1+.
5. The reaction product of a fatty acid or oil with ethylenic monomer is presumed to be a liquid and is placed in Class 560, Organic Compounds, subclasses 1+ or Class 562, Organic Compounds, subclasses 400+, respectively. If formation of a solid polymer (prior to air drying) is disclosed, the material is classified in Class 526.
6. A fatty acid or oil + maleic acid yields a reaction product. The reaction product admixed with styrene is presumed to yield a liquid product, which product is classified in Class 560 Organic Compounds, subclasses 76+ or Class 562, Organic Compounds, subclasses 488+, respectively. A solid would be placed in Class 526.
7. Polymerized fatty acids or oil (e.g., dimer or trimer fatty acids, etc.) are not treated as fatty acids for purposes of the Class 520 Series. However, in recognition of the historical treatment of these materials as derivatives of fats, the following rules are offered (a) solid materials produced from dimerized or trimerized fatty acids together with a SPFI or SICP or ethylenic monomer other than solely a fatty acid or oil are treated as solid polymers for the purpose of the Class 520 Series, and (b) materials produced from dimerized or trimerized fatty acids with otherwise incomplete specified polymer-forming system (SPFI) are classified as appropriate in Class 562, Organic Compounds, subclasses 400+ without regard to consideration of solid or liquid.
D. RULES PERTAINING TO NATURAL RESINS OR DERIVATIVES
In the absence of disclosure to the contrary, the following rules apply:
1. Natural resin and natural resin derivative is defined in the Glossary.
2. Reaction of natural resins with ethylenic reactants is presumed to produce a solid polymer proper for Class 527, subclass 600, with the exception that reaction products of natural resins or derivatives (including hydrogenated rosin) with solely terpenes and/or polycarboxylic acids, anhydrides, or halides are treated, per se, as derivatives of natural resins for purposes of Class 530, subclass 214. The practical effect of this is that abietic acid copolymerized with malelic anhydride is classified, per se, in Class 530, subclass 214. However, rosin + maleic + styrene is classified in Class 526, subclass 238.3.
3. A natural resin reacted with an ingredient which introduces ethylenic unsaturation (other than in section B, 2 situation above), e.g., rosin ester of allyl alcohol, is a monomer for purposes of Class 526. Thus, polymers of these materials are classified in Class 526; however, natural resins are assumed to be ethylenically unsaturated. For example, the reaction product of a natural resin + SICP is presumed to be a solid and is classified in Class 525.
4. A natural resin reacted with a material to introduce a functional group provided for on the SPFI listing is not presumed to be a solid polymer for purposes of Class 520 unless admixed with any additional ingredient necessary to complete the SPFI system.
5. A reaction product of a natural resin with SPFI ingredients is presumed to be a solid and proper for Class 527, subclasses 600+.
6. A reaction product of a natural resin with ethylenic reactant (other than those excluded in section B, 2 above) with nonethylenic reactant is presumed to produce a solid polymer proper for Class 527.
7. The reaction product of a natural resin with a reactant which introduces functional groups that are part of a SPFI system and wherein the other necessary ingredient of the SPFI system is subsequently introduced, produces a product which is presumed to be solid and is proper for Class 527.
E. RULES PERTAINING TO NATURAL RUBBER, PROTEIN, NATURAL GUM, BITUMINOUS MATERIAL, LIGNIN OR TANNIN, NONVOLATILE RESIDUE OF DISTILLATION PROCESSES, WAXES, AND MODIFIED FORMS THEREOF, AND CARBOHYDRATES OR DERIVATIVES
In the absence of disclosure to the contrary, the following rules apply (For purposes of convenience, the group of materials above has been treated as a single entity and has been categorized as being selected materials. The rules that follow relating to selected materials apply to each of the materials above.):
1. The reaction of a "selected material" with SICP (or SICP ingredients) is presumed to be a solid and is classified in Class 527.
2. The reaction product of a selected material with SPFI ingredients is presumed to be a solid polymer and proper for Class 527.
3. The reaction product of a selected material with ethylenic reactant is presumed to be a solid polymer and proper for Class 527.
4. The reaction product of a selected material with ethylenic reactant + nonethylenic reactant is presumed to produce a solid polymer proper for Class 527.
5. A selected material which is reacted so as to increase or decrease ethylenic unsaturation, but not to leave the material devoid of any unsaturation, produces a monomer proper for Class 526 which, if homopolymerized or copolymerized with an ethylenic reactant only, produces a polymer proper for Class 526.
6. The reaction product of selected material which introduces functional groups that are part of a SPFI system and wherein the other necessary ingredient of the SPFI system is subsequently introduced, produces a product which is presumed to be solid and is proper for Class 527. An example of such a situation is the treatment of sucrose with ethylene oxide to produce a sucrose polyether polyol which is subsequently treated with a polyisocyanate.
The reactions enumerated above, when dealing with multiple materials, may be simultaneous or sequential. For example, a selected material may be reacted with a polyol and then subsequently halogenated and, in turn, reacted with a polycarboxylic acid. The polycarboxylic acid and polyol reactant are a SPFI system. Therefore, the product produced is presumed to be solid and proper for Class 527.
The reactions enumerated above all pertain to SPFI, SICP, ethylenic reactants, or to materials which introduce functional groups which are part of a SPFI system, and to which another material is added that completes the necessary ingredients that comprise a SPFI system. Any other reaction with the above materials, other than where the patentee specifically states that materials are present which will form a solid polymer under the conditions of reaction if the materials enumerated above were absent, is excluded from being a solid synthetic resin proper for the Class 520 Series.
F. PROCESS: THE FOLLOWING GENERAL LINES EXIST BETWEEN THE CLASS 520 SERIES AND OTHER CLASSES PROVIDING FOR PROCESSES
Class 264, Plastic and Nonmetallic Article Shaping or Treating: Processes. Refer particularly to the main section of Class 264 for the line between Class 264 and the composition classes. In general, patents which include both the preparation and a significant molding or working treatment of a compound or composition classifiable in Class 520 are placed in Class 264 and cross-referenced to this Class 520 when desirable. However, where the molding is only nominally recited or is merely incidental to the preparation of a Class 520 composition, the patent is placed in this Class 520 according to the following guidelines.
1. Patents limited to process claims reciting a broad or nominal molding step only (a) where a composition classifiable in Class 520, per se, is molded and there is no disclosure as to a chemical reaction being present, the patent goes to Class 264; (b) where a chemical reaction, mixing or blending of ingredients to form a composition of matter classifiable in Class 520 is recited to take place in a mold or during the molding or shaping step, the patent goes to Class 520, even if temperature and pressure conditions are set out; (c) similarly, where a chemical reaction, mixing or blending of ingredients to form a composition classifiable in Class 520 is recited to take place prior to the nominal shaping or molding step, the patent goes to Class 520.
2. Patents containing both composition claims classifiable in Class 520 and process claims reciting nominal molding only (a) patents containing both claims to a composition classifiable in Class 520 and also claims reciting broad or nominal molding of said composition go to Class 520; (b) patents containing both claims to a composition classifiable in Class 520 and claims reciting broad or nominal molding of said composition wherein there is a chemical reaction, blending, or mixing of ingredients of said composition during or prior to the molding step, will go to Class 520, even if temperature or pressure conditions are set out; (c) where patents contain both claims to a composition classifiable in Class 520 and claims reciting a nominal or broad molding of said composition, per se, and there is no disclosure of any chemical reaction taking place, and specific temperature and/or pressure conditions are set out, the patent will go to Class 264.
Class 427, Coating Processes. Refer to the class definition of Class 427 particularly the section, Nonsignificant Coating Processes. In general, nonsignificant or nominal coating methods are classified with a compound or composition if claims are present for both compound or composition.
1. A patent containing a claim to a coating composition or compound, which claim is, per se, classified in Class 520, and also a claim to a nonsignificant process of utilizing the claimed compound or composition to coat a substrate, is classified with the claimed Class 520 composition. The following guidelines are used to determine if a process step is significant.
a. Any pretreatment or post-treatment of a base or applied coating is a significant process step; processes limited to etching or making a base more compatible with, or adherent to, the coating wherein the base is the substrate (work) onto which a coating is applied are included (e.g., curing, drying, or smoothing a coating, or cleaning or drying a base.
b. A specific recitation of how the coating is applied (e.g., brushing, dipping, spraying, immersion, etc.) is significant. General statements of applying, covering, or coating, etc., are not significant.
c. Processes resulting in plural coatings are considered significant.
d. A process resulting in a coating having a specific thickness or lack of uniformity is considered significant.
e. Specific recitation as to the condition of a coating being applied is generally significant except (1) a condition also included in an independent composition claim (e.g., pH concentration, etc.) is not significant, and (2) statements that a coating material is molten or in an organic, inorganic, or aqueous solution is not significant unless accompanied by a recitation of specific times or temperatures or chemically defined solvents.
2. Patents containing only claims to a process of coating a substrate wherein no significant process steps are recited are classified in Class 428, Stock Material or Miscellaneous Articles, according to the product produced by the process. Guidelines for use in determining if a process is significant are the same as set out under step 1 above.
3. Patents containing (a) a claim to a composition which is classifiable in Class 520, (b) a coated product claim which does not have significant structure for Class 428 and is classifiable only as a nonstructural laminate in Class 428, and (c) a claim to a significant coating process which is classifiable in Class 427 are classified as an original in Class 428 (see particularly Class 428, subclass 411.1
G. Composition: The Following General Lines Exist Between Class 520 and Other Composition Classes or With Classes Providing Patents Wherein the Claims Recite a Composition Limited to an Art Use Provided for in That Class
1. A composition having no art use claimed, but disclosed as having a single art use is classified with the art use.
2. Compositions which are disclosed as having a plurality of functions provided for in different main classes and only a single use, property, or function is claimed, are placed as original in the composition class providing for such claimed use, property, or function and are cross-referenced to other classes for disclosed uses, properties, or functions when desirable.
3. Compositions which are disclosed as having a plurality of uses, properties, or functions provided for in different main classes, and there are claims to a plurality of such several uses, properties, or functions, are placed in the composition class coming first in the following order of superiority:
Class 504, Plant Protecting and Regulating Compositions
Class 424, Drug, Bio-Affecting and Body Treating Compositions
Class 426, Food or Edible Material: Processes, Compositions, and Products.
Class 71, Chemistry: Fertilizers.
Class 149, Explosive and Thermic Compositions or Charges.
Class 430, Radiation Imagery Chemistry: Process, Composition, or Product Thereof.
Class 252, Compositions, subclasses 9+.
Class 44, Fuel and Related Composition.
Class 148, Metal Treatment.
Class 252, Compositions (special uses or functions) to subclass 194.
Class 502, Catalyst or Solid Sorbent.
Class 252, Compositions (special uses or functions), subclass 478 and those following, except subclasses 302+, 363.5+, 364, 365+, 367+, 372, 378 R,P.
Class 8, Bleaching and Dyeing; Fluid Treatment and Chemical Modification of Textiles ad Fibers.
Class 429, Chemistry: Electrical Current Producing Apparatus, Product, and Process.
Class 204, Chemistry: Electrical and Wave Energy.
Class 106, Compositions: Coating or Plastic, subclasses 1.05 to 38.9.
Class 501, Compositions: Ceramic.
Class 106, Compositions: Coating or Plastic, subclasses 600-316.
Class 451, Abrading.
Class 75, Specialized Metallurgical Processes, Compositions for Use Therein, Consolidated Metal Powder Compositions, and Loose Metal Particulate Mixtures (Alloys).
Class 420, Alloys or Metallic Compositions
Class 75, Specialized Metallurgical Processes, Compositions for Use Therein, Consolidated Metal Powder Compositions, and Loose Metal Particulate Mixtures (rest of class).
Class 518, Chemistry: Fischer-Tropsch Processes; or Purification or Recovery of Products Thereof
Class 520, Synthetic Resins or Natural Rubbers: Series of Classes.
Class 260, Chemistry of Carbon Compounds: Series of Classes.
Class 208, Mineral Oils: Processes and Products.
Class 252, Compositions (nonspecial uses or functions) i.e., subclasses 182.11+, 183.11+, 302+, 363.5, 364, 365+, 367, 372, 378 R,P, 380, 408.1, 600.
Class 585, Chemistry of Hydrocarbon Compounds (mixture subclasses).
This superiority list is not intended as a complete list and will be expanded or added to as the relationship between classes containing resin compositions and Class 520 is determined.
Class 435 is not on the above list and may contain a resin composition and see the notes between Class 520 and Class 435.
4. Class 520, subclass 1 is the residual home for compositions containing a solid synthetic resin or nonreactant proper for Class 520. Any composition containing a polymer proper for the Class 520 Series and having a claimed utility, property, or function which is unprovided for in any of the classes superior to the Class 520 Series on the (B, 3) list above is proper for Class 520. The Class 520 Series is also the proper home for any composition having a polymer proper for the series where the disclosure is limited to a single disclosed utility, property, or function which is unprovided for in any of the classes superior to the series on the (B, 3) list. A claim that fails to recite a utility, property, or function and wherein the disclosure is generic to a number of utilities, properties, or functions, some of which are provided for specifically by classes higher on the list than the Class 520 Series and others that are provided in Class 520 Series, or that are not provided for in any other composition area on the list, should generally be cross-referenced into the Class 520 Series on the basis of the unprovided or provided for utilities, properties, or functions enumerated in the disclosure.
5. In Class 430, certain disclosures have been excluded, (see the Class Definition of Class 430). In the circumstance enumerated in Class 430, the patent would be classified in Class 520 rather than Class 252 when the composition contains a polymer proper for Class 520.
6. It is a general rule of classification to classify a process of preparing a composition along with the composition. In these circumstances where only a process of preparing a composition is claimed and there is no claim to a composition, the claims would be classified identically as if there were a composition claimed. An exception to the general rule above is Class 435, Chemistry: Molecular Biology and Microbiology, wherein an enzymatic reaction may have produced a particular resin composition. The composition is properly classified in Class 520, while the process is classified in Class 435.
7. Class 520 provides for a composition containing a synthetic resin for treating textile materials, as for example, compositions for oiling or lubricating, rendering antistatic, softening, and silk-soaking, excepting detergent, bleaching and mere wetting compositions. Patents which claim a resin composition intended for textile treating and also claim (a) processes involving no more than mere application of the composition to a textile material, or mere application combined with a broadly stated textile operation, and/or (b) textile products characterized essentially by the application of the composition, are classified herein. For patents which claim only processes of application and/or products thereof, or a significant process of applying these compositions, see Class 8, Bleaching and Dyeing; Fluid Treatment and Chemical Modification of Textiles and Fibers. Class 427, Coating Processes, and Class 428, Stock Material or Miscellaneous Articles. For the lines between these classes, see the definition of the respective classes.
8. This class (520) specifically provides the Si-C or Si-H containing compositions containing reactants which are claimed as being moisture curable; see Class 528, subclasses 10+, and for moisture curable N=C=X (X is chalcogen) and liquid polysulfide compositions, respectively.
See the section Lines With Other Classes in the class definition of Class 428 for a treatment of the lines between Class 428 and the Class 520 Series of Classes.
SECTION III - REFERENCES TO OTHER CLASSES
SEE OR SEARCH CLASS:
| 117, | Single-Crystal, Oriented-Crystal, and Epitaxy Growth Processes; Non-Coating Apparatus Therefor, for processes for growing therein-defined single-crystal of all types of materials, including inorganic or organic. |
| 204, | Chemistry: Electrical and Wave Energy, appropriate subclasses for producing a product of this class by other than a wave energy process. Class 204 is superior to this class, therefore a patent claiming in the alternative a process of preparing an organic compound and a synthetic resin or natural rubber in the presence of wave energy is classified for original purposes in Class 204 and cross-referenced to this Class 520 Series on the basis of wave energy and the synthetic resin or natural rubber species. In the situation where an alternative process claim is presented as well as a specific process claim to the nonsynthetic resin or nonnatural rubber species, and the process is directed to wave energy, the same rule of original patent placement applies. Any process step involving electrolysis, electric current, electro-osmosis, electrophoresis, electrostatic field, electrical discharge, or magnetic field and also involving the preparing or treating or a synthetic resin or natural rubber is properly classified in Class 204, even if the wave energy step involved is subsequent to the 204 step. Combination of chemical processes falling within the definition of Class 520 (other than wave energy) and those methods falling within the the definitions of Class 204, are classified in Class 204 when the chemical process steps are preparatory to the Class 204 process and are classified in Class 520 when the 204 method is precedent to the Class 520 chemical step. |
| 252, | Compositions, subclasses 182.11+ and 183.11+ for a polymerizable composition which is devoid of a necessary reactant or catalyst needed to produce the desired synthetic resin. |
| 430, | Radiation Imagery Chemistry: Process, Composition, or Product Thereof, for a polymerizable composition which is radiation sensitive and limited by claims or disclosure for use in radiation imagery. |
| 435, | Chemistry: Molecular Biology and Microbiology, for mere processes of making, separating, or purifying carbon compounds by operations that include fermentations and compositions and apparatus that are specialized for use therein, and processes of making such compositions for such use; also ferments that are carbon compounds (enzymes), respectively, and processes of making products resulting from biological processes (fermentation) are classified in the Office according to the products of those processes. subclasses 174+ for a carrier-bound or immobilized enzyme or cell, even if attached to a solid synthetic resin; and appropriate subclasses, for a process of preparing a solid synthetic resin utilizing a microorganism, tissue cell culture, or enzyme. |
| 506, | Combinatorial Chemistry Technology: Method, Library, Apparatus, for a chemical or biological library or a process of creating said library. |
| 530, | Chemistry: Natural Resins or Derivatives; Peptides or Proteins; Lignins or Reaction Products Thereof, subclass 200 (the definition thereof) for a complete discussion of what is intended by natural resin. |
| 560, | Organic Compounds, for styrene copolymerized with a fatty acid glyceride to form a liquid material. |
| 562, | Organic Compounds, for styrene copolymerized with a fatty acid to form a liquid material. |
SECTION IV - GLOSSARY
ACYCLIC
Denotes a compound devoid of any ring-containing moiety.
ALCOHOL
Denotes an organic compound having the general structure C-OH wherein the carbon atom bound to the oxygen atom of the hydroxyl group cannot be double bonded to oxygen, sulfur, selenium, or tellurium, or triple bonded to nitrogen. The terms as used herein include phenols.
ALDEHYDE
Denotes an organic compound having the group -C(=O)H [i.e., -CHO] bonded directly to hydrogen or to an additional carbon,which carbon is not double bonded to chalcogen (i.e., oxygen, sulfur, selenium, or tellurium), or triple bonded to nitrogen.
ALDEHYDE DERIVATIVE
Denotes the following: A. Compounds having a X-CH2-OH group where X is other than carbon or hydrogen (e.g., paraformaldehyde, methyol derivatives of urea, etc.); B. Heterocyclic compounds having only carbon and oxygen as alternating ring members (the number of ring carbon atoms must equal the number of ring oxygen atoms). An example is trioxane, which is shown as Figure 1 at the end of the "Aldehyde Derivative" definition; C. Hexamethylene tetramine (i.e., urotropine) or derivatives thereof. Hexamethylene tetramine per se is shown as Figure 2 at the end of the "Aldehyde Derivative" definition. A derivative, for purposes of this definition, requires the basic hexamethylene tetramine ring structure, where substitution has been made for the hydrogens bonded to the ring carbons. Compounds having a -CH2OH bonded to atoms other than C, H, or oxygen are regarded as being two compounds; for instance, a methylol derivative of melamine is regarded as a mixture of melamine and formaldehyde, and methylol urea is regarded as being a mixture of urea and formaldehyde. A structurally unspecified novolak is proper for this area in that it is considered as a mixture of a phenol and an aldehyde. If a novolak of specified structure is claimed as prepared from specific reactants, then classification is proper on the basis of the specific reactants.
FIGURE 1. Trioxane
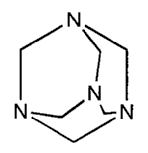
FIGURE 2. Hexamethylene tetramine
ALDEHYDE-TYPE
An aldehyde-type reactant is limited to an aldehyde derivative or methylol derivative.
AMINE
Denotes an organic compound having a nitrogen atom single or double bonded to a carbon atom and wherein the carbon atom bonded to the nitrogen atom is devoid of a double bond to oxygen, sulfur, selenium, or tellurium or triple bonded to nitrogen. In addition, those compounds wherein the same nitrogen atom is bonded to a -C(=X)- group (X is O, S, Se, or Te) and to a carbon atom which is not double bonded to oxygen, sulfur, selenium, or tellurium, are not considered as being amines, e.g., -C(=X)NH-CH3, etc. Although amides may be considered chemically as amines, it has been found expedient for these classes to exclude compounds containing only amide nitrogen herefrom. Therefore, as used throughout this area, the term amide is not to be confused as being an amine. A compound, however, which contains a nitrogen atom bonded to a non -C(=X)- carbon atom and which contains either a nitrogen atom bonded to a -C(=X)- group of an amide group, is considered as being an amine. An organic amine salt of a carboxylic acid has been classified as if it were a mixture of an amine and a carboxylic acid. An organic diamine salt of a dicarboxylic acid where the amine salt-forming groups are identical is considered as being a single amine compound, whereas, if the amine groups are different then they are regarded as two amine compounds. Where the amine groups contain two or more nitrogen atoms bonded to the same or different noncarbonyl carbon atom then they are to be regarded as polyamines.
ARYL
Denotes a benzene ring or a carboxylic ring system having a benzene ring as part of the system.
BIOLOGICALLY ACTIVE POLYPEPTIDE
Denotes polypeptide chains which have been built up primarily from alpha- or beta-amino carboxylic acids and which exhibit biological activity similar to naturally occurring proteins or polypeptides; such activity may be, for example, hormone activity (e.g., insulin, etc.), immuno-activity (e.g., antigen or antibody, etc.), antibiotic activity (e.g., bacitracin or bleomycin, etc.), or antiviral activity (e.g., interferon, etc.). Mere statements that a material demonstrates any of these activities is sufficient to create a presumption that a biologically active polypeptide is present. On the other hand, a material merely disclosed as a polypeptide which has been built-up from amino acids will not be presumed to have biological activity, and will be placed in Class 520 according to the disclosed structure and function as appropriate.
BLOCK COPOLYMER
A. The structure is given, i.e., a long polymer backbone has attached or coupled to one or both of its terminal ends one or more polymers at least three reactant units in length or; B. The copolymer is named as a block providing that the disclosure is otherwise silent as to its structure or if the structure is likewise given, it is consistent with that described above or; C. The structure can be ascertained from the following limiting process conditions (a) Treating a nonterminated solid polymer, that is, one which is terminally active or "living", with an ethylenic reactant with subsequent polymerization to form additional blocks. The process may be continued to produce higher order block copolymers. For example, treating dilithiated polystyrene with butadiene to yield an ABA block copolymer; and (b) two or more nonidentical solid polymer chain ends are coupled directly or through the use of a chemical agent. For example, the coupling of hydroxy terminated solid polybutadiene with hydroxy terminated polyethylene glycol terephthalate with phosgene.
BLOCK-TYPE COPOLYMER
The structure is given, i.e., to a long solid polymer backbone possessing terminally active sites (i.e., functional groups), or that is a "living polymer" is attached or coupled, through chemical reaction with those functional groups or sites, an ethylenic reactant containing one or more functional groups or sites; an example is to contact hydroxy terminated polybutadiene glycol with allyl isocyanate, or two or more identical solid polymer chain ends are coupled directly or through the use of a chemical agent. For purposes here, identical means those polymer segments which contain the same carbon backbone but differ in stereo regularity (e.g., isotactic, syndiotactic, atactic) optical activity, or degree of polymerization. Thus, coupling lithium terminated polystyrene segments with molecular weights of 25,000 and 100,000, respectively, with stannic chloride is proper for this area.
CARBOHYDRATES
Denotes polyhydroxy aldehydes (i.e., aldoses) and polyhydroxy ketones (i.e., ketoses) of the empirical formula Cx(H2O)x where x is five or more; and substances hydrolyzable to said polyhydroxy (aldehydes or ketones). Included herein for example are the following: (a) monosaccharide sugars such as pentoses (e.g., arabinose, arabinulose, etc.) hexoses (e.g., glucose, levulose, etc.) and the heptoses (e.g., mannoheptose, etc.); (b) disaccharides (e.g., lactose, maltose, sucrose, cellobiose, etc.); (c) trisaccharides (e.g., raffinose, etc.); (d) polysaccharides (e.g., starches, celluloses, dextrins, hemicelluloses, glycogen, insulin, etc.); (e) complex polysaccharides (e.g., gum arabic, pectins, etc.). Excluded herefrom are lignin, tannin, and derivatives thereof. Also excluded are the simple "triose" (i.e., glyceradehyde di-hydroxy acetone) or "tetrose" (i.e., erythrose, threose and keto tetroses) sugars since these sugars have less than five carbons; such materials are treated as polyhydroxy (aldehydes or ketones).
CARBOHYDRATE DERIVATIVE
Denotes reaction products of carbohydrates wherein the carbon skeleton of the carbohydrate is not destroyed. Included herein are cellulose nitrate, cellulose acetate, cellulose ethers, viscose, cellulose xanthate, chitin, etc.
CARBOXYLIC ACID OR DERIVATIVE
A. A carboxylic acid denotes the carboxyl group, represented as -COOH or -C(=O)OH, bonded to: (1) a carbon atom that is not double-bonded to sulfur, selenium, or tellurium, or triple bonded to nitrogen; (2) hydrogen; or (3) [-C(O=)-]n, where n is an integer (e.g., oxalic acid, etc.). A carboxylic acid derivative is limited to: 1. nitride; 2. carboxylic acid ester; 3. carboxylic acid anhydride; 4. carboxylic acid salt; 5. carboxylic acid amide; 6. carboxylic acid imide; 7. carboxylic acid lactam; 8. carboxylic acid halide; and 9. lactone. B. A carboxylic acid anhydride denotes the basic structure -C(=O)-O-C(=O)-, the carbons of which may independently be bonded to: (1) hydrogen; (2) a carbon atom that is not double bonded to sulfur, selenium, or tellurium; or (3) [-C(=O)-]n, where n is an integer. In either of (2) or (3), supra, the -C(=O)-O-C(=O)- group may be part of a ring. C. A carboxylic acid ester denotes the structure -C(=O)-O-C-, where the carbon atom bonded to the -O- of the -C(=O)-O- group may not be double bonded to chalcogen (i.e., oxygen, sulfur, selenium, or tellurium), or triple bonded to nitrogen, and the carbonyl carbon of the -C(=O)-O-C- group may be bonded to (1) hydrogen; (2) a carbon atom that is not double bonded to sulfur, selenium, or tellurium, or triple bonded to nitrogen; or (3) [-C(=O)-]n, where n is an integer. D. A nitride denotes the structure -CbN bonded to carbon, which carbon may not be double bonded to chalcogen (i.e., oxygen, sulfur, selenium, or tellurium), or triple bonded to nitrogen. E. A carboxylic acid amide denotes the structure -C(=O)-NH2, where substitution may be made for the hydrogens, and the carbonyl carbon may be bonded to (1) hydrogen; (2) a carbon atom that is not double bonded to sulfur, selenium, or tellurium, or triple bonded to nitrogen; or (3) [-C(=O)-]n, where n is an integer. F. A carboxylic acid halide denotes the structure -C(=O)-hal, where hal is halogen and the carbonyl carbon may be bonded to (1) hydrogen; (2) [-C(=O)-]n, where n is an integer; or (3) a carbon atom that is not double bonded to sulfur, selenium, or tellurium, or triple bonded to carbon. G. The imide of a dicarboxylic acid is a heterocyclic ring having as ring members the group -C(=O)-NH-C(=O)-, where substitution may be made for hydrogen; all remaining ring members are carbon atoms. H. The lactam of a carboxylic acid is a heterocyclic ring having as ring members the group -NH-C(=O)-, where substitution may be made for hydrogen; all remaining ring members are carbon atoms. I. The lactone of a carboxylic acid is a heterocyclic ring having as ring members the group -C(=O)-O-; all remaining ring members are carbon atoms, and the carbon atoms bonded to either the carbon or oxygen or the -C(=O)O- group may not themselves be double bonded to chalcogen (i.e., oxygen, sulfur, selenium, or tellurium). J. A carboxylic acid salt denotes the structure -C(=O)-O-θX⊕, where X is a cation and ionic bonding exists between the cation, X, and the -C(=O)O- group. The carbon of the -C(=O)O- group may be bonded to: (1) hydrogen; (2) a carbon atom that is not double bonded to sulfur, selenium, or tellurium, or triple bonded to nitrogen; or (3) [-C(=O)-]n, where n is an integer. In the above definitions of carboxylic acids and their derivations, certain derivations may technically fit into more than one derivative grouping. A lactone, for example, is a species of an ester, and a lactam is a species of an amide. Compounds that are themselves multifunctional (i.e., possess more than one functional group) are classified on the basis of the first appearing functional group in the hierarchy. A polycarboxylic reactant requires the presence of at least two carboxylic acid groups, or of at least one carboxylic acid group and at least one carboxylic acid derivative, or at least two identical carboxylic acid derivatives, or at least two different carboxylic acid derivatives.
A cyclic carboxylic anhydride having the group -C(=O)-O-C(=O)- as members of a ring is considered as a polycarboxylic acid. Compounds having both a cyclic anhydride group and a free carboxyl (-COOH) group are considered as tricarboxylic acids. An example is trimellitic anhydride, which is shown as Figure 1 at the end of the "Carboxylic Acid or Derivative" definition. A compound containing two cyclic anhydride groups is considered a tetracarboxylic acid. An example is pyromellitic dianhydride, which is shown in Figure 2 at the end of the "Carboxylic Acid or Derivative" definition.
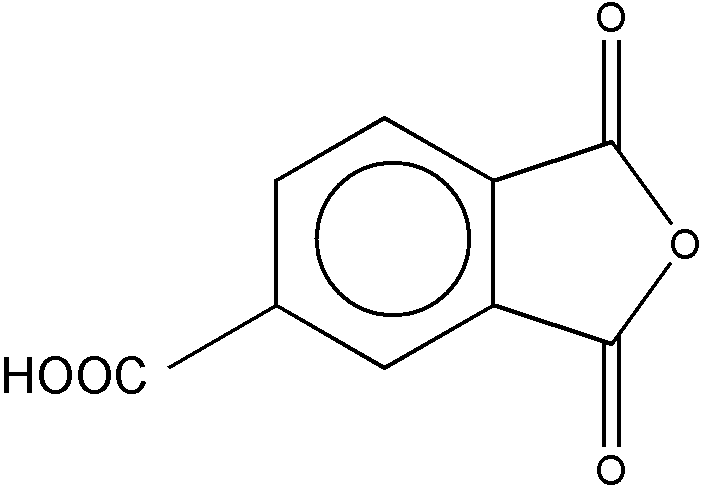
FIGURE 1. Trimellitic Anhydride
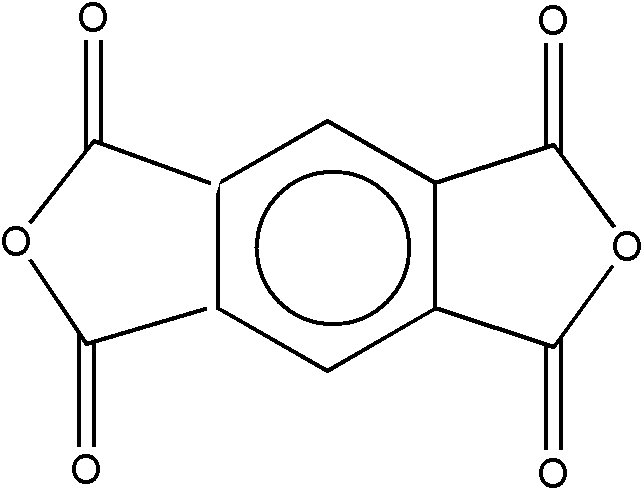
FIGURE 2. Pyromellitic Dianhydride
CHEMICAL TREATING AGENT
Denotes a chemical material which is added to the formed solid polymer and which causes or is present during a process wherein a change in a bond of the polymer is effected. Claims reciting a "chemical treating agent" are classified on the basis of the first-appearing material utilized as part of the chemical agent. No attempt has been made to classify on the basis of functionality of the chemically active material and therefore all materials in a composition are regarded equally (e.g., diluent, reactant, catalyst, etc.). Processes and products which refer to mere cross-linking, curing, or vulcanizing will be classified on the basis of the product treated.
DIMER OR TRIMER OF AN ALIPHATIC MONOCARBOXYLIC ACID
Denotes dimeric or trimeric fatty acids prepared, e.g., by free radical, ionic, thermal polymerization, etc., of a monomeric fatty acid which can be saturated or unsaturated monocarboxylic acid having at least eight carbon atoms. So-called "polymeric fatty" acids in the absence of other disclosure are presumed to be a mixture of dimers and trimers of aliphatic monocarboxylic acids. Included herein are reaction products of dimers or trimers wherein the dimer or trimer structure is not destroyed. Dimers or trimers of ethylenically unsaturated aliphatic monocarboxylic acids are presumed to be unsaturated in the absence of disclosure to the contrary.
ETHER
Denotes an organic compound characterized by the presence of an oxygen atom bonded directly to two carbon atoms, where the carbon atoms may not be double bonded to chalcogen (i.e., oxygen, sulfur, selenium, or tellurium), or triple bonded to nitrogen. An example is dimethyl ether, CH3-O-CH3.
ETHYLENICALLY UNSATURATED
Requires the presence of two carbon atoms bonded to each other by a double or triple bond, provided that the double bond is not part of a benzene ring. Indane (Fig. 1) is not within the scope of olefinically unsaturated; coumarone (Fig. 2) and indene (Fig. 3) are within said scope.
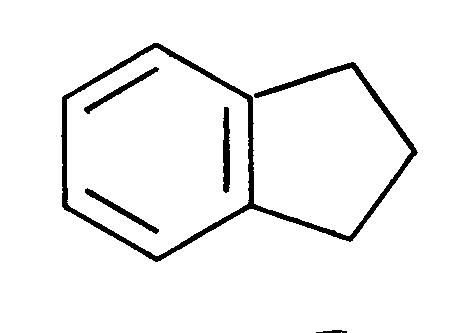
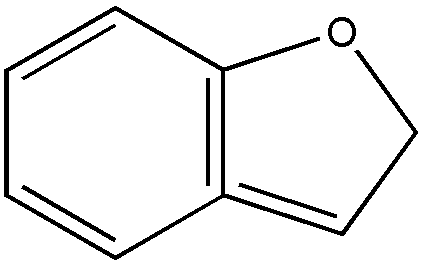
FIGURE 2. Coumarone

FIGURE 3. Indene
FATTY ACID
Denotes an aliphatic monocarboxylic acid having an unbroken chain of at least seven acyclic carbon atoms bonded to the carboxyl group. Fatty acid glycerol ester denotes a mono-, di-, or tri-ester of a fatty acid with glycerol. The so-called drying or semidrying oils are naturally occurring fatty acid glycerol esters (i.e., glycerides). The drying or semidrying property relates to the degree of ethylenic unsaturation. Naturally occurring fatty acid glycerides (i.e., fats and fatty oils) include but are not limited to linseed oil, perilla oil, olive oil, oititica oil, soybean oil, fish oil, castor oil, tallow, and other natural glycerides: alfalfa, apricot kernel, beechnut, bontio, Brazil nut, candlenut, cedar nut, chaulmoogra, cherry kernel, coconut, corn, cottonseed, croton seed, grapefruit seed, grapeseed, hempseed, isano, ivory wood seed, jute seed, mustard seed, oat, orange seed, plum kernel, poppyseed, poyok, rice, rye, safflower, sesame, stillingia, sunflower, teaseed, thistle seed, tobacco seed, tomato seed, walnut, wheat, wild rose seed. Naturally occurring fats and fatty oils are treated in this Class 520 Series as fatty acid glycerol esters. A fatty acid derived from naturally occurring glyceride denotes the carboxylic acids normally obtained by saponification of the naturally occurring glycerides (i.e., oleic, linoleic, linolenic, licanic, eleostearic, ricinoleic, arachidic, stearic, palmitic, lauric, erucic, palmitoleic, capric, caprylic, myristic and clupanodonic acids). Carboxylic acids specifically enumerated above will be treated as fatty acids derived from naturally occurring glycerides only where there is specific disclosure that the acid is derived from a naturally occurring glyceride source. Tall oil denotes the mixture of fatty acids, rosin, and unsaponifiable material obtained by treatment of Kraft (or sulfite) process black liquor. In this Class 520 Series, tall oil is usually treated as if it were a mixture of fatty acids derived from naturally occurring glycerides unless otherwise specifically stated; see appropriate subclass definitions for exceptions. Fatty acid derived from tall oil denotes the fatty acid portion of tall oil In this series, derivatives or modifications of the fatty acid glycerol ester, fatty acid derived from a naturally occurring glyceride, tall oil, or fatty acids derived from tall oil are excluded, except as specifically provided for in the following list: salts of the fatty acid moiety, blown oils, refined oils and acids, stand oils, boiled oils, bodied oils, hydrogenated oils or acids, dehydrogenated oils or acids, dehydrated castor oil or dehydrated castor oil fatty acids. Synthetically produced fatty acids having the same structure as fatty acids derived from naturally occurring glycerides are included herein. Dimerized or trimerized or "polymeric" fatty acids are excluded as "fatty acids" for purposes of this Class 520 Series; similarly, adducts of fatty acids or fatty acid glycerol esters with alpha, beta ethylenically unsaturated carboxylic acids are excluded as "fatty acids".
FUSED OR BRIDGED RING SYSTEM
Denotes a ring system having at least two rings which (a) share with each other two adjacent ring atoms, or (b) share with each other three or more ring atoms and wherein each ring having shared atoms is either a heterocyclic ring or a carbocyclic ring.
GLASS
An amorphous, hard, brittle, often transparent material comprising a fused mixture of the silicates of the alkali alkaline earth, or heavy metals.
GRAFT COPOLYMER
The structure is given, i.e., a long solid polymer backbone (substrate) is attached to a pendant (nonterminal) polymer or copolymer (superstrate) having at least three reactant units in length or; The copolymer is so named as a graft providing that the disclosure is otherwise silent as to the structure or, if structure is likewise recited, it is consistent with that required in A. above, or the structure can be ascertained from the following limiting process conditions: (a) the disclosure states there is a reaction between a solid polymerized unsaturated reactant and an unpolymerized unsaturated reactant in the presence of a catalyst or; (b) the disclosure does not state whether or not any reaction has occurred between the solid polymerized unsaturated reactant and the unpolymerized unsaturated reactant, but relates that a product is obtained which is inseparable by a variety of physical techniques such as, extraction, precipitation, ion exchange, etc. In the absence of one or more of these requirements the reaction is considered to produce a polymeric blend.
GRAFT-TYPE COPOLYMER
The structure is given, i.e., a long solid polymer backbone (substrate) possessing nonterminal active sites or functional groups is attached (grafted) through a chemical reaction of these functional groups or sites to an ethylenic reactant containing one or more functional groups or active sites. The reaction product may or may not possess unsaturated pendant groups depending on the mode of chemical reaction. The following examples will illustrate this point:
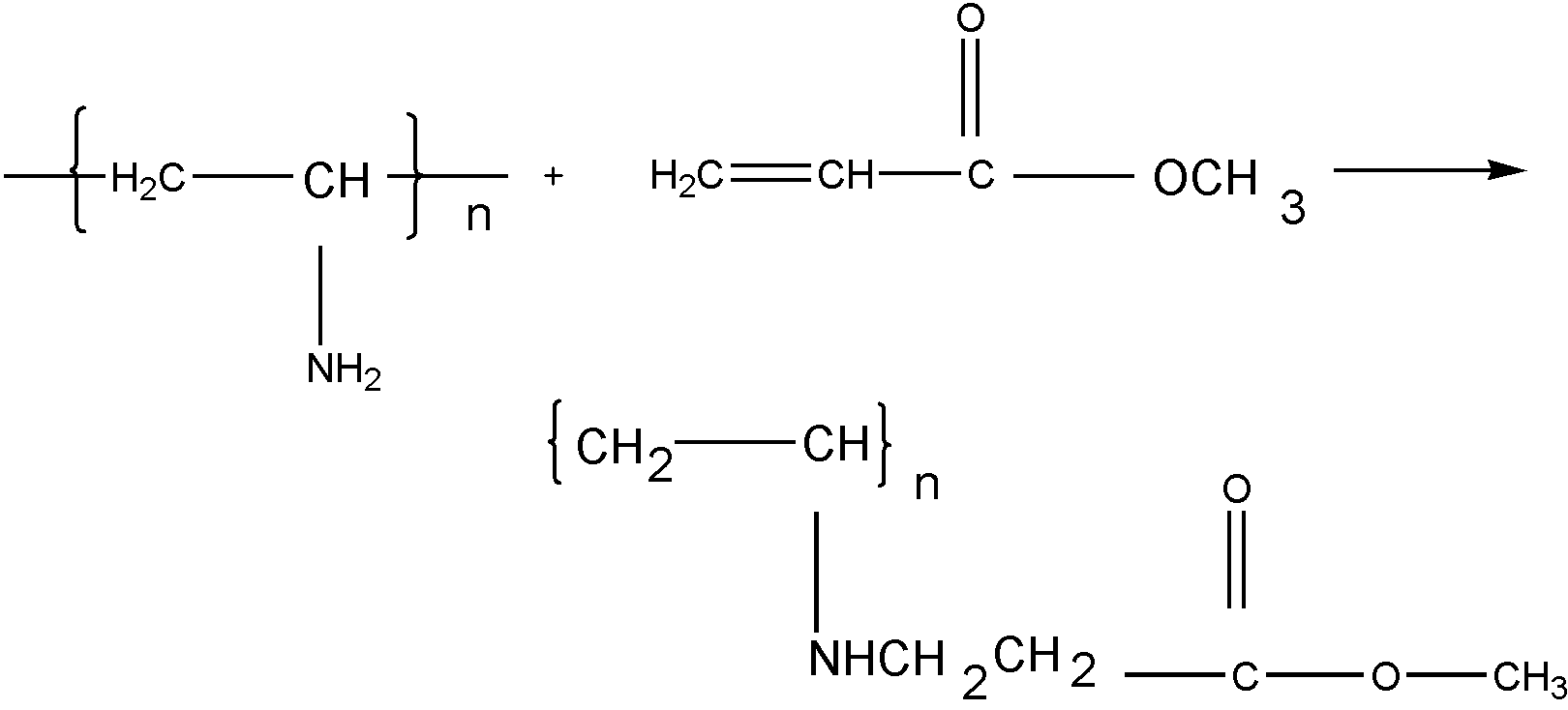
(a)
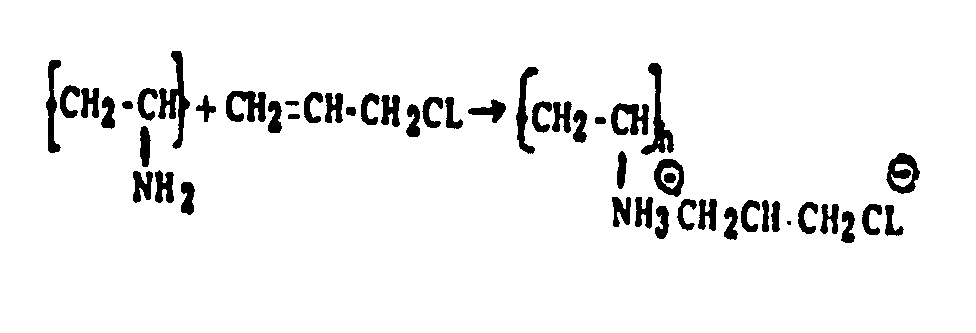
(b)
The structure can be ascertained when:
There is disclosed a reaction between the solid polymerized unsaturated reactant and the unpolymerized unsaturated reactant which reaction uses specific art-recognized terms, e.g., "esterification, acylation, sulfonylation, cyanoethylation, addition to, reaction or condensation with, halogenation, nitration, sulfonation, alkylation, amination, etc.". Examples of these reactions would be:
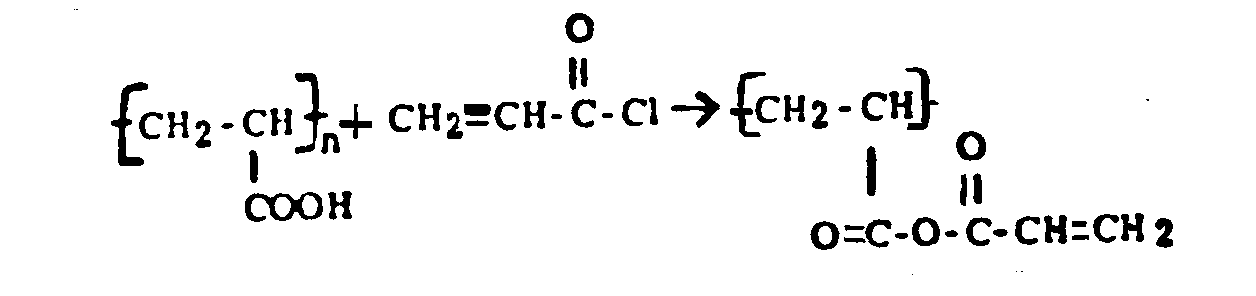
(a)
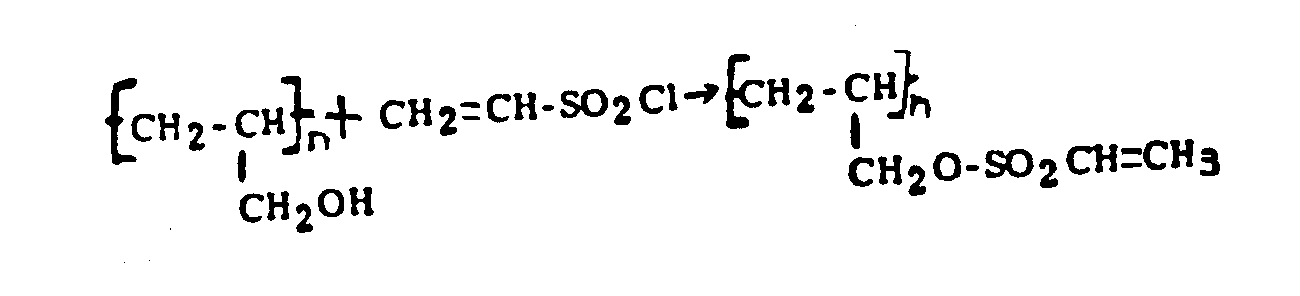
(b)
There is disclosed an interaction between two or more solid polymers through their respective nonterminal functional groups or through the use of an intermediate reactant or chemical agent, e.g., causing salt, ester, amide, urea formation. Examples of these reactions would be (a) Contacting polypropylene and polyethylene with benzoyl peroxide. (b) Contacting polyvinylamine and polyallylamine with glyoxal. (c) Contacting chloromethylated polystyrene with polyvinylamine. (d) Contacting polyacrylic acid with polyvinylamine.
In the absence of one or more of these requirements the reaction is considered to produce a polymeric blend. See the Glossary for the definition of the terms "specified intermediate condensation product" (SICP) and "specified polymer-forming intermediates" (SPFI).
HALOGENATED HYDROCARBON
Denotes a compound containing only carbon, hydrogen, and halogen, or only carbon and halogen.
HETEROCYCLIC
Denotes an organic compound wherein one or more carbon atoms are covalently bonded in a ring system with at least one hetero atom of oxygen, sulfur, nitrogen, selenium, or tellurium and there are no other different atoms in the ring.
KETONE
Denotes an organic compound having the general structure n (n is 1 or more) and wherein the carbon atoms bonded to the n group are not double bonded to oxygen, sulfur, selenium, or tellurium. Ketone as used throughout includes ketene, i.e., CH2=C=O
LIGNIN
Denotes a material usually derived during paper pulp manufacture by separation of the cellulose from wood. Lignin is considered to be the binder for cellulose in wood. Lignin includes crude mixtures of lignose, lignone and lignin. Lignin per se is a complex structure having some aromatic rings and phenolic groups.
LIGNIN DERIVATIVE
Denotes materials not otherwise provided for, derived from lignin or from sulfite or soda paper pulping processes, e.g., sodium lignosulfonate, waste sulfite liquor, black liquor, etc.
METALS
Are limited to elements of atomic numbers 3, 4, 11-13, 19-33, 37-51, 55-84, 87, and higher.
| (1) Note. The Group IA metals are Li, Na, K, Rb, Cs, Fr. |
| (2) Note. The Group IIA metals are Be, Mg, Ca, Sr, Ba, Ra. |
| (3) Note. The Group IIIA metals are Ai, Ga, In, Ti. |
| (4) Note. The Group IVA metals are Ge, Sn, Pb. |
| (5) Note. The Group VA metals are As, Sb, Bi. |
| (6) Note. The Group VIA metal is Po. |
| (7) Note. The Group IB metals are Cu, Ag, Au. |
| (8) Note. The Group IIB metals are Zn, Cd, Hg. |
| (9) Note. The Group IIIB metals are Sc, Y, La, Ac. |
| (10) Note. The Group IVB metals are Ti, Zr, Hf. |
| (11) Note. The Group VB metals are V, Nb, Ta. |
| (12) Note. The Group VIB metals are Cr, Mo, W. |
| (13) Note. The Group VIIB metals are Mn, Tc, Re. |
| (14) Note. The Group VIII metals are Fe, Ru, Os, Co, Rh, Ir, Nb, Pd, Pt. |
| (15) Note. "Transition metal" is limited to elements of atomic numbers 21-29, 39-47, 57-79, 89, and higher and does not include Zn, Cd, and Hg. |
METHYLOL OR METHYLOL DERIVATIVE
Methylol or methylol derivative is limited to
(a) A compound containing a
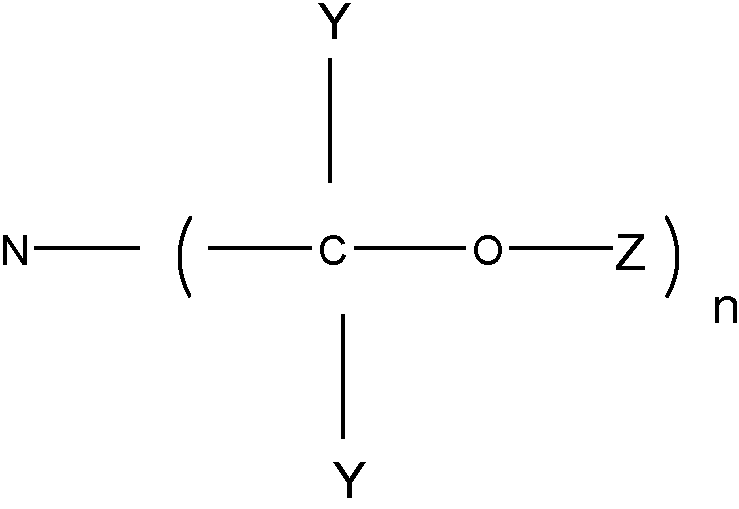
(b) A compound containing a T-(-O-A)n
(c) A compound containing a
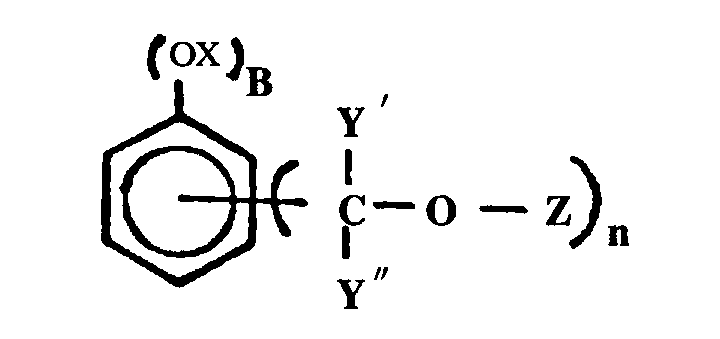
Y" and Y" are H or hydrocarbon groups; Z is H or a carbon atom. T - is an atom other than C or H. A - is a hydrogen or a carbon atom and which carbon atom is bonded to only H, carbon, or single bonded oxygen atoms. X - is H, part of an ether group, or an inorganic salt. n - is two or more, B is one or more.
NATURAL RESIN
Denotes the following: Those materials which have been customarily employed as such in the paint, lacquer, varnish, adhesive, and ink trades. Thus, "natural resin" is a term of art.
Natural resins include but are not limited to shellac, copals from various sources, e.g., Congo, manila, etc.; amber, dammar, dead dammar, rosin (colophony), gum rosin, wood rosin, burgundy pitch, gurjun balsam, Canada balsam, sandarac, mastic, accroides, benzoin, elemi, gamboge, gum thus, venice turpentine, bordeaux turpentine, abietic acid, pimaric acid, etc. Tall oil, per se, is not treated as a natural resin unless the rosin content is specifically stated. In the event the rosin content is not stated, tall oil is treated as an unsaturated fatty acid derived from naturally occurring glycerides. Unless indicated to the contrary, "rosin" is presumed to be primarily abietic acid.
NATURAL RESIN DERIVATIVE
Denotes the following:
A. Heat decomposition products of natural resins which contain a hydroaromatic nucleus and which are unprovided for elsewhere. B. Materials described by patentee as natural resins. C. Modifications of natural resins wherein the carbon structure of the abietyl nucleus is retained, including but not limited to: abietyl amine, dehydroabietyl amine, abietyl alcohol, zinc abietate, hydrogenated rosin, dehydroabietic acid, disproportioned rosin, rosin esters, ester gum (i.e., triglyceride of rosin), polymerized ester gum, hydrogenated ester gum, oxidized ester gum, etc. D. Other modified natural resins; for example, aceto- and butyro-copal, copal ester, etc.
NONMETALS
Denotes boron, oxygen, carbon, selenium, tellurium, nitrogen, sulfur, phosphorous, silicon, hydrogen, fluorine, chlorine, bromine, iodine, astatine, helium, neon, argon, krypton, xenon, and radon.
ORGANIC COMPOUND
Denotes all compounds having carbon therein and which are further characterized by the presence of (a) two carbon atoms bonded together, or (b) one atom of carbon bonded to at least one atom of hydrogen or halogen, or (c) one atom of carbon bonded to at least one atom of nitrogen by a single or double bond, with the proviso that hydrocyanic acid, cyanogen, isocyanic acid, cyanamide, cyanogen halides, isothiocyanic acid, and metal carbides are excluded from being organic compounds.
PHENOLIC REACTANT
The term phenolic reactant as used is intended to include the subject matter enumerated below:
A phenol for purposes of this subclass requires one or more -OH groups directly bonded to a nuclear carbon atom of a substituted or unsubstituted benzene ring, which benzene ring can be an individual benzene ring or can be part of a polycyclic ring system.
A phenol ether for purposes of this subclass requires one or more -O-C groups wherein the oxygen atom of the -O-C group is directly bonded to a nuclear carbon atom of a substituted or unsubstituted benzene ring and wherein the carbon atom of the -O-C group is not double bonded to oxygen, sulfur, selenium, or tellurium or triple bonded to nitrogen. The benzene ring may be an individual benzene ring or may be part of a polycyclic ring system. The following examples of phenol ether are within the definition set out above:
(a) The -O-C group may itself be part of a cyclic ring system, e.g., see Fig. 1 below, etc.
(b) The carbon of the -O-C group may be a ring atom of a cyclic or aromatic ring, e.g., see Fig. 2 below, etc.
(c) An inorganic phenolate is an inorganic salt of a phenol (see phenol 1 above) wherein the hydrogen atom of a -OH group is replaced by a metal or an inorganic group. Tannin or tannic acid is considered to be a polyhydroxy polycyclic carboxy-containing phenol. Crysylic acid is considered to be cresol. Coal tar extracts are considered to be an indefinable mixture of ingredients some of which are phenolic in nature. Cardanol and anacardic acids are phenolic derivatives.
(d) The carbon of the -O-C group may be a terminal carbon atom, e.g., Figure 3 below; or may be the carbon atom of a chain, e.g., Figure 4 below, etc.
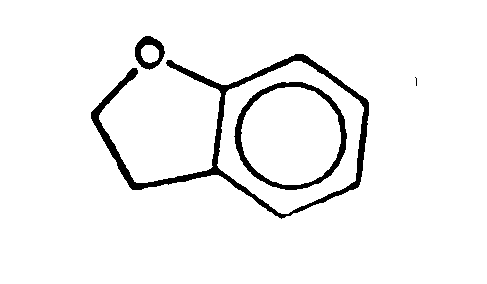
Figure 1, Cyclic Ring System
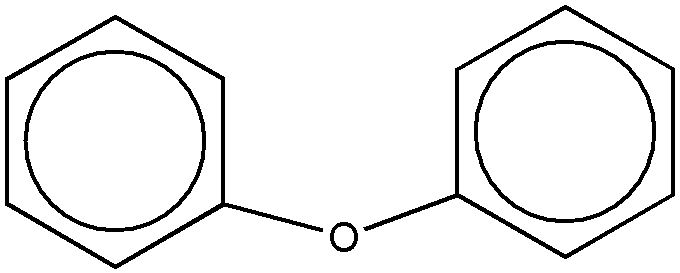
Figure 2, ring atom of a cyclic or aromatic ring
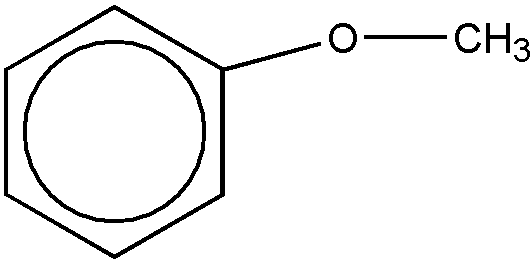
Figure 3, terminal carbon atom
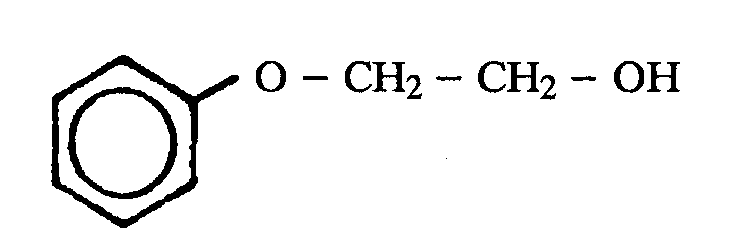
Figure 4, carbon atom of a chain
POLYEPOXIDE
Denotes a material having more than one 1,2-epoxy group per molecule.
PROTEIN
Denotes polypeptides composed of more than 100 amino acids or having molecular weights greater than 10,000.
Included herein are, for example:
(a) The so-called simple proteins which yield alpha-amino acids upon hydrolysis (e.g., albumins, globulins, glutelins, prolamines, histones, sceleroproteins, etc.).
(b) The conjugated proteins wherein protein is bound to some other molecule or group (e.g., nucleoproteins, glucoproteins, etc.).
(c) Derived proteins which are the cleaved products of proteins, excluding the monomeric alpha-amino acids themselves (e.g., proteins, metaproteins, coagulated proteins, proteoses, peptones, peptides, etc.).
(d) Reaction products wherein the protein has been reacted and wherein the final reaction product retains peptide linkages.
SATURATED
Denotes nonethylenically unsaturated; thus, for purposes of this class, materials containing an aryl structure (e.g., benzene, naphthalene, etc.) are treated as saturated materials unless otherwise excluded either specifically or hierarchically. Compare ethylenically unsaturated.
SPECIFIED INTERMEDIATE CONDENSATION PRODUCT (SICP)
Specified intermediate condensation product is limited to:
(a) Reaction of an aldehyde or derivative and an amine or compound containing a N- or N group where R is hydrogen or hydrocarbon radical.
(b) Reaction of an aldehyde or derivative and a phenolic material.
(c) Reaction of an aldehyde or derivative and a ketone.
(d) A compound containing a

N-(-C-O-Z)n group.
(e) A compound containing a T-(-O-A)n group.
(f) A compound containing a group.

Y" and Y" are H or hydrocarbon groups, Z is H or a carbon radical.
T - is an atom other than H or C.
A - is a hydrogen or carbon atom and which carbon atom is bonded to only H, carbon, or single bonded oxygen atoms.
X - is H, part of an ether group, or an inorganic cation.
n - is two or more, a is one or more.
SPECIFIED MATERIAL
Denotes the intentional and deliberate presence of a material (other than as a reactant monomer) during the polymerization reaction, which material may be removed subsequent to the polymerization, or which may remain with or in the final desired polymeric product.
The term "specified material" is limited to an amount of a material (e.g., 2 percent of a material, etc.).
A recitation of at least one specified element in a compound or in elemental form (e.g., oxygen-liberating compound, peroxy compound, chlorine-containing, etc.)
Groups of elements which can be identified from the periodic table, other than metal or nonmetal (e.g., Group IA, transition metal, halogen-containing, etc.)
Compounds which have identified art meaning (e.g., alcohol, ethers, esters, etc.)
Examples of material, which are described in mere functional terms and are thereby excluded as being a "specified material" since they do not meet the parameters set out above, are terms such as free radical catalyst, redox catalyst, emulsifier, dispersant, base, acid, organic medium, etc.
Water in any of its physical forms: inert gases (Group VIIIA), hydrocarbons, and chlorinated hydrocarbons are specifically excluded from this area as being specified material even if specifically recited as to name (e.g., chloroform, etc.) or as to amount (e.g., 2 percent of chloroform, etc.). A search for these materials requires a search of the appropriate product area. However, specific provision has been made in Class 526, subclass 208 for a mixture of a chlorinated hydrocarbon and water, and in Class 526, subclass 207 for a mixture of hydrocarbon and water.
Terms such as complex, coordination complex, chelate, sequestered, or adduct, and terms which are exemplary of these but which are not limited to the enumerated examples, such as sequestered complex, chelated compound, etc., are classified as are compounds, per se, when they are products of a metal or metal compound and a nonmetal organic compound. These materials are classified as separate compounds or elements when (a) the product is the reaction product of at least two or more metals, metal-containing compounds, or mixtures thereof (e.g., alloy, etc.) or (b) when the product is the reaction of at least a metal or metal compound and an inorganic material.
Patents in this area are to be classified on the basis of the claimed final compound or composition that is introduced into the reaction zone and is in direct contact with any of the monomers therein. If it is not possible to so classify the introduced material, classification is then made on the basis of the individual reactants used in the preparation of the unknown material. In the event that the claims recite both the individual reactants and identify the product formed therefrom, then the original classification should be made in the subclass that provides for the known product and a cross-reference should be placed in the appropriate subclass that provides for the reactants.
Patents which claim an "in situ" preparation of "specified material" in the presence of the monomer are originally classified on the basis of the introduced reactants and cross-referenced to the prepared "specified material".
SPECIFIED POLYMER-FORMING INGREDIENTS (SPFI)
Specified polymer-forming ingredients are limited to:
(a) Aldehyde or derivative and a phenolic material.
(b) Aldehyde or derivative and an amine.
(c) Aldehyde or derivative and a compound containing N-.
(d) Aldehyde or derivative and a hydrocarbon.
(e) Polyepoxides.
(f) Polyisocyanates and a polyol.
(g) Polyisocyanates and a polyamine.
(h) Polyisocyanates and a polycarbocyclic acid or anhydride.
(i) Carbonic acid or carbonate and a polyol.
(j) Hal--hal and a polyol.
(k) Polycarboxlic acid or derivative and a polyol.
(l) Polycarboxylic acid or derivative and a polyamine.
(m) Aldehyde or derivative and a compound containing N group.
TRANSITION METAL
Denotes elements of atomic numbers 21-29, 39-47, 57-79, 89, and higher and does not include Zn, Cd, and Hg.
SUBCLASSES
![[List of Patents for class 520 subclass 1]](../ps.gif) 1 1 | SYNTHETIC RESINS OR NATURAL RUBBERS: |
| Subject matter involving synthetic resins, or natural rubbers preparation, or treatment thereof; compositions containing synthetic resins or natural rubbers preparation or treatment thereof. | |