![[Search a list of Patent Appplications for class 532]](../as.gif) CLASS 532, CLASS 532, | ORGANIC COMPOUNDS -- PART OF THE CLASS 532 - 570 SERIES |
| Click here for a printable version of this file | |
SECTION I - CLASS DEFINITION
Class 532 is the parent class for the Class 532 series of classes, the hierarchical arrangement of which is shown below in Lines With Other Classes And Within This Class.
Class 532 and its integral classes (i.e., the classes of the 532 series) embrace monomeric organic carbon compounds and polymeric organic carbon compounds that are not solid synthetic resins, natural rubbers, or hydrocarbons.
For a more complete description of the relationship of this class and its integral classes to the remainder of the United States Patent Classification System as it relates to chemical compounds nd compositions classes, see Lines With Other Classes Within This Class.
SECTION II - LINES WITH OTHER CLASSES AND WITHIN THIS CLASS
A. HIERARCHICAL RELATIONSHIPS
The arrangement of Class 532 and its integral classes under the Class 260 umbrella, and its hierarchical relationship with classes therein, is as follows:
Class 260 Hierarchy
Class 260/1 (Miscellaneous Organic Carbon Compounds)
Class 518 (Fischer-Tropsch Processes)
Class 520/1 (Synthetic Resins & Natural Rubbers)
. Class 521 (Ion-Exchange Polymers, Cellular Products, Waste Polymer Recovery)
. Class 522 (Wave Energy Polymer Chemistry)
. Class 523/1 (Synthetic Resin Compositions with Nonreactant Material)
. . Class 524
. Class 525 (Chemically Treated Synthetic Resins, Compositions of Plural Synthetic Resins)
. Class 526 (Miscellaneous Processes, Synthetic Resins from Only Ethylenically Unsaturated Monomers)
. Class 527 (Synthetic Resins from Specified Natural Sources)
. Class 528 (Synthetic Resins from Plant Material of Unknown Constitution or Specified Reactant)
Class 530 (Natural Resins, Peptides, Proteins, Lignins)
Class 532 (No Patents Here at Present; Intended Future Residual Subclass After Abolition of Class 260)
. Class 534 (Radioactive or Rare Earth Metal Compounds, Azo and Diazo Compounds)
. Class 536 (Carbohydrates)
. Class 540/1 (Heterocyclic Carbon Compounds: Cyclopentanohydrophenanthrene Containing, Four-Membered Lactams, Porphyrins, Azaporphyrins, Nitrogen Hetero Rings with Seven or More Hetero Atoms)
. . Class 544 (Six-Membered Nitrogen Hetero Rings with Two or More Hetero Atoms)
. . Class 546 (Six-Membered Hetero Rings with One Ring Nitrogen)
. . Class 548 (Five-, Four-, or Three Membered Nitrogen Hetero Rings)
. . Class 549 (Oxygen or Sulfur Hetero Rings)
. Class 552 (Azides, Triphenylmethanes, Quinones, Hydroquinones, Steroids)
. Class 554 (Fats, Fatty Derivatives)
. Class 556 (Heavy Metal, Aluminum or Silicon Compounds)
. Class 558 (Esters)
. Class 560 (Esters, including Carboxylic)
. Class 562 (Acids, Acid Halides, Acid Anhydrides, Selenium & Tellurium Compounds)
. Class 564 (Amino Nitrogen Compounds)
. Class 568 (Boron, Phosphorus, Sulfur, or Oxygen Compounds)
. Class 260/665R (Carbon-Light Metal Compounds)
. Class 570 (Halogen Compounds)
Class 585 (Hydrocarbons)
B. REFERENCE TO CLASS 260 CLASS DEFINITION
The Class 260 class Definition is applicable, in almost all respects, to the subject matter of the Class 532 series of classes. The Class 260 class definition provides a comprehensive discussion of such topics as:
1. what constitutes an organic compound,
2. mixtures classifiable in compound subclasses,
3. patent placement rule,
4. genus-species relationships,
5. tautomeric forms,
6. intermediate - final product relationships, and other topics.
The Class 260 definition must be read to gain a complete understanding of the subject matter of Class 532 and its integral classes.
Class 532, at present, has only one subclass. This subclass has no patents. When reclassification of Class 260 is complete, and Class 260 no longer exists, this subclass will be the residual home for subject matter of the Class 532 series of classes.
C. MULTI-STEP SYNTHESIS CLAIMS
Many of the schedules in this Series of Classes provide process subclasses. These are indented under compound subclasses and embrace processes for the production of the compounds provided for in said compound subclasses.
The process subclasses are usually based on a type of reaction (such as "by hydrogenation") or on a type of starting material (such as "from nitro containing compound") or on a combination of the above (such as "by hydrogenation of nitro containing compound").
Many patents claim multistep processes. The problem of which step of a multistep process claim determines classification is resolved as follows: The word "directly" will be appropriately employed in the subclass title when the last step of forming the compound is to control. If "directly" does not appear in the title the step which determines classification may be prior to the final preparation step. Consider the following two claims.
Claim I - A method of making X by alkylating A to make B, isomerizing B to make C, and reducing C to make X.
Claim II - A method of making X by reducing A to make B, alkylating B to make C, and isomerizing C to make X.
which are to be placed in the following schedule:
1 . Compounds X
2 . . Prepared directly by reduction
3 . . Prepared by alkylation
Claim I is placed in subclass 2, because use of "directly" in a process claim means that the step involved results in directly obtaining the desired compound.
Claim II is placed in subclass 3. It is not placed in subclass 2 because, in claim II, the reduction step does not result in direct production of compound X, i.e., reduction is not the last step in making the compound. Subclass 3, which does not specify "directly", will take claim II because of the alkylating step.
This point is further illustrated by placement of claims I and II, supra, in the following schedule:
4 . Compounds X
5 . . Prepared by alkylation
6 . . Prepared directly by reduction
Claim I and Claim II must both be placed in subclass 5 since that subclass provides for alkylation irrespective of whether compound X is produced directly by the alkylation step or not. It would not be appropriate to classify the claims in subclass 6 because any process of making compound X which includes alkylation is provided for in subclass 5.
Purification or recovery steps would not effect classification in the above illustrations. Classification is determined by the controlling synthesis step.
D. SPECIAL RULES FOR CLASSIFYING SALTS
The rule to be utilized in classifying and cross referencing generic claims to salts in this series of Classes is clarified here. This rule applies only to salts and is not to be considered analogous to nor does it apply to other types of claimed disclosure.
A patent wherein the controlling claim is to a "compound" (e.g., acid or base) and wherein the claim includes a generic reference to salts, such as: "and the pharmacologically acceptable salt thereof", "or therapeutically useful acid addition salts thereof", "and nontoxic heterocyclic amine salts thereof", etc., will have its original classification determined by the "compound" without regard to the generic reference to the salts thereof. A patent in which the generic reference to salts is in a separate claim which is dependent on a claim to the "compound" is considered equivalent and will also have its original classification determined by the "compound" without regard to the generic reference to the salts thereof.
Cross-referencing of such a patent for a salt is mandatory only when it is clear that the specific salt was actually made as evidenced by: (a) a "working example" of a specific salt, (b) a property of a specific salt, such as its melting point, infrared scan, nuclear magnetic resonance, etc., or (c) an example of using a specific salt, such as in the treatment of animal life. Other cross referencing of salts, such as those which are part of a list in the disclosure, is optional and should be made only when clearly useful.
When a specific salt is set forth in a claim, the entire compound will be considered in determining the original classification, i.e., the original will be placed on the basis of the first appearing subclass providing for the acid, base, or salt. A specific salt is considered to be set forth in a claim when the structure of the salt forming moiety is clear from the claim or when the claim specifies that a heavy metal or a specific hetero ring (e.g., "and substituted morpholine containing salts thereof", etc.) is present in the salt forming moiety.
Other claims are treated the same as the controlling claim when considering where to cross reference, i.e., any generic reference to salts is disregarded as explained above.
E. CLASSIFYING COMPOUNDS OF UNKNOWN STRUCTURE WITHIN THIS SERIES OR CLASSES.
Classifying compounds of unknown structure in this Series of Classes is accomplished by considering two possible methods for classifying them and employing the one which results in the highest classification in the Series. The two methods are: 1. Classify according to an element or group of elements known to be part of the compound. 2. Classify based on an organic reactant utilized to make the compound.
When considering the first method, compounds are classified based on any partial structure of the compound which is known or which can be found by looking up a named compound in published sources. For example, if a specific alkaloid is named in a patent and if the structure or partial structure for that alkaloid can be found, the patent is classified according to that structure or partial structure. Patents claiming unnamed alkaloids in general have been classified in Class 546, subclass 1 on the assumption that alkaloids usually include a ring consisting of one nitrogen and five carbons.
Another situation involving unknown structures involves "oxidized hydrocarbon" in which there is no disclosure as to the structure of the products. These are placed in Class 568 in an indent under "oxygen containing". All that is known about them are the elements they contain. However, sulfurized carbohydrates of unknown structure are placed with carbohydrates based on the organic starting material. The "sulfur containing" subclasses are lower in the Series than carbohydrates in Class 568. Sulfurized nitro containing organic compounds are classified with "sulfur containing" because that is higher in the Class 568 schedule than "nitro containing".
Compounds which are disclosed as carbohydrates, proteins, lignins, starch, etc., and which are provided for according to titles of the Series are considered known structures, even though the exact structure isn"t set forth in the patent. They will be classified as known compounds and will not be treated as compounds of unknown structure or undetermined constitution.
F. LINES BETWEEN COMPOSITION CLASSES AND THIS SERIES OF CLASSES
In general, the 532-570 Series of Classes takes mixtures of organic compounds only if the mixtures:
(A) result from a chemical process or synthesis wherein a plurality of carbon compounds are simultaneously formed,
(B) result from a separation process wherein a plurality of carbon compounds are isolated simultaneously from a natural source, or
(C) result from the admixture of organic compounds with preserving or stabilizing agents whose sole function is to prevent chemical or physical change in the carbon compounds.
In contrast, the Composition Classes, in general, take mixtures which include organic carbon compounds if the mixtures are formed by simple physical admixing of preformed compounds (except those preserved or stabilized mixtures in (C) above).
The following rules are intended to provide guidance in placing patents in accord with the principles stated above, but are superseded by any specific class lines to the contrary:
(1) Where a mixture normally classifiable in the 532-570 Series is chemically treated as a whole, (where the entire mixture behaves in the reaction as a compound) the resulting product will still be classifiable in the 532-570 Series. (See 5 and 9, infra.).
(2) Where a mixture is treated so as to separate its components, followed by a reblending thereof, the mixture will be considered classifiable in an appropriate composition class based on the disclosed utility.
(3) Where a mixture is altered in composition by adding an ingredient not originally present, the resulting product will be considered classifiable in an appropriate composition class based on disclosed utility.
(4) Where a mixture is treated to remove a component, the claimed product, if still a mixture, will be considered classifiable in an appropriate composition class based on disclosed utility.
(5) Where a mixture is chemically treated so that only some of the components react (by disclosure), the resulting mixture of reacted and unreacted components will be considered classifiable in an appropriate composition class based on disclosed utility. (See 1, supra.).
(6) Where a mixture is claimed which by disclosure can be obtained either (1) as a reaction mixture or (2) by physically admixing the ingredients, and there are no reaction limitations in the product claims and no reaction process claims, or if there are claims to both methods of making the product, the mixture will be classified in the appropriate composition class based on disclosed utility.
(7) Where a reaction between two or more compounds is carried out in the presence of an additional compound which remains intentionally as a significant part of the final mixture, the mixture will be classified in the appropriate composition class based on disclosed utility. (e.g. A + B + D Æ C + D).
(8) Where two or more compounds are reacted under conditions where an excess of one of the reactants is used so that it will remain as a significant part of the composition, the resulting mixture will be classified in the appropriate composition class based on disclosed utility. (e.g., A + B in excess Æ C + B).
(9) Where a mixture of compounds is reacted with another compound and results in a mixture, it will be classified in the appropriate composition class based on disclosed utility, except where the original mixture is one, per se, classifiable in the 532-570 Series (e.g., (A + B + C) + D Æ AD + BD + CD) (See 1, supra.).
(10) Where two or more compounds are reacted under controlled conditions to give a desired resultant mixture, it will be classified in the appropriate composition class based on disclosed utility. The intention must be to get a mixture having utility as that specific mixture. Reaction mixtures, in general, are classified as compounds according to the desired compound produced. Many reaction mixtures have utility because of a desired compound formed by the reaction while the other components of the mixture serve no purpose; these reaction mixtures are classified as compounds. At least two components of a reaction mixture must be necessary for a disclosed utility for the reaction mixture to be classified as a composition.
The 532-570 Series of Classes also includes carbon compounds, provided for by the Series, when admixed with or dissolved in a solvent which served as the reaction medium for the synthesis of the organic compound, unless the mixture or solution thus formed is claimed, or is solely disclosed, as having a function or utility provided for in a composition class.
A water solution of a compound provided for in the 532-570 Series of Classes, whether preserved or not, is classified in this Series unless the water solution"s use is claimed or a single use for it is disclosed, in which case classification is in the Class indicated. If plural uses are disclosed, see the hierarchical rules set forth in the section LINES WITH OTHER CLASSES AND WITHIN THIS CLASS, in the Class 252 Definition. However, a gel or other colloid system consisting of a water solution of a compound provided for in the 532-570 Series of Classes is classified in Class 516 (see subclasses 98+ for gels), even though no use is claimed or disclosed.
Patents containing claims to a novel carbon compound dye and claims to a fiber or material dyed therewith are classified in the 532-570 Series of Classes, except where the dying process is also claimed.
The 532-570 Series of Classes also includes carbon compounds, provided for by the Series, when admixed with a preserving agent whose sole function is to prevent physical or chemical change in the carbon compound unless the mixture thus formed is claimed as having a function or utility provided for in a composition class. "Preserving agent" is intended to include an agent which inhibits chemical decomposition of, corrosion by, or caking of the carbon compound to which it is added. Preserved compounds classifiable in the 532-570 Series of Classes are classified on the basis of the carbon compound preserved even though the preserving agent is itself a carbon compound provided for in a preceding subclass.
SECTION III - REFERENCES TO OTHER CLASSES
SEE OR SEARCH CLASS:
| 506, | Combinatorial Chemistry Technology: Method, Library, Apparatus, for a chemical or biological library or a process of creating said library. |
SECTION IV - GLOSSARY
ACYCLIC
This term denotes a compound which does not contain a ring.
ACYCLIC ATOM
This term denotes an atom which is not a ring member. Figure 1 contains acyclic nitrogen while Figure 2 does not contain acyclic nitrogen
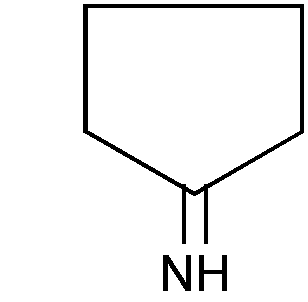
Figure 1
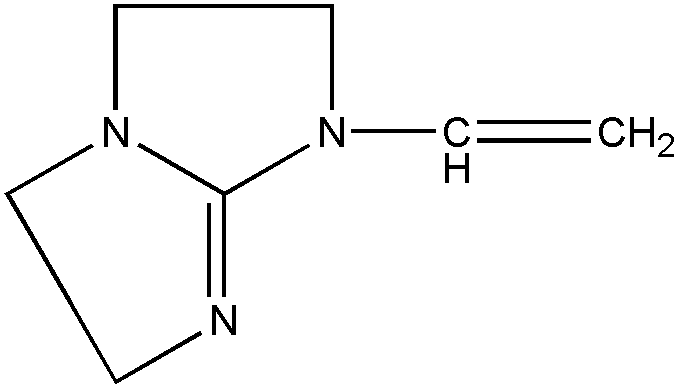
Figure 2
ACYCLIC BONDING
As used in regard to bonding or attachment of specified moieties, this term denotes that the moieties are connected to each other exclusively by atoms and bonds which are not part of a ring. The compounds in Figure 3 show oxygen attached to a hetero ring by acyclic bonding. Note that the sulfur-containing compound also has nitrogen and sulfur attached to the hetero ring by acyclic bonding.
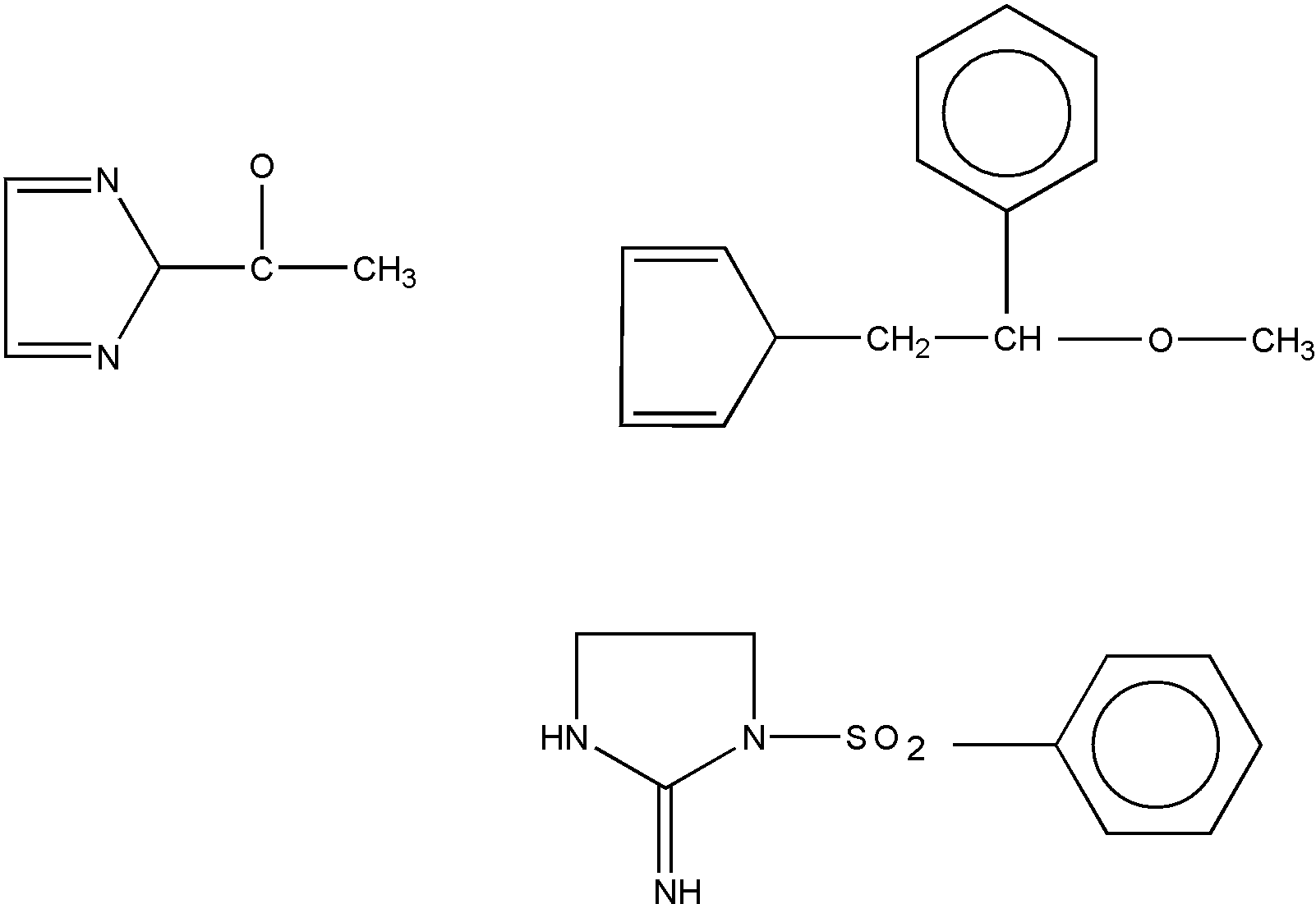
Figure 3
The compounds in Figure 4 would be excluded from a subclass requiring oxygen attached to a hetero ring by acyclic bonding since a carbocyclic ring is between the oxygen and the hetero ring in each structure.
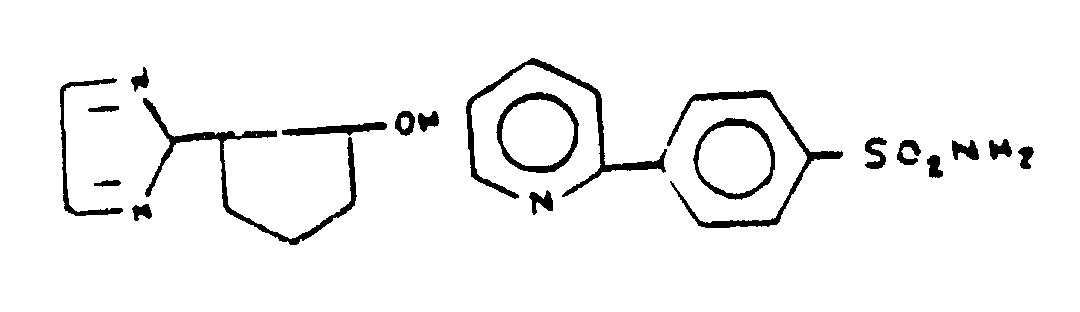
Figure 4
ALICYCLIC RING OR RING SYSTEM
This term denotes a carbocyclic ring which is not a benzene ring or a polycyclo carbocyclic ring system which does not have a benzene ring as one of the cyclos.
ALKENYL
This term denotes an acyclic carbon chain which contains a carbon-to-carbon double bond and is represented by the formula -CnH2n-1.
ALKENYLENE
This term denotes an acyclic carbon chain which contains a carbon-to-carbon double bond and is represented by the formula -(CnH2n-2)-.
ALKYL
This term denotes an acyclic carbon or a saturated acyclic carbon chain represented by the formula -CnH2n+1.
ALKYLENE
This term denotes an acyclic carbon or a saturated acyclic carbon chain represented by the formula CnH2n-.
ALKYNYL
This term denotes an acyclic carbon chain which contains a carbon-to-carbon triple bond and is represented by the formula -(CnH2n-3)-.
ALKYNLENE
This term denotes an acyclic carbon chain which contains a carbon-to-carbon triple bond and is represented by the formula -(CnH2n-4).
AMINO NITROGEN
Denotes any nitrogen in an organic compound other than a nitrogen in an inorganic ion of an addition salt, a nitro (-NO2) or nitroso (-NO). Component parts of an "adduct" will be considered to be attached to each other ionically except if it is clear that the mode of attachment is nonionic.
ARYL RING OR RING SYSTEM
This term denotes a benzene ring or a polycyclo carbocyclic ring system having a benzene ring as one of the cyclos.
ATTACHED DIRECTLY OR BONDED DIRECTLY
These terms are used to show that specified moieties are connected by bonds only.
ATTACHED INDIRECTLY
This term denotes that at least one atom, as well as bonds, connects specified moieties.
BENZENE RING
This term includes, in all cases except where there are explicit limitations to the contrary, substituted benzene rings, including substitution in the form of an additional fused or bridged ring or ring system.
Thus, for example, if a subclass reads: "Benzene ring bonded directly to the five-membered hetero ring", the moiety bonded directly to the hetero ring may be phenyl, chlorophenyl, dinitrophenyl, naphthyl, etc. All that is necessary to satisfy the terminology of the subclass is that a substituted or unsubstituted benzene ring be bonded directly to the hetero ring.
CARBOCYCLIC
This term denotes a ring or ring system where all ring members are carbons.
CHAIN
This term denotes a plurality of atoms which connect specified groups or atoms. The atoms of the chain must be nonionically attached to each other and to the specified groups or atoms. If the chain may not include any ring members it will be designated as acyclic. When the chain may include ring members the title will state that the chain may include a ring. The chain ends where it attaches to the specified groups or atoms and does not include any part of them. The chain may have substituents but the substituents are not part of the chain.
CLATHRATES AND INTERCALATES (INCLUSION COMPOUNDS)
Clathrates and intercalates (inclusion compounds), per se, are classified hierarchically and subject to the limitations set forth in the compound (element) classes based both on the encapsulant and encapsulate. For example, a clathrate of urea and hydrogen peroxide is classified in Class 564, subclass 32, urea and an organic compound in Class 564, subclass 1.5, dextran and iodine in Class 536, subclass 112, etc. Where a patent does not state that a material is either a clathrate or an intercalate, the assumption is made that the material is either a coated or encapsulated product classified in Class 428, subclasses 402+.
CONTAINING
This term is to be interpreted broadly. In a subclass which specifies halogen containing, for example, the halogen may be attached to other parts of the compound by ionic bonding or nonionic bonding.
Further, the element contained in a material may be in any form. In a subclass such as-- Heavy metal containing catalyst (or material) utilized--, the metal may be in elemental or compound form.
CYCLO
This term refers to a ring of a polycyclo ring system.
HEAVY METAL
This term denotes any metal having a specific gravity greater than four and includes arsenic.
HETERO RING
This term denotes a ring having carbon and at least one atom from the group consisting of nitrogen, oxygen, sulfur, selenium and tellurium as ring members; and contains no other element as a ring member.
To qualify as a hetero ring, nonionic bonding must exist between all ring members. Inner salt compounds such as betaines, sulfobetaines, etc., wherein two ring members are attached to each other by ionic bonding, are not regarded as hetero rings.
INCLUDING HYDROGENATED
This term, as a parenthetical expression, is used following the name of a heterocyclic ring or ring system which is unsaturated, e.g., oxazoles, etc. For example, if a subclass is entitled "1, 3-Oxazoles (including hydrogenated)",
the parenthetical expression "(including hydrogenated)" means that the subclass is generic to fully unsaturated 1,3-oxazoles and to 1,3-oxazoles wherein one or two of the ring double bonds have been replaced by a single bond; i.e., the subclass is generic to oxazoles, oxazolines and oxazolidines.
When the name of such a heterocyclic ring is used in indents where no degree of ring saturation or unsaturation is specified, the name of the heterocyclic ring will again be construed as generic to all possible degrees of ring saturation and unsaturation.
If, for example, a subclass entitled "Nitrogen bonded directly to ring carbon of the oxazole ring were indented under "1, 3-Oxazoles (including hydrogenated" the nitrogen subclass be construed as embracing nitrogen bonded directly to the ring carbon of an oxazole, of an oxazoline, or of an oxazolidine.
When used following the name of a heterocyclic ring system, such as quinoline, the parenthetical expression indicates that the subclass is generic to compounds having the fully unsaturated form of the ring system, as well as to compounds wherein any number of ring double bonds of the ring system have been replaced by single bonds. The statement made above, re indents, is also applicable to heterocyclic ring systems.
INCLUSION COMPOUNDS
See CLATHRATES AND INTERCALATES (INCLUSION COMPOUNDS).
INTERCALATES
See CLATHRATES AND INTERCALATES (INCLUSION COMPOUNDS).
LIGHT METAL
This term denotes any metal having a specific gravity less than four.
METALS AND NONMETALS
Hydrogen, boron, carbon, silicon, nitrogen, phosphorous, oxygen, sulfur, selenium, tellurium, the noble gases and the halogens, including astatine, are considered to be nonmetals. All other elements, including arsenic, are considered metals.
MONOCYCLIC RING
This term denotes a ring which is not part of a polycyclo ring system.
NITROCYCLIC
This term denotes a ring or ring system where all ring members are nitrogens.
NONIONIC BONDING
As used in regard to bonding or attachment of specified moieties denotes the absence of ionic bonding between the moieties. If the moieties are attached directly, the bonds between them must be covalent or coordinate. If the moities are attached indirectly, each atom of the connecting chain must be attached by covalent or coordinate bonding to another atom of the connecting chain or to one of the moieties. However, the connecting chain may have substituents thereon which include ionic bonding. Some examples will be given of compounds which could be classified in a subclass having the following title: "Oxygen attached indirectly to the six-membered hetero ring by nonionic bonding". Two typical compounds which would be classified in such a subclass are shown in Figure 5.
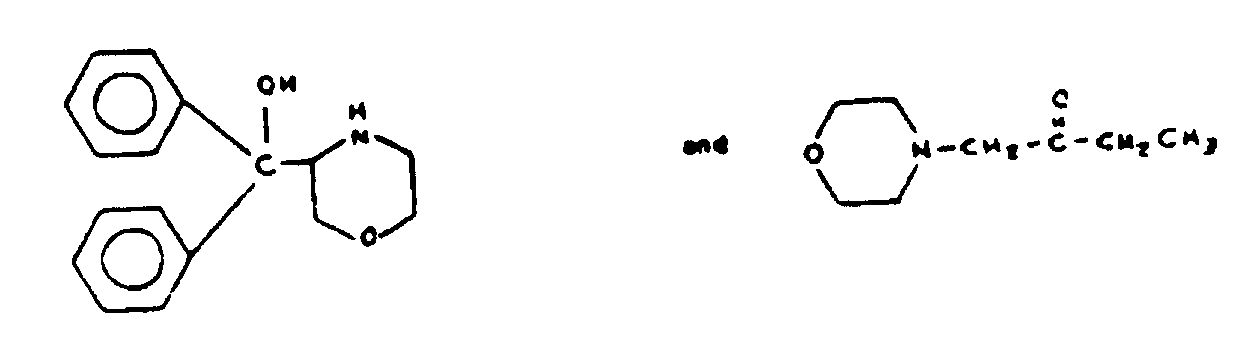
Figure 5
The three compounds shown in Figure 6 would also be classified in such a subclass but they are not typical.
The three atypical examples in Figure 6 are considered to meet the title since there is a chain of atoms between the hetero ring and the oxygen in which each atom is connected to the hetero ring, the oxygen, or another atom of the chain by nonionic bonding. The ionic bonding between the ring nitrogen and the oxygen in the two betaine inner salts is additional and does not keep the betaines out of such a subclass.
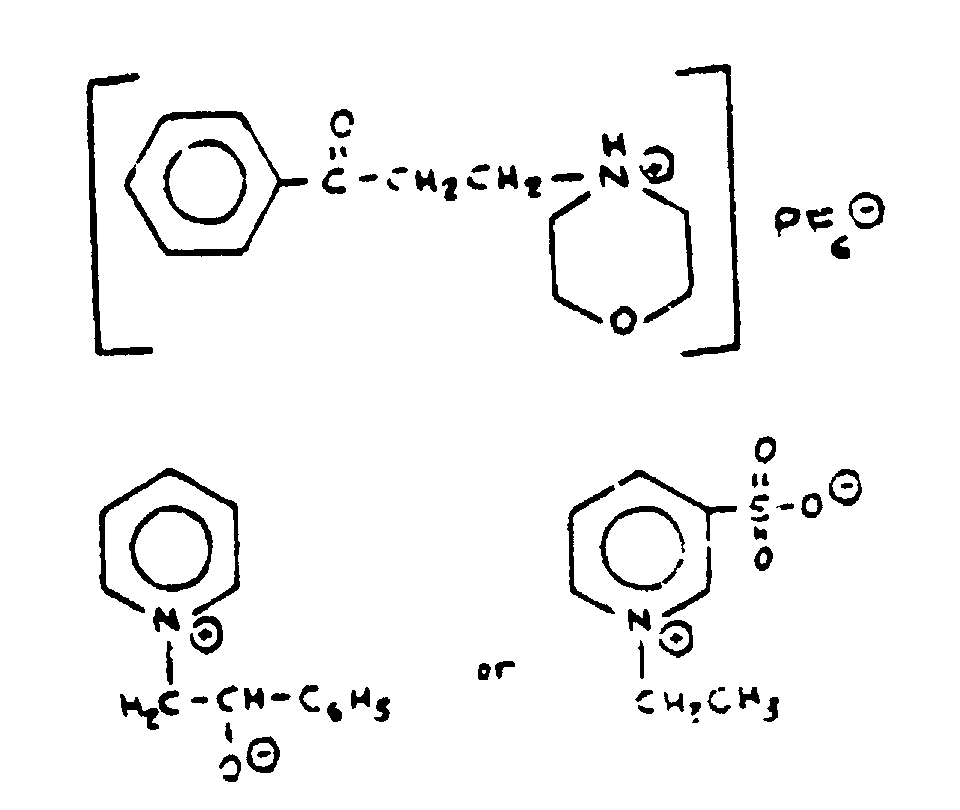
Figure 6
However, a structure such as that shown in Figure 7 is excluded since no oxygen is attached indirectly (or directly) to the six-membered hetero ring by nonionic bonding.
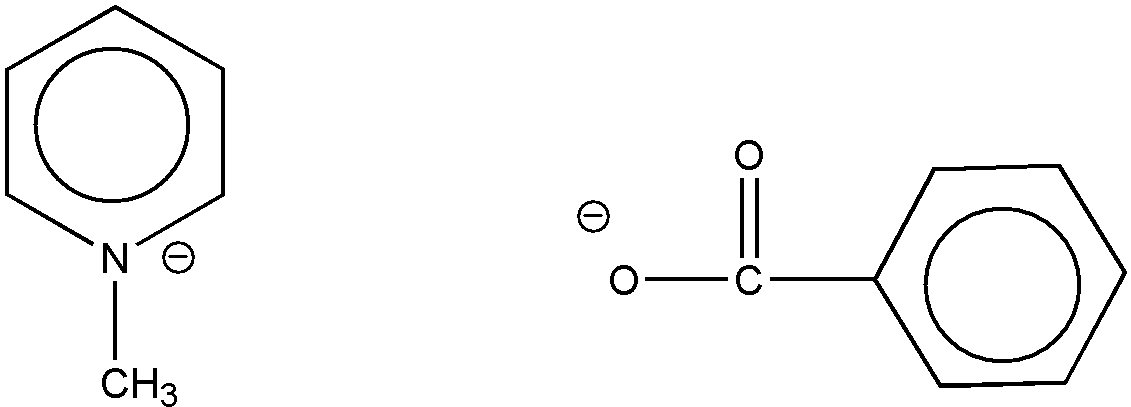
Figure 7
The oxygen of an N-oxide, as shown in Figure 8, is considered attached to the ring by nonionic bonding (coordinate bonding).
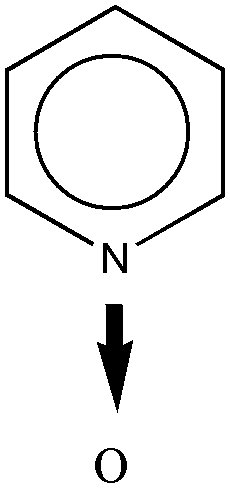
Figure 8
POLYCYCLO RING SYSTEM
This term denotes a compound which contains fused or bridged rings. The polycyclo ring system must contain at least two rings and each ring of the system must share two or more of its atoms with another ring of the system. All ring members must be attached to each other by nonionic bonding. The polycyclo ring system is usually only a moiety within a compound. Indents such as bicyclo and tricyclo limit the number of rings or cyclos in the polycyclo ring system to exactly two rings and three rings, respectively. For polycyclo systems having bridges, the system is regarded as composed only of the smallest number of smallest rings that will account for all atoms and valences. This is in accord with the nomenclature employed by The Ring Index, Second Edition, (1960). An example of the use of this system of nomenclature is shown in Figures 9 and 10, which show two different possible methods of representing the same compound.
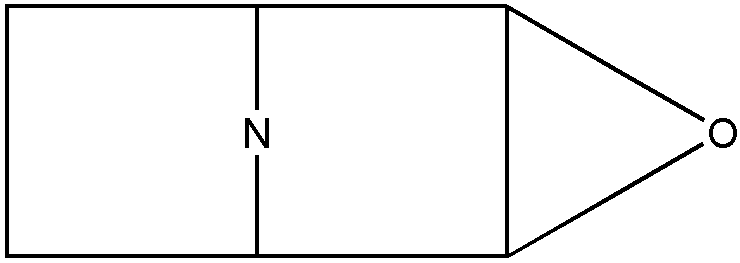
Figure 9
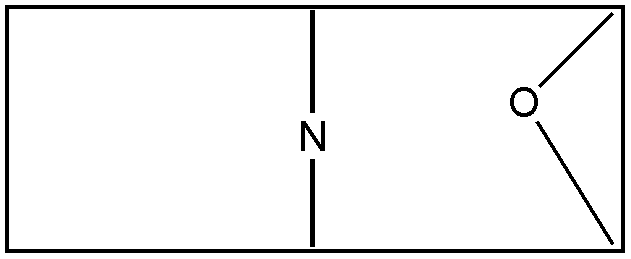
Figure 10
The represented compound should be considered as a (C4N-C4N-C2O) tricyclo system as in Figure 9, rather than as a (C4N-C4NO-C2O) tricyclo system as possibly seen in Figure 10. The former interpretation is the one with the smallest number of smallest rings that accounts for all atoms and valences.
A further example of the principle set forth above is illustrated by the compound of Figure 11, which is considered as a bicyclo system composed of a C2N2O ring and a C4O ring; it is not considered a diazine ring for classification.
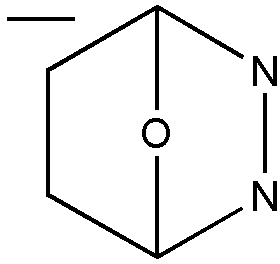
Figure 11
Another example is 3-azabicyclo [3.1.0] hexane, shown in Figure 12, which is classified with pyrrolidines by considering the structure a five-membered ring and a three-membered ring rather than with piperidines which would require considering it a six-membered ring.
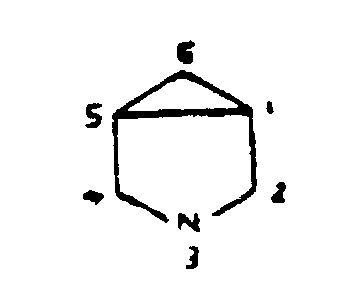
Figure 12
Similarly, the structure shown in Figure 13 is considered to be a pentacyclo ring system having three six-membered carbocyclic rings, one five-membered hetero ring consisting of one ring oxygen and four ring carbons, and one six-membered ring consisting of one ring nitrogen and five ring carbons.
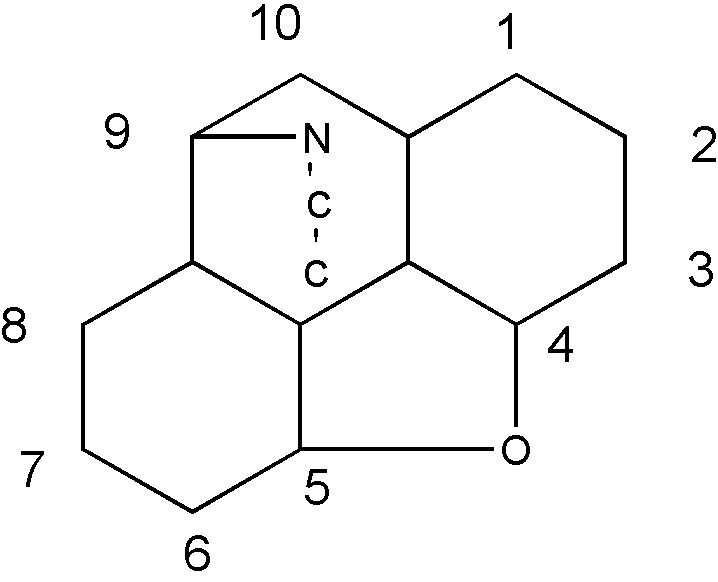
Figure 13
Betaine salts are sometimes shown as ring structures, e.g., as shown in Figure 14.
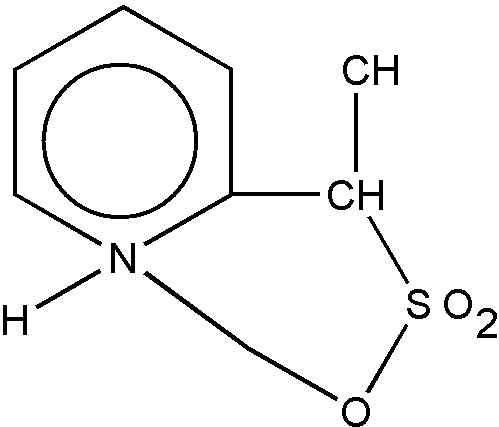
Figure 14
However, Figure 14 is not a polycyclo ring system because nonionic bonding does not exist between the N and O atoms. To be regarded as a hetero ring, nonionic bonding must exist between all members of a ring. The bonding between the N and O atoms in Figure 14 is ionic and such a compound is classified as represented by Figure 15.
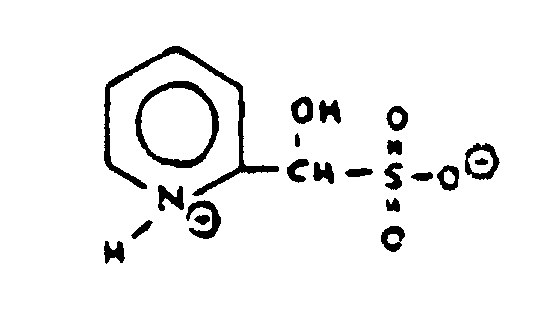
Figure 15
A structure of the type shown in Figure 16 is considered to be a polycyclo ring system composed of five rings: C4N-C4O-C4N-C4N-C4O-C12N2O2.
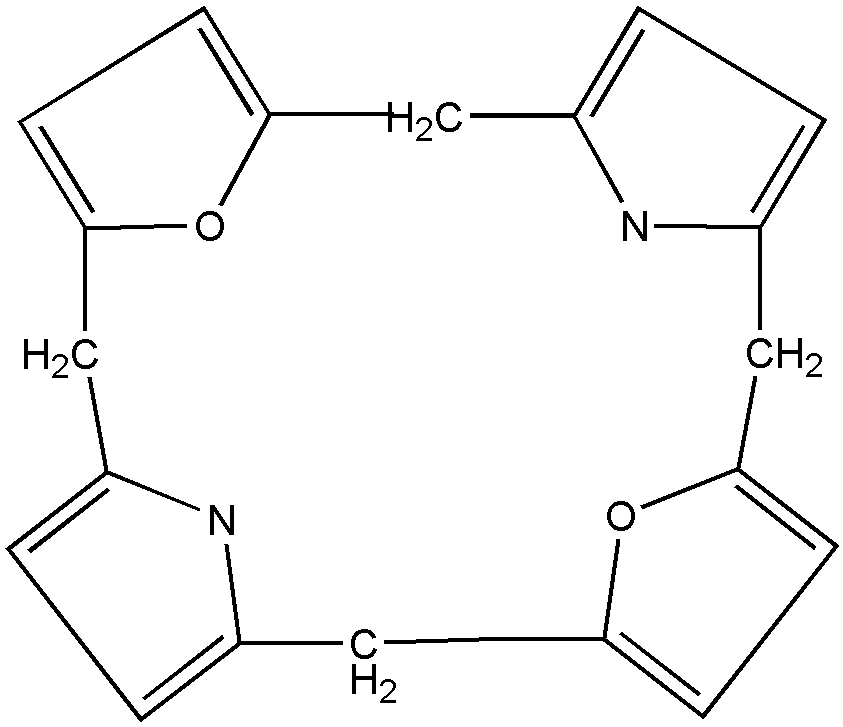
Figure 16
RING HETERO ATOM
This term denotes nitrogen, oxygen, sulfur, selenium or tellurium as a ring member, i.e., as one of the members which forms a hetero ring.
RING NITROGEN
This term denotes that nitrogen is one of the members which form a ring. Nitrogen bonded directly to a ring is not a ring nitrogen. Terms such as ring sulfur and ring oxygen are used similarly.
SPIRO and SPIRO RING SYSTEM
These terms denote the sharing of one common ring member only by exactly two rings. The following two structures shown in Figure 17 are illustrative of spiro systems:
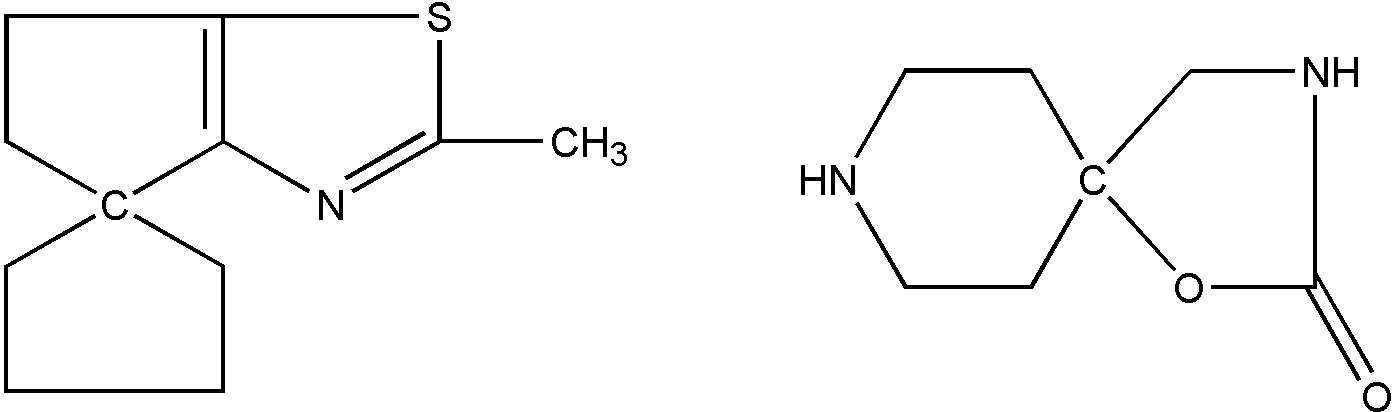
Figure 17
A structure such as that of Figure 18 is not spiro because the "C" shared by the two rings is also shared by a third ring.
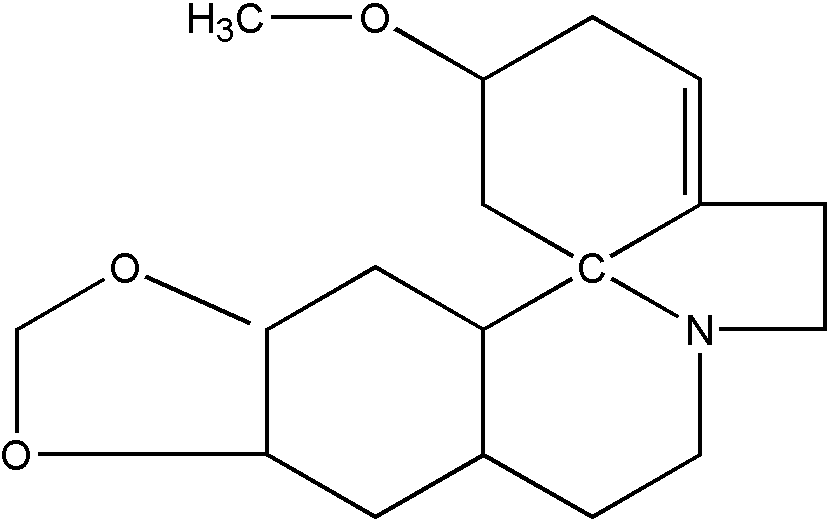
Figure 18
The common ring member must be attached to two ring members of each of the rings by nonionic bonding, and nonionic bonding must exist between all members of a ring in order for the ring to be regarded as a hetero ring. Thus, the structures shown in Figures 19 and 20 are excluded from the category of "spiro ring systems" because ionic bonding exists between the nitrogen hetero ring atom and an acyclic atom (an oxygen atom in each case) in the formation of these betaine inner salts.
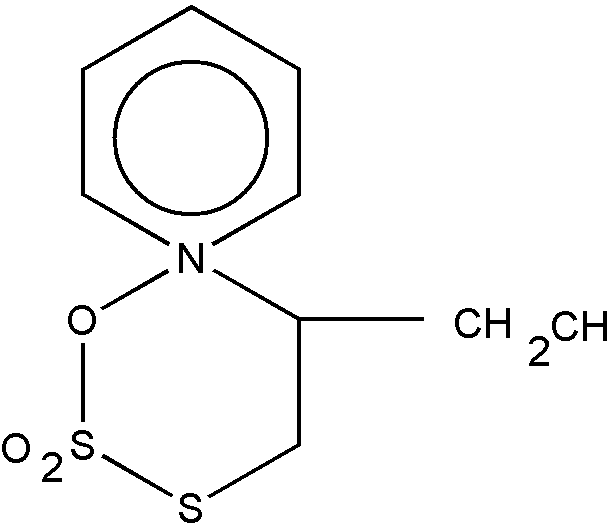
Figure 19
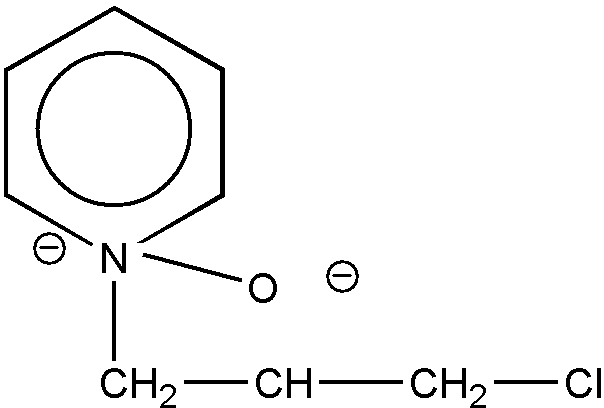
Figure 20
These two structures will be considered and classified in the forms depicted, respectively, as Figures 21 and 22.
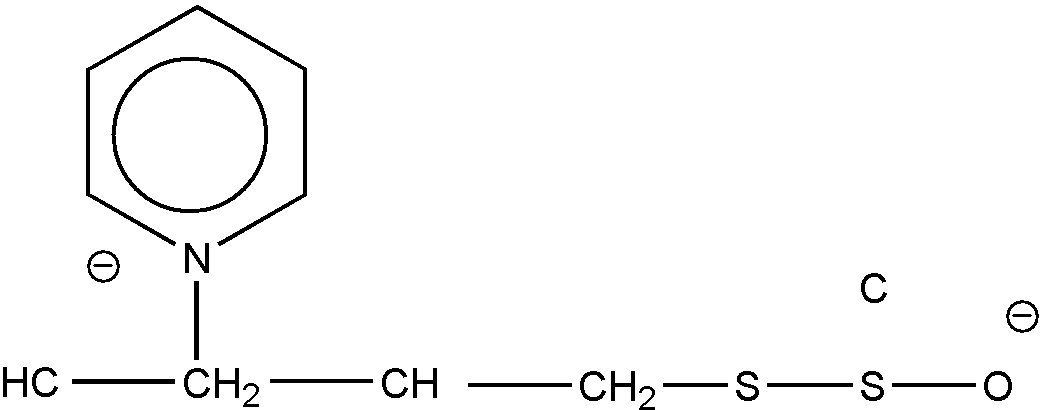
Figure 21
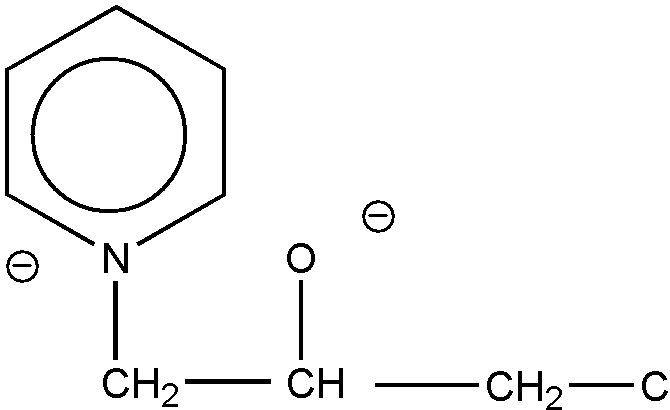
Figure 22