| CPC A61K 31/495 (2013.01) [A61K 9/008 (2013.01); A61K 9/0019 (2013.01); A61K 9/08 (2013.01); A61K 9/2027 (2013.01); A61K 9/2054 (2013.01); A61K 9/2059 (2013.01); A61K 9/4858 (2013.01); A61K 31/138 (2013.01); A61K 31/282 (2013.01); A61K 31/337 (2013.01); A61K 31/496 (2013.01); A61K 31/553 (2013.01); A61K 31/704 (2013.01); A61K 31/7048 (2013.01); A61K 33/24 (2013.01); A61K 33/243 (2019.01); A61K 38/05 (2013.01); A61K 45/06 (2013.01); A61K 47/10 (2013.01)] | 6 Claims |
|
1. A method of treating cancer comprising administering to a subject in need thereof an effective amount of PAC-1:
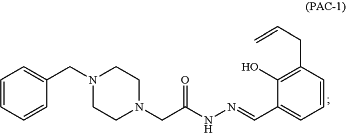 and an effective amount of sunitinib, wherein sunitinib is synergistic with PAC-1 in the treatment of the cancer, wherein PAC-1 has a concentration of about 2.5 μM to about 10 μM, sunitinib has a concentration of about 100 nM to about 10 μM, and the cancer is a carcinoid tumor.
|