| CPC C25B 3/07 (2021.01) [C25B 3/23 (2021.01); C25B 3/26 (2021.01); C25B 9/19 (2021.01); C25B 11/032 (2021.01); C25B 11/075 (2021.01)] | 13 Claims |
|
1. A process for the electrochemical formation of formate, wherein the process is performed in an electrochemical cell comprising a cathode compartment containing a cathode, an anode compartment containing a nickel-based anode and an ion exchange membrane separating the anode compartment from the cathode compartment, and wherein the process comprises:
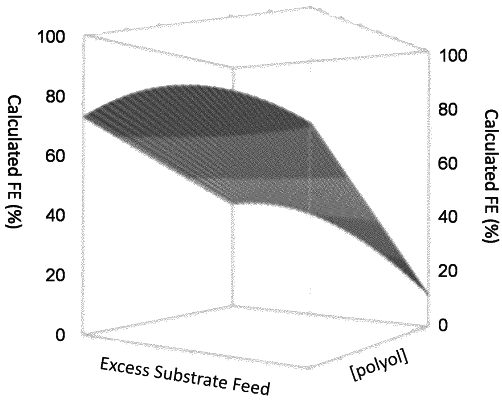
(a) feeding an anolyte comprising at least one polyol to the anode compartment at an excess polyol substrate feed from 5 to 200, wherein the excess polyol substrate feed is defined as the ratio between a molar polyol substrate feed at the anode and a maximum rate of polyol substrate conversion at the anode, which excess polyol substrate feed is expressed by the equation:
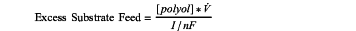 wherein, [polyol] is the steady state polyol concentration in the anolyte (M), V is the anolyte flow rate (L s−1), I is the current (A), n is the number of electrons involved in the oxidation of one polyol molecule and F is the Faraday constant;
(b) feeding a catholyte comprising carbon dioxide to the cathode compartment; and
(c) applying a voltage difference between the cathode and the anode, wherein, at the cathode, carbon dioxide is reduced to formate and at the anode the at least one polyol is oxidized to formate.
|