| CPC C09K 19/588 (2013.01) | 6 Claims |
|
1. A liquid-crystalline material comprising at least one chiral dopant selected from the group consisting of the structures
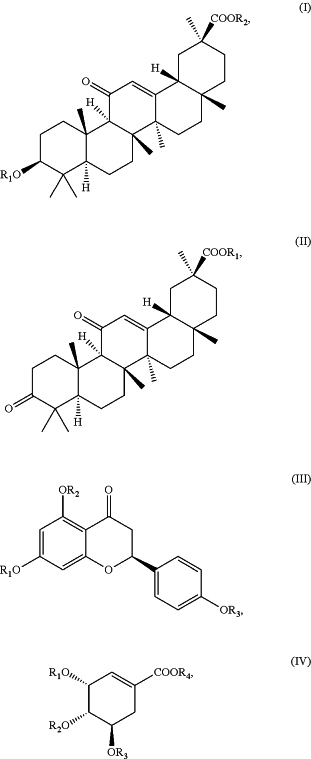 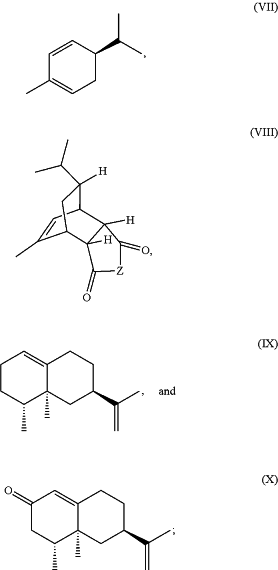 wherein R1, R2, R3, and R4 are each independently hydrogen, an aliphatic moiety, an aryl moiety, an arylalkylene moiety, an alkyl arylene moiety, an alkanoyl moiety, an arylalkanoyl moiety, or any halogenated derivative of the foregoing moieties, and
wherein Z is selected from C(H)R5, —CR5═CR5—, O, S, or NRs, wherein R5 is independently hydrogen, an aliphatic moiety, an aryl moiety, an arylalkylene moiety, an alkyl arylene moiety, an alkanoyl moiety, an arylalkanoyl moiety, or any halogenated derivative of the foregoing moieties, and
a nematic or a nematogenic substance.
|