| CPC C07F 5/025 (2013.01) [A61P 3/00 (2018.01); A61P 25/28 (2018.01); A61P 27/06 (2018.01); A61P 29/00 (2018.01); A61P 37/00 (2018.01); A61P 37/06 (2018.01)] | 48 Claims |
|
1. A compound represented by formula I or II:
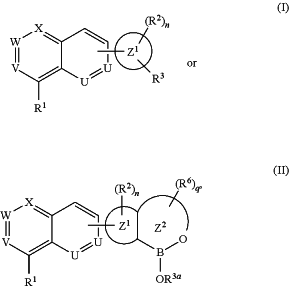 or a pharmaceutically acceptable salt thereof, wherein:
R1 is hydrogen, halogen, amino, hydroxyl, alkoxy, or alkylthio;
W is N;
V and X are selected such that either V is CRa and X is N, or V is N and X is CRb;
Ra is hydrogen, halogen, nitro, cyano, amino, hydroxyl, alkoxy, alkylthio, or alkyl;
Rb is hydrogen, halogen, nitro, cyano, amino, hydroxyl, alkoxy, alkylthio, alkyl, alkenyl, alkynyl, aralkyl, heteroaralkyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl;
each U independently is N or CRc;
each Rc independently is hydrogen, halogen, alkyl, or alkoxy;
ring Z1 is a five- or six-membered aryl or heteroaryl;
ring Z2 is a five- or six-membered heterocycle;
each R2 independently is halogen, nitro, cyano, amino, acylamino, amido, hydroxyl, alkoxy, alkylthio, acyl, amidino, azido, carbamoyl, carboxyl, carboxyester, guanidine, haloalkyl, haloalkoxy, heteroalkyl, imino, oxime, phosphonate, dialkylphosphine oxide, sulfonyl, sulfonamido, sulfonyl urea, sulfinyl, sulfinic acid, sulfonic acid, thiocyanate, thiocarbonyl, alkyl, aralkyl, heteroaralkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl; or two vicinal R2, together with the intervening carbon atoms to which they attach, combine to form a 5- or 6-membered carbocycle, 5- or 6-membered heterocycle, 5- or 6-membered aryl, or 5- or 6-membered heteroaryl;
n is 0 or an integer selected from 1-4, as valency permits;
each R6 independently is halogen, nitro, cyano, amino, acylamino, amido, hydroxyl, oxo, carboxyl, alkoxy, alkylthio, acyl, amidino, azido, carbamoyl, carboxyl, carboxyester, guanidine, haloalkyl, haloalkoxy, heteroalkyl, imino, oxime, phosphonate, dialkylphosphine oxide, sulfonyl, sulfonamido, sulfonyl urea, sulfinyl, sulfinic acid, sulfonic acid, thiocyanate, thiocarbonyl, alkyl, alkenyl, alkynyl, aralkyl, heteroaralkyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl; or any two R6, together with the intervening carbon atom(s) to which they attach, combine to form a carbocycle or heterocycle;
q is 0 or an integer selected from 1-4, as valency permits;
R3 is
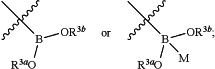 M is N(R8)3, N(R8)2, OR8 or SR8;
each R8 is independently hydrogen, alkyl, aralkyl, heteroaralkyl, alkenyl, alkynyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl; and
R3a and R3b independently are hydrogen, alkyl, acyl, alkenyl, alkynyl, aralkyl, heteroaralkyl, carbocyclyl, heterocyclyl, aryl, or heteroaryl; or R3a and R3b, together with the boron atom and the two intervening oxygen atoms that separate them, combine to form a monocyclic or polycyclic heterocyclyl; or R3a, R3b, and M, together with the boron atom and the intervening oxygen atoms, combine to form a polycyclic heterocycle.
|