| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01); C07D 215/54 (2013.01); C07D 401/12 (2013.01); C07D 417/12 (2013.01); C07D 487/04 (2013.01)] | 19 Claims |
|
1. A compound of Formula I:
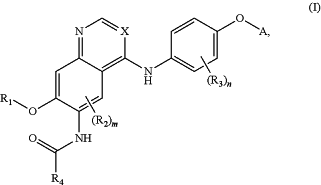 or a pharmaceutically acceptable salt or solvate thereof, wherein:
A is
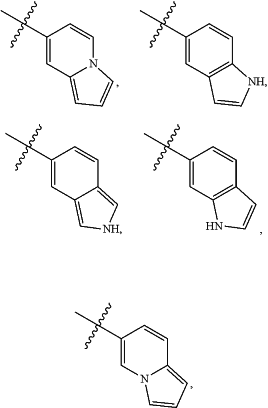 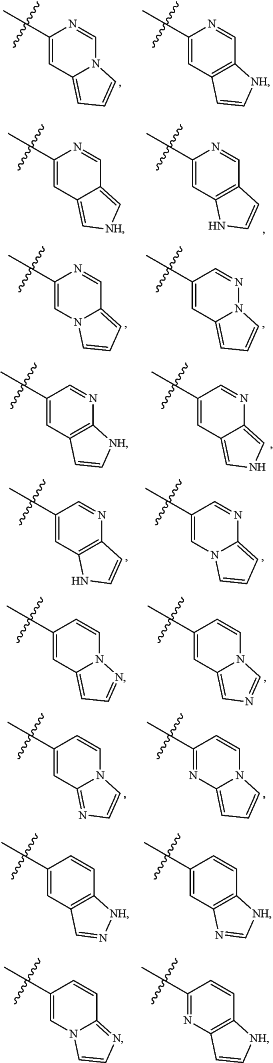 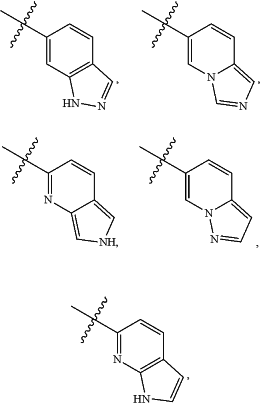 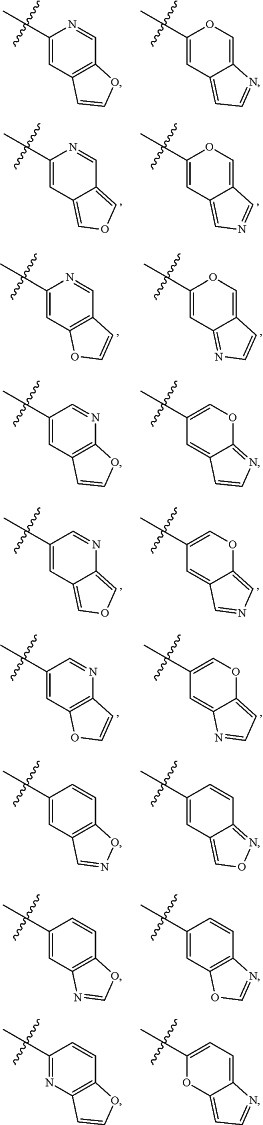 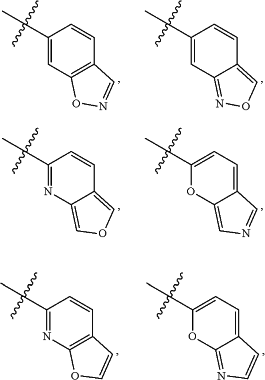 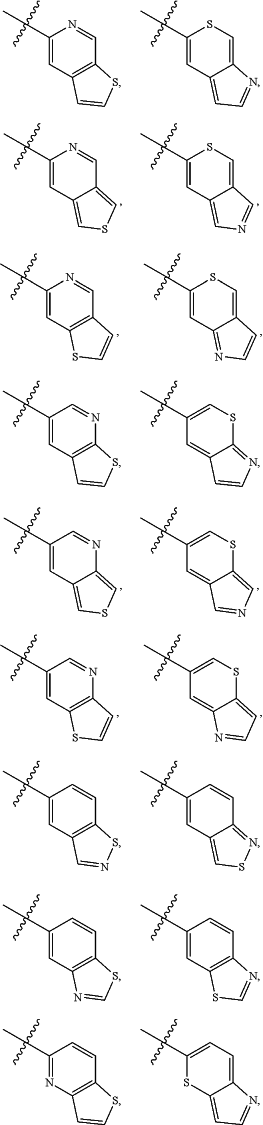 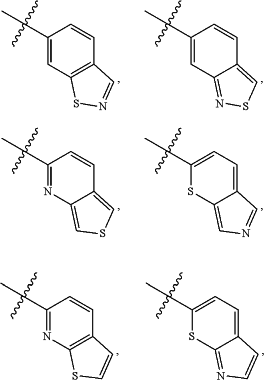 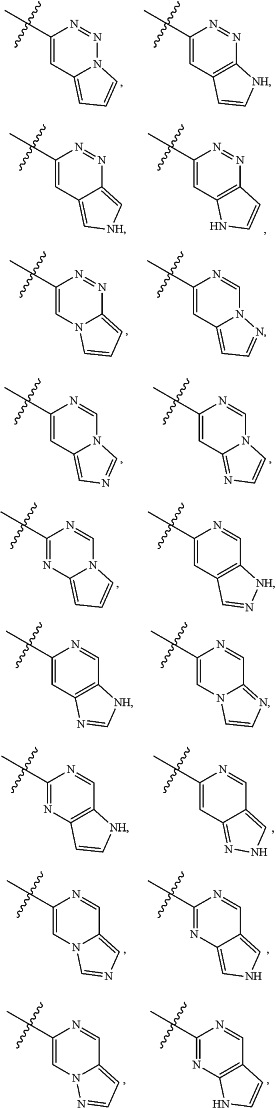 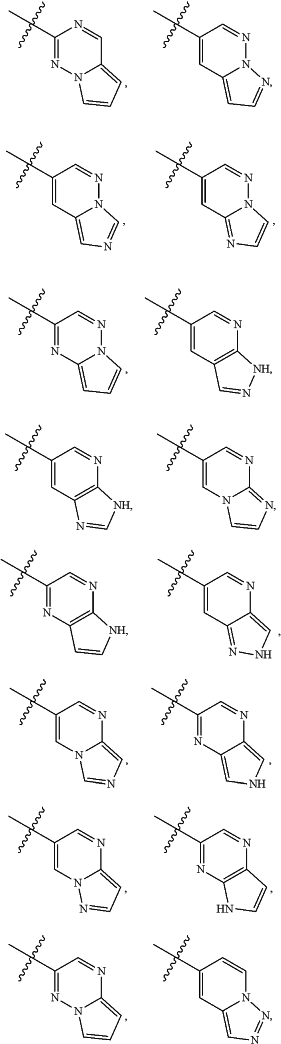 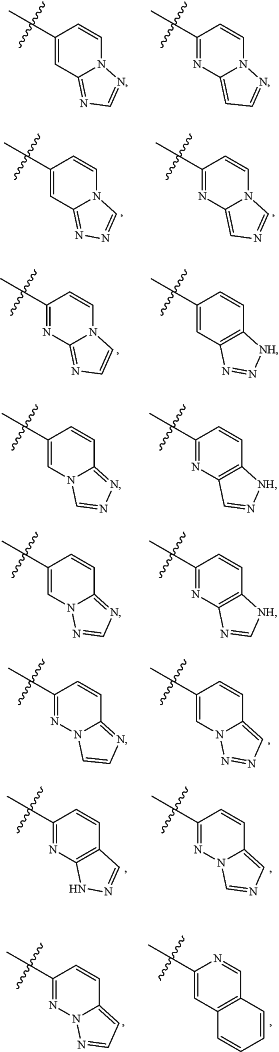 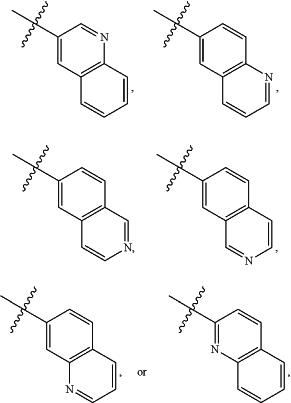 and is optionally substituted with one or more RA;
each RA is independently C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, halogen, OH, CN, NO2, NH2, C1-C6 alkylamino, or di-C1-C6 alkylamino;
X is C—CN;
R1 is C1-C6 alkyl or (CH2)1-3-heterocyclyl comprising one 5- or 6-membered ring and 1-3 heteroatoms selected N and O and at least one N, wherein the alkyl is optionally substituted with one or more RB′ and the heterocyclyl is optionally substituted with one or more RB;
m is 0, 1, or 2;
each R2 is independently C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, halogen, OH, CN, NO2, NH2, C1-C6 alkylamino, di-C1-C6 alkylamino, or NHC(O)R5;
n is 0, 1, 2, 3, or 4;
each R3 is independently C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkoxy, C1-C6 haloalkoxy, halogen, OH, CN, NO2, NH2, C1-C6 alkylamino, or di-C1-C6 alkylamino;
R4 and R5 are each independently C2-C4 alkenyl optionally substituted with one or more Rx;
each Rx is independently NH2, C1-C3 alkylamino, or di-C1-C3 alkylamino;
each RB′ is independently C1-C6 alkoxy, C1-C6 haloalkoxy, halogen, OH, CN, NO2, NH2, C1-C6 alkylamino, or di-C1-C6 alkylamino; and
each RB is independently C1-C6 alkyl, C1-C6 haloalkyl, or RB′,
wherein when A is 4-quinolinyl and n is 1, then R3 is not halogen.
|