| CPC C07D 403/12 (2013.01) [C07D 207/16 (2013.01); C07D 401/06 (2013.01); C07D 401/12 (2013.01); C07D 401/14 (2013.01); C07D 403/06 (2013.01); C07D 405/06 (2013.01); C07D 413/06 (2013.01); C07D 413/14 (2013.01); C07D 417/06 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 487/10 (2013.01); C07D 491/048 (2013.01); C07D 491/08 (2013.01); C07D 498/10 (2013.01)] | 41 Claims |
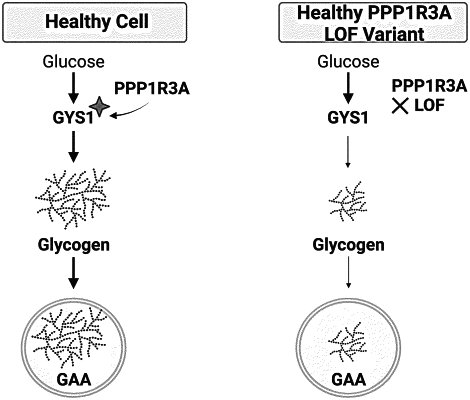
|
1. A compound of formula (I-A):
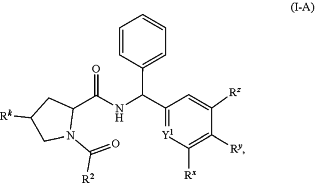 or a stereoisomer or tautomer thereof, or a pharmaceutically acceptable salt of any of the foregoing, wherein:
Y1 is CH or N;
Rx and Rz are independently H, halo, C1-6alkyl, or —NH2, wherein, when Y1 is CH, the C1-6alkyl of Rx or Rz may be optionally substituted with one or more halo;
Ry is (i) C1-6alkyl, or (ii) C3-10cycloalkyl, wherein the C3-10cycloalkyl is optionally substituted with one or more halo or C1-6alkyl;
Rk is H, halo, —OH, —NH2, or —NH—C(O)C1-6alkyl:
R2 is C1-6alkyl, wherein the C1-6alkyl of R2 is substituted with one or more Ra, wherein Ra is —OH or 5-20 membered heteroaryl, wherein the 5-20 membered heteroaryl of Ra is optionally substituted with one or more Rb; and
Rb is halo, C1-6alkyl, C1-6alkoxy, —NH2, —NH(C1-6alkyl), —N(C1-6alkyl)2, C3-10cycloalkyl, 3-15 membered heterocyclyl, or —C(O)—C1-6alkoxy, wherein the C1-6alkyl of Rb is optionally substituted with one or more halo, —NH2, —NH(C1-6alkyl), —N(C1-6alkyl)2, —NH—C(O)C1-6alkyl, or —NH—C(O)—C1-6alkoxy, and the 3-15-membered heterocyclyl of Rb is optionally substituted with one or more halo or —C(O)—C1-6alkoxy.
|