| CPC A61K 31/655 (2013.01) [A61K 33/24 (2013.01); A61K 39/42 (2013.01); A61K 45/06 (2013.01); A61P 31/00 (2018.01)] | 18 Claims |
|
1. A method of treating a pulmonary bacterial infection caused by a bacteria, comprising administering an effective antibacterial amount of a compound having the following formula:
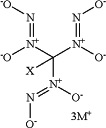 Formula I
wherein:
X is H or D,
and M+ is a pharmaceutically-acceptable cation selected from the group consisting of sodium, potassium, lithium, and quaternary ammonium salts,
to a patient in need of treatment thereof, wherein an effective antimicrobial amount of the compound is between 1 and 100 mg/kg, and
wherein the pulmonary bacterial infection is selected from the group consisting of Acetinobacter baumannii, Burkolderia cenocepacia, Escherichia coli, Haemophilus influenza, Mycobacterium abcessus, Pseudomonas aeruginosa, Staphylococcus aureus, and Streptococcus pyogenes.
|