| CPC A61K 31/496 (2013.01) [A61K 31/5355 (2013.01); A61K 45/06 (2013.01); C07D 205/04 (2013.01); C07D 207/12 (2013.01); C07D 211/46 (2013.01); C07D 211/58 (2013.01); C07D 241/04 (2013.01); C07D 295/112 (2013.01); C07D 295/13 (2013.01); C07D 413/12 (2013.01); C07D 471/04 (2013.01); C07D 519/00 (2013.01)] | 17 Claims |
|
1. A method for treating a cancer or a tumor comprising administering an effective amount of a compound of Formula (I), or a pharmaceutically acceptable salt thereof, or a pharmaceutical composition comprising an effective amount of the compound, or a pharmaceutically acceptable salt thereof, and a pharmaceutically acceptable carrier, diluent, excipient or combination thereof, to a subject having the cancer or the tumor, wherein the cancer or the tumor is selected from a bladder cancer, a brain cancer, a breast cancer, a bone marrow cancer, a cervical cancer, a colorectal cancer, an esophageal cancer, a hepatocellular cancer, a lymphoblastic leukemia, a follicular lymphoma, a lymphoid malignancy of T-cell or B-cell origin, a melanoma, a myelogenous leukemia, a Hodgkin's lymphoma, a Non-Hodgkin's lymphoma, a head and neck cancer (including oral cancer), an ovarian cancer, a non-small cell lung cancer, a chronic lymphocytic leukemia, a myeloma, a prostate cancer, a small cell lung cancer, a spleen cancer, a polycythemia vera, a thyroid cancer, an endometrial cancer, a stomach cancer, a gallbladder cancer, a bile duct cancer, a testicular cancer, a neuroblastoma, an osteosarcoma, an Ewings's tumor and a Wilm's tumor, Formula (I) having the structure:
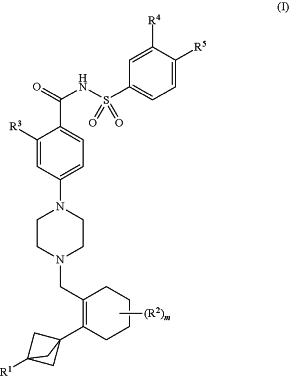 wherein:
R1 is selected from the group consisting of hydrogen, halogen, a substituted or unsubstituted C1-C6 alkyl, a substituted or unsubstituted C1-C6 haloalkyl, a substituted or unsubstituted C3-C6 cycloalkyl, a substituted or unsubstituted C1-C6 alkoxy, an unsubstituted mono-C1-C6 alkylamine and an unsubstituted di-C1-C6 alkylamine;
each R2 is independently selected from the group consisting of halogen, a substituted or unsubstituted C1-C6 alkyl, a substituted or unsubstituted C1-C6 haloalkyl and a substituted or unsubstituted C3-C6 cycloalkyl; or
when m is 2 or 3, each R2 is independently selected from the group consisting of halogen, a substituted or unsubstituted C1-C6 alkyl, a substituted or unsubstituted C1-C6 haloalkyl and a substituted or unsubstituted C3-C6 cycloalkyl, or two R2 groups taken together with the atom(s) to which they are attached form a substituted or unsubstituted C3-C6 cycloalkyl or a substituted or unsubstituted 3 to 6 membered heterocyclyl;
R3 is selected from the group consisting of X—R3A,
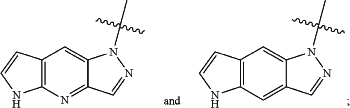 R3A is a substituted or unsubstituted 5 to 10 membered heteroaryl;
R4 is selected from the group consisting of NO2, S(O)R6, SO2R6, halogen, cyano and an unsubstituted C1-C6 haloalkyl;
R5 is selected from the group consisting of —X1-(Alk1)n-R7 and —X2(CHR8)-(Alk2)p-X3—R9;
Alk1 and Alk2 are independently selected from an unsubstituted C1-C4 alkylene and a C1-C4 alkylene substituted with 1, 2 or 3 substituents independently selected from fluoro, chloro, an unsubstituted C1-C3 alkyl and an unsubstituted C1-C3 haloalkyl;
R6 is selected from the group consisting of a substituted or unsubstituted C1-C6 alkyl, a substituted or unsubstituted C1-C6 haloalkyl and a substituted or unsubstituted C3-C6 cycloalkyl;
R7 is selected from a substituted or unsubstituted C1-C6 alkoxy, a substituted or unsubstituted C3-C10 cycloalkyl, a substituted or unsubstituted 3 to 10 membered heterocyclyl, hydroxy, amino, a substituted or unsubstituted mono-substituted amine group, a substituted or unsubstituted di-substituted amine group, a substituted or unsubstituted N-carbamyl, a substituted or unsubstituted C-amido and a substituted or unsubstituted N-amido;
R8 is selected from a substituted or unsubstituted 3 to 10 membered heterocyclyl(C1-C6 alkyl), a substituted or unsubstituted di-C1-C6 alkylamine(C1-C6 alkyl) and a substituted or unsubstituted mono-C1-C6 alkylamine(C1-C6 alkyl);
R9 is selected from a substituted or unsubstituted 5 to 10 membered heteroaryl and a substituted or unsubstituted C6-C10 aryl;
m is 0, 1, 2 or 3;
n and p are independently selected from 0 and 1;
X1 and X2 are —NH—; and
X and X3 are independently selected from the group consisting of —O—, —S— and —NH—.
|