| CPC A61K 31/436 (2013.01) [A61K 9/0014 (2013.01); A61K 9/06 (2013.01); A61K 47/06 (2013.01); A61K 47/12 (2013.01); A61K 47/14 (2013.01); A61K 47/20 (2013.01); A61K 47/22 (2013.01); A61K 47/44 (2013.01); A61P 17/00 (2018.01)] | 20 Claims |
|
1. A formulation for topical delivery of a therapeutic agent, comprising:
(a) a therapeutically effective amount of sirolimus, and
(b) a solvent comprising dimethyl sulfoxide (DMSO) and propylene carbonate;
(c) one or more pharmaceutically acceptable carriers, wherein the one or more pharmaceutically acceptable carriers comprise caprylocaproyl polyoxyl-8 glycerides ranging from about 1% to about 10% by weight in the formulation;
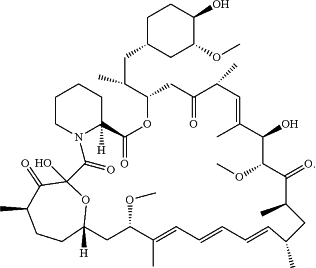
wherein the DMSO and the propylene carbonate are in a ratio selected from a range of from about 1:1 to about 1:10 and, at a temperature of 30° C. and a humidity of 65%, less than 5% of isomer C is detected in the formulation in about 2 weeks or less than 0.8% seco-rapamycin is detected in the formulation in about 2 weeks, wherein the isomer C is represented by the structure below
 |