| CPC G01N 33/6893 (2013.01) [G01N 33/6869 (2013.01); G16B 25/10 (2019.02); G01N 2333/521 (2013.01); G01N 2333/5421 (2013.01); G01N 2333/575 (2013.01); G01N 2400/40 (2013.01); G01N 2800/085 (2013.01)] | 1 Claim |
|
1. A method of diagnosing Non-Alcoholic Steatohepatitis (NASH) and/or the hepatic fibrosis status of a subject, wherein the method comprises:
quantifying, in a serum or plasma sample obtained from the subject, levels of IL-8, CXCL10 and hyaluronic acid (HA), said quantifying comprising contacting said sample with antibodies that bind to said at least three biomarkers and determining the levels of said IL-8, CXCL10 and HA in said sample;
calculating a value for f1(NASH) and/or f1(fibrosis), wherein
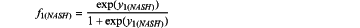 wherein f1(NASH):
y1(NASH)=(−2.3083+0.4079×IL8−0.0129×HA+0.0019×CXCL10)
and
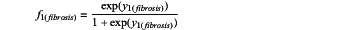 where
y1(fibrosis)=(−7.0457+1.0511×IL8+0.067×HA+0.0004×CXCL10)
and
when f1(NASH)>0.4997, the subject is diagnosed with NASH and when f1(NASH)≤0.4997, the subject is diagnosed with non-alcoholic fatty liver (NAFL) and when f1(fibrosis)>0.1953, the subject is diagnosed with advanced liver fibrosis and when f1(fibrosis)≤0.1953, the subject is diagnosed with early-moderate liver fibrosis,
said method further comprising administering to the subject having f1(NASH)>0.4997 and identified as having NASH a therapeutic agent selected from the group consisting of:
(a) elafibranor in an amount of about 50 mg/d to about 120 mg/d;
(b) saroglitazar in an amount of about 4 mg/d to about 20 mg/d;
(c) obeticholic acid in an amount of about 10 mg/d to about 25 mg/d;
(d) tropifexor in an amount of about 2 mg/d to about 20 mg/d;
(e) SEQ ID NO: 1 in an amount of about 1 mg/d to about 6 mg/d;
(f) aramchol in an amount of about 400 mg to about 600 mg;
(g) selonsertib in an amount of about 6 mg/d to about 40 mg/d alone or in combination with simtuzumab in an amount of about 125 mg per week or every two weeks;
(h) selonsertib in an amount of about 6 mg/d to about 40 mg/d alone or in combination with fenofibrate in an amount of about 30 mg/d to about 200 mg/d;
(i) cenicriviroc in an amount of about 75 mg/day to about 150 mg/d;
(j) emricasan in an amount of about 5 mg/BID to about 50 mg/BID;
(k) simtuzumab in an amount of about 75 mg to about 700 mg per week or every two weeks or and amount of about 75 mg to about 200 mg per week or every two weeks;
(l) galactoarabino rhamnogalacturonate in an amount of about 2 mg/d to 10 mg/kg lean body mass every other week;
(m) pioglitazone in an amount of about 30 mg/d alone or in combination with vitamin E in an amount of about 800 IU/d;
(n) pioglitazone in an amount of about 45 mg/d;
(o) aramchol in an amount of about 100 mg/d to about 300 mg/d;
(p) liraglutide in an amount of about 1.8 mg/d;
(q) pegylated FGF21 in an amount of about 5 mg/d to about 50 mg/day, administered subcutaneously;
(r) EDP-305 in an amount of about 5 mg/s to about 50 mg/d;
(s) encapsulated hyperimmune bovine colostrum enriched with anti-LPS antibodies in an amount of about 300 mg to about 1500 mg up to three times daily;
(t) lanifibranor in an amount of about 200 mg/d to about 1200 mg/d or about 400 mg/d;
(u) LMB763 in an amount of about 5 mg/d to about 750 mg/d;
(v) licogliflozin in an amount of about 100 mg/d to about 300 mg/d;
(w) MGL-3196 in an amount of about 60 mg/d to about 100 mg/d;
(x) MSDC 0602K in an amount of about 50 mg/d to about 300 mg/d;
(y) PF-05221304 in an amount of about 2 mg/d to about 60 mg/d;
(z) SAR425899 in an amount of about 20 microgram to about 300 microgram administered once daily by subcutaneous injection;
(aa) selonsertib+GS-0976+GS-9674 in an amount of about 15 mg/d to about 30 mg/d for each active agent;
(bb) semaglutide in an amount of about 0.1 mg to about 0.5 mg administered subcutaneously once daily; and
(cc) volixibat in an amount of about 5 mg/d to about 50 mg/d.
|