| CPC C07D 401/14 (2013.01) [A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61K 31/5377 (2013.01); A61K 31/55 (2013.01); A61K 45/06 (2013.01); C07D 405/14 (2013.01); C07D 413/14 (2013.01); C07D 417/14 (2013.01); C07D 487/08 (2013.01); C07D 491/107 (2013.01)] | 40 Claims |
|
1. A compound having Formula I:
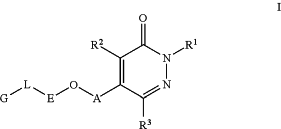 or a pharmaceutically acceptable salt, a solvate, a tautomer, a stereoisomer, or a deuterated analog thereof, wherein:
A is a 5-6 membered aromatic ring or a 4-7 membered nitrogen containing heterocycloalkyl, wherein A is substituted with 0-3 R4, provided that when ring A is a 4-7 membered nitrogen containing heterocycloalkyl, then the pyridazinone moiety of Formula I is attached to a nitrogen atom of A;
E is phenyl or a 5 or 6 membered heteroaryl, wherein E is substituted with 0-3 Q and 0-1 R11, provided that when E is a 5 or 6 membered heteroaryl, O is not attached to a heteroatom of E;
L is absent, —C(O)N(H)—, C0-C3alkylene, —N(H)—, or —O—;
G is one of the following groups:
(a) cycloalkyl substituted with 0-4 T1 and 0-1 T2;
(b) cycloalkenyl substituted with 0-4 T1 and 0-1 T2;
(c) a bridged carbocylic ring substituted with 0-4 T1 and 0-1 T2;
(d) a carbocyclic spiro ring containing two cycloalkyl groups joined by one common spiro carbon atom, wherein the carbocyclic spiro ring is substituted with 0-4 T1 and 0-1 T2;
(e) a heterocyclic spiro ring containing two cyclic groups with at least one heteroatom, wherein the two cyclic groups are joined by one common spiro carbon atom, wherein the heterocyclic spiro ring is substituted with 0-3 T5, 0-1 T6;
(f) phenyl substituted with 0-4 T1 and 0-1 T4;
(g) heterocycloalkyl substituted with 0-4 T5 and 0-1 T6;
(h) heterocycloalkenyl substituted with 0-4 T5 and 0-1 T6;
(i) a bridged heterocylic ring substituted with 0-4 T5 and 0-1 T6; or
(j) heteroaryl substituted with 0-3 T5 and 0-1 T3;
each Q is independently halogen, CN, or alkyl optionally substituted with 1-3 halogens;
each T1 is independently halogen, hydroxyl, alkyl optionally substituted with 1-3 Rb, alkenyl optionally substituted with 1-3 Rb, alkynyl optionally substituted with 1-3 Rb, CN, cyanoalkyl, alkoxyl optionally substituted with 1-3 Rb, or alkoxyalkyl optionally substituted with 1-3 Rb;
T2 is —(CH2)0-3—N(R9)SO2—R7, —(CH2)0-3—SO2—R7, —(CH2)0-3—SO2N(R9)R9, —(CH2)0-3—N(R9)SO2N(R9)R9, —(CH2)0-3—N(R9)C(O)N(R9)R9, —(CH2)0-3—N(R9)C(O)R8, —(CH2)0-3—N(R9)C(O)OR9, —(CH2)0-3—N(R9)R9, —(CH2)0-3—C(O)N(R9)R9, —(CH2)0-3—C(O)OR9, —(CH2)0-3—C(O)R10, —(CH2)0-3—C(O)H, —(CH2)0-3—N(R9)C(O)R10, —(CH2)0-3cycloalkyl optionally substituted with 1-4 Z3, —(CH2)0-3-phenyl optionally substituted with 1-3 Z5, or —(CH2)0-3heteroaryl optionally substituted with 1-3 Z5;
T3 is —(CH2)0-3—C(O)N(R9)R9, —(CH2)0-3—N(R9)R9, —(CH2)0-3—C(O)OR9, —(CH2)0-3-cycloalkyl, —(CH2)0-3-cycloalkenyl, —(CH2)0-3-heterocycloalkyl, —(CH2)0-3-heterocycloalkenyl, —O-heterocycloalkyl optionally substituted with 4-chloropyridazin-3-one-5-yl, or —(CH2)0-3-bridged carbocyclic ring, wherein the —(CH2)0-3-cycloalkyl, —(CH2)0-3-cycloalkenyl, —(CH2)0-3-heterocycloalkyl, —(CH2)0-3-heterocycloalkenyl, or —(CH2)0-3-bridged carbocyclic are each optionally substituted with 1-3 Z5 and 0-1 Z1, provided that when T3 is attached to a heteroatom of G, G cannot be attached to an oxygen or nitrogen atom of T3;
T4 is —(CH2)0-3C(O)OR9, —(CH2)0-3—N(R9)C(O)R9, —(CH2)0-3—N(R9)SO2—R7, —(CH2)0-3—SO2—R7, —(CH2)0-3—SO2N(R9)R9, —(CH2)0-3—N(R9)C(O)N(R′)R9, or N(Ra)2;
each T5 is independently halogen, hydroxyl, alkyl optionally substituted with 1-3 Rb, alkenyl optionally substituted with 1-3 Rb, alkynyl optionally substituted with 1-3 Rb, CN, cyanoalkyl, alkoxyl optionally substituted with 1-3 Rb, or alkoxyalkyl optionally substituted with 1-3 Rb, provided that when T5 is attached to a heteroatom of G, T5 cannot be halogen, hydroxyl, CN, or alkoxyl optionally substituted with 1-3 Rb;
T6 is —(CH2)0-3—N(R9)SO2—R7, —(CH2)0-3—SO2—R7, —(CH2)0-3—SO2N(R9)R9, —(CH2)0-3—N(R9)SO2N(R9)R9, —(CH2)0-3—N(R9)C(O)N(R9)R9, —(CH2)0-3—N(R9)C(O)R9, —(CH2)0-3—N(R9)C(O)OR9, —(CH2)0-3—N(R8)R9, —(CH2)0-3—C(O)—N(R8)R9, —(CH2)0-3—C(O)OR9, —(CH2)0-3—C(O)R10, —(CH2)0-3—N(R9)C(O)R10, —N(H)C(H)C═O, —(CH2)0-3cycloalkyl optionally substituted with 1-4 Z3, —(CH2)0-2heterocycloalkyl optionally substituted with 1-4 Z3, —(CH2)0-3heteroaryl optionally substituted with 1-3 Z5, or 4-chloropyridazin-3-one-5-yl, provided that when T6 is attached to a heteroatom of G, G cannot be attached to an oxygen or nitrogen atom of T6;
Ra is H or alkyl;
Rb is halogen, CN, CF3, or hydroxyl, provided that not more than 1 Rb can be CF3;
R1 is C1-C4alkoxyC1-C4alkyl, C2-C4alkenyl substituted with 1-3 Z2, or C2-C4alkyl substituted with 1-3 Z2;
R2 is H, halogen, alkyl, alkenyl, alkoxyl, haloalkyl, CF3, or CN;
R3 is H, halogen, alkyl, CN, or haloalkyl;
each R4 is independently halogen, CN, or alkyl optionally substituted with 1-3 halogens;
R7 is alkyl optionally substituted with 1-4 Z4, —C0-C3alkyl-cycloalkyl optionally substituted with 1-4 Z3, —C0-C3alkyl-phenyl optionally substituted with 1-4 Z3, —C0-C3alkyl-heteroaryl optionally substituted with 1-3 Z5, or —C0-C3alkyl-heterocycloalkyl optionally substituted with 1-3 Z5;
R8 is H, alkyl optionally substituted with 1-4 Z4, alkenyl optionally substituted with 1-4 Z4, —C0-C3alkyl-cycloalkyl optionally substituted with 1-4 Z3, —C0-C3alkyl-phenyl optionally substituted with 1-4 Z3, —C0-C3alkyl-heteroaryl optionally substituted with 1-3 Z5, —C0-C3alkyl-heterocycloalkyl optionally substituted with 1-3 Z5, or a bridged carbocylic ring substituted with 0-5 T1;
each R9 is independently H or alkyl optionally substituted with 1-4 Z4;
R10 is alkyl substituted with 0-4 Z4, —C0-C3alkyl-cycloalkyl optionally substituted with 1-4 Z3, —C0-C3alkyl-phenyl optionally substituted with 1-4 Z3, —C0-C3alkyl-heteroaryl optionally substituted with 1-3 Z5, or —C0-C3alkyl-heterocycloalkyl optionally substituted with 1-3 Z5;
R11 is NH2;
Z1 is cyanoalkyl, —(CH2)0-2—C(O)OR9, —(CH2)0-2—C(O)—N(R8)R9, provided that when Z1 is attached to a heteroatom, then Z1 is not C(O)OR9;
each Z2 is independently hydroxyl, halogen, NH2, or CN, provided that not more than 1 Z2 can be NH2;
each Z3 is independently alkyl, halogen, haloalkyl, hydroxyl, hydroxyalkyl, alkoxyl, alkoxyalkyl, or CN;
each Z4 is independently hydroxyl, halogen, alkoxyl, or CN; and
each Z5 is independently alkyl, haloalkyl, hydroxyl, hydroxyalkyl, halogen, alkoxyl, alkoxyalkyl, CN, or cyanoalkyl, provided that when Z5 is attached to a heteroatom, then Z5 is not halogen, hydroxyl, alkoxyl, or CN.
|