| CPC A61K 47/6803 (2017.08) [A61K 47/6849 (2017.08); A61K 47/6851 (2017.08); A61K 47/6855 (2017.08); A61K 47/6857 (2017.08); A61K 47/6863 (2017.08); A61K 47/6865 (2017.08); C07K 16/30 (2013.01)] | 18 Claims |
|
1. A compound of one of the formulae below:
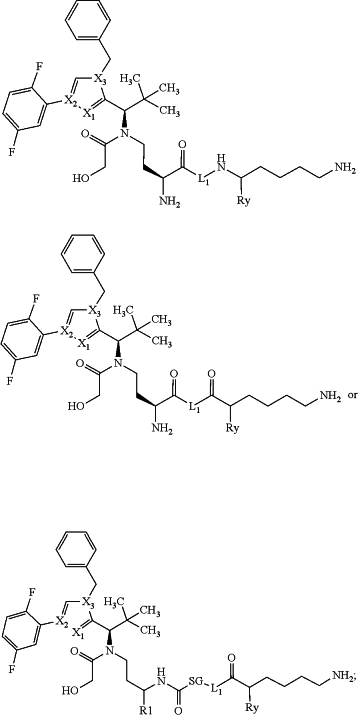 or a salt, solvate, salt of a solvate, or epimer thereof, wherein:
X1 is N, X2 is N and X3 is C;
or X1 is N, X2 is C and X3 is N;
or X1 is CH or CF, X2 is C and X3 is N;
or X1 is NH, X2 is C and X3 is C;
or X1 is CH, X2 is N and X3 is C;
L1 is an organic group not cleavable in vivo;
SG is an in vivo cleavable 2-8 oligopeptide group;
Ry is —H, —C(═O)—NH-alkyl, —NH—C(═O)-alkyl, —C(═O)—NH2, or —NH2;
R1 is —H, -MOD, or —(CH2)0-3Z;
wherein:
-MOD is the group —(NR10)n-(G1)o-G2-G3;
wherein:
R10 is —H or C1-C3 alkyl;
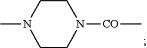
G1 is —NH—C(═O)—, —C(═O)NH— or
 G2 is a linear and/or branched hydrocarbon group which has 1 to 10 carbon atoms and which may be interrupted once or more than once by one or more of the groups —O—, —S—, —S(═O)—, —S(═O)2—, —NRy—, —NRyC(═O)—, —C(═O)—NRy—, —NRyNRy—, —S(═O)2—NRyNRy—, —C(═O)—NRyNRy—, —C(═O)—, —CRx═N—O—, and where the hydrocarbon chain is optionally substituted with —NH—C(═O)—NH2, —COOH, —OH, —NH2, NH—CNNH2, sulphonamide, sulphone, sulphoxide or sulphonic acid;
wherein:
Ry is —H, phenyl, C1-C10-alkyl, C2-C10-alkenyl or C2-C10-alkynyl, each of which may be substituted by —NH—C(═O)—NH2, —COOH, —OH, —NH2, NH—CN—NH2, sulphonamide, sulphone, sulphoxide or sulphonic acid;
Rx is —H, C1-C3-alkyl or phenyl;
G3 is —H or —COOH; wherein MOD has at least one —COOH group;
n is 0 or 1; and
o is 0 or 1;
Z is —H, halogen, —OY3, —SY3, —NHY3, —C(═O)—NY1Y2 or —C(═O)—OY3,
Y1 is —H, —NH2, —(CH2CH2O)0-3—(CH2)0-3Z′ or —CH(CH2W)Z′
Y2 is —H, —NH2, —(CH2CH2O)0-3—(CH2)0-3Z′ or —CH(CH2W)Z′,
Y3 is —H or —(CH2)0-3Z′,
wherein:
W is —H or —OH,
Z′ is —H, —NH2, —SO3H, —COOH, —NH—C(═O)—CH2—CH2—CH(NH2)COOH or —(C(═O)—NH—CHY4)1-3COOH, and
Y4 is linear or branched C1-6-alkyl which is optionally substituted by —NH—C(═O)—NH2,
or Y4 is an aryl or benzyl, which are each optionally substituted by —NH2.
|