| CPC A61K 39/12 (2013.01) [C07K 7/06 (2013.01); G01N 33/505 (2013.01); G01N 33/6893 (2013.01)] | 11 Claims |
|
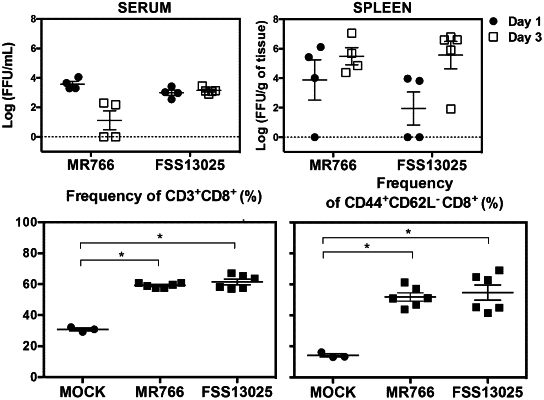
1. A method of inducing, enhancing, or sustaining an immune response against Zika virus in a subject, the method comprising contacting T cells of the subject with an effective amount of a composition comprising an acceptable carrier or diluent, and one or more peptides selected from the group of a peptide consisting of an amino acid sequence set forth in any one of SEQ ID NO: 125 to SEQ ID NO: 129.
|