| CPC A61K 31/497 (2013.01) [A61K 31/4985 (2013.01); A61K 31/501 (2013.01); A61K 31/506 (2013.01); A61P 25/28 (2018.01); C07D 403/14 (2013.01); C07D 413/14 (2013.01); C07D 471/04 (2013.01); C07D 487/04 (2013.01); C07D 519/00 (2013.01)] | 37 Claims |
|
1. A compound of Formula I:
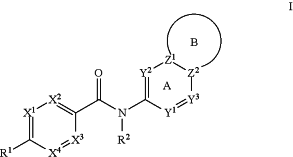 or an isotopically enriched analog, pharmaceutically acceptable salt, tautomer, stereoisomer, or a mixture of stereoisomers thereof, wherein:
X1, X2, X3, and X4 are CR4 or N, wherein at least two but no more than three of X1, X2, X3, and X4 are N;
each R4 is independently hydrogen, halo, hydroxy, C1-6alkyl, C1-6haloalkyl, or C1-6alkoxy;
Y1 is CR5 or N;
R5 is hydrogen, cyano, halo, hydroxy, C1-6alkyl, C1-6haloalkyl, C1-6alkylthio, C1-6alkoxy, C1-6haloalkoxy, heterocyclyl, —NH2, —NHR17, or —N(R17)2, and optionally substituted on an available nitrogen atom with C1-6alkyl or C1-6haloalkyl;
Y2 is absent, CR6 or N;
R6 is hydrogen, cyano, halo, hydroxy, C1-6alkyl, C1-6haloalkyl, C1-6alkylthio, C1-6alkoxy, C1-6haloalkoxy, heterocyclyl, —NH2, —NHR17, or —N(R17)2, and optionally substituted on an available nitrogen atom with C1-6alkyl or C1-6haloalkyl; and
Y3 is CR3 or N;
R3 is hydrogen, cyano, halo, hydroxy, C1-6alkyl, C1-6haloalkyl, C1-6alkylthio, C1-6alkoxy, C1-6haloalkoxy, heterocyclyl, —NH2, —NHR17, or —N(R17)2, and optionally substituted on an available nitrogen atom with C1-6alkyl or C1-6haloalkyl;
each R17 is independently C1-4alkyl, or two R17 join, with any intervening atoms, to form a 3- to 6-membered heterocyclyl;
each of Z1 and Z2 is C or N;
Ring A and Ring B together form a 9-membered bicyclic heteroaryl containing 1 to 3 ring nitrogen atoms;
Ring B contains 1 to 3 heteroatoms independently selected from N and O, and is substituted on available carbon atom(s) with 1 to 3 substituents independently selected from halo, hydroxy, C1-6alkyl, C1-6haloalkyl, C1-6alkoxy, and C1-6haloalkoxy, or is substituted on an available nitrogen atom with C1-6alkyl or C1-6haloalkyl;
R1 is -L1-R11, wherein L1 is —O—, —S—, —S(O)—, —S(O)2—, —N(R12)—, —C1-3alkylene-, —O—C1-3alkylene-, —N(R12)—C1-3alkylene, or absent, and R11 is C2-6alkynyl, C3-10cycloalkyl, heteroaryl, or heterocyclyl, wherein R11 is optionally substituted with 1 to 4 R13 groups;
R12 is hydrogen or C1-6alkyl;
each R13 is independently selected from halo, cyano, hydroxy, C1-6alkyl optionally substituted with R16, C1-6haloalkyl, C1-6hydroxyalkyl, C3-10cycloalkyl optionally substituted with R16, C3-10cycloalkyl-C1-6alkyl optionally substituted with R16, C6-10aryl optionally substituted with R16, C6-10aryl-C1-6alkyl optionally substituted with R16, heteroaryl optionally substituted with R16, heteroaryl-C1-6alkyl optionally substituted with R16, heterocyclyl optionally substituted with R16, heterocyclyl-C1-6alkyl optionally substituted with R16, OR14, —NH2, —NHR14—N(R14)2, —C1-6alkylene-NH2, —C1-6alkylene-NHR14, —C1-6alkylene-N(R14)2, —C(O)R15, —C(O)OR15, —C(O)NHR15, —C(O)N(C1-4alkyl)R15, —S(O)2R15, —S(O)R15, —NHC(O)R15, —N(C1-4alkyl)C(O)R15, —NHS(O)R15, —N(C1-4alkyl)S(O)R15, —NHS(O)2R15, and —N(C1-4alkyl)S(O)2R15;
each R14 is independently selected from C1-6alkyl, C3-10cycloalkyl, C6-10aryl, heteroaryl, and heterocyclyl; and each R14 is optionally substituted with one to six halo, C1-3alkyl, C1-3alkoxy, C3-10cycloalkyl, or —NHSO2-aryl-N(CH3)2;
each R15 is independently hydrogen, —OH, C1-6alkyl, C1-6haloalkyl, C3-10cycloalkyl, C6-10aryl, heteroaryl, or heterocyclyl;
each R16 is independently halo, cyano, hydroxy, —NH2, —NHR21, —N(R21)2, C1-6alkyl, C1-6haloalkyl, OR21, or C3-10cycloalkyl;
each R21 is independently selected from C1-6alkyl, C3-10cycloalkyl, C6-10aryl, heteroaryl, and heterocyclyl, and each R21 is optionally substituted with one to six halo or C1-3alkoxy; and
R2 is hydrogen or C1-6alkyl.
|