| CPC A61K 31/4709 (2013.01) [A61K 9/0053 (2013.01); A61K 31/519 (2013.01); A61P 21/00 (2018.01)] | 5 Claims |
|
1. A method for modulating DUX4 activity in a subject in need thereof comprising administering to the subject in need thereof a composition comprising a compound, or a pharmaceutically acceptable salt thereof, having the structure of Formula (III):
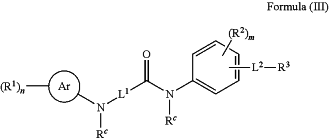 wherein:
Ar is phenyl or 5- or 6-membered heteroaryl;
each R1 is independently hydrogen, halogen, —CN, —OH, —OR20, —SH, —SR20, —NO2, —NR21R21, —S(═O)2R20, —NR21S(═O)2R20, —S(═O)R20, —S(═O)2NR21R21, —C(═O)R20, —OC(═O)R20, —C(═O)OR21, —OC(═O)OR21, —C(═O)NR21R21, —OC(═O)NR21R21, —NR21C(═O)NR21R21, —NR21C(═O)R20, —NR21C(═O)OR21, unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted C2-C6 alkenyl, unsubstituted or substituted C2-C6 alkynyl, unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, unsubstituted or substituted heteroaryl, unsubstituted or substituted —C1-C6-alkylene-cycloalkyl, unsubstituted or substituted —C1-C6-alkylene-heterocycloalkyl, unsubstituted or substituted —C1-C6-alkylene-aryl, or unsubstituted or substituted —C1-C6-alkylene-heteroaryl;
n is 0-5;
L1 is absent or *—(CRaRb)—C(═O)—, wherein * denotes attachment point to the carbonyl carbon;
Ra and Rb are independently selected from hydrogen, C1-C6 alkyl, or C1-C6 haloalkyl;
or Ra and Rb are taken together with the carbon to which they are attached to form a 3-, 4-, 5-, or 6-membered cycloalkyl or a 3-, 4-, 5-, or 6-membered heterocycloalkyl;
each R2 is independently hydrogen, halogen, —CN, —OH, —OR20, —NR21R21, —C(═O)R20, —OC(═O)R20, —C(═O)OR21, —C(═O)NR21R21, —NR21C(═O)R20, C1-C6 alkyl, or C1-C6 haloalkyl;
or two R2 on adjacent atoms are taken together with the atoms to which they are attached to form an unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, or unsubstituted or substituted heteroaryl;
m is 0-4;
L2 is absent, —O—, —O—(C1-C4 alkylene)-, or —NRc—C(═O)—;
each Rc is independently selected from hydrogen, C1-C6 alkyl, or C1-C6 haloalkyl;
R3 is unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, or unsubstituted or substituted heteroaryl; wherein when R3 is substituted, it is substituted by 1-3 R4;
each R4 is independently hydrogen, halogen, —CN, —OH, —OR20, —SH, —SR20, —NO2, —NR21R21, —S(═O)2R20, —NR21S(═O)2R20, —S(═O)R20, —S(═O)2NR21R21, —C(═O)R20, —OC(═O)R20, —C(═O)OR21, —OC(═O)OR21, —C(═O)NR21R21, —OC(═O)NR21R21, —NR21C(═O)NR21R21, —NR21C(═O)R20, —NR21C(═O)OR21, unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted C2-C6 alkenyl, unsubstituted or substituted C2-C6 alkynyl, unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, or unsubstituted or substituted heteroaryl;
each R20 is independently selected from unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted C2-C6 alkenyl, unsubstituted or substituted C2-C6 alkynyl, unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, or unsubstituted or substituted heteroaryl;
each R21 is independently selected from hydrogen, unsubstituted or substituted C1-C6 alkyl, unsubstituted or substituted C1-C6 alkenyl, unsubstituted or substituted C1-C6 alkynyl, unsubstituted or substituted cycloalkyl, unsubstituted or substituted heterocycloalkyl, unsubstituted or substituted aryl, or unsubstituted or substituted heteroaryl;
or two R21 on the same N atom are taken together with the N atom to which they are attached to form an unsubstituted or substituted N-containing heterocycle;
wherein when any R1, R2, R4, R20, or R21 is substituted, substituents on the R1, R2, R4, R20, or R21 are independently selected at each occurrence from halogen, —CN, —NO2, —OR22, —CO2R22, —C(═O)R23, —C(═O)N22R22, —NR22R22, —NR22C(═O)R23, —NR22C(═O)OR22, —SR22, —S(═O)R23, —SO2R23, —SO2NR22R22, C1-C6 alkyl, C1-C6 haloalkyl, monocyclic cycloalkyl, monocyclic heterocycloalkyl, phenyl, benzyl, 5-membered heteroaryl, and 6-membered heteroaryl; or two substituents on the same carbon atom are taken together to form a C═O or C═S;
each R22 is independently selected from hydrogen, C1-C6 alkyl, C3-C6 cycloalkyl, phenyl, benzyl, 5-membered heteroaryl, and 6-membered heteroaryl;
or two R22 groups are taken together with the N atom to which they are attached to form a N-containing heterocycle; and
each R23 is independently selected from C1-C6 alkyl, C3-C6 cycloalkyl, phenyl, benzyl, 5-membered heteroaryl, and 6-membered heteroaryl.
|