| CPC A61J 3/00 (2013.01) [G16H 20/13 (2018.01); G16H 20/17 (2018.01); A61J 2200/70 (2013.01); A61J 2205/10 (2013.01); A61J 2205/20 (2013.01)] | 20 Claims |
|
1. A system to create one or more personalized individual unit doses associated with treatment of an individual, the system comprising:
a polymer carrier matrix selected from the group consisting of polyethylene oxide, polyvinyl pyrrolidone, polyvinyl alcohol, hydrophilic cellulosic polymer, and combinations thereof;
an active;
an apparatus configured to combine the polymer carrier matrix and the active into a self-supporting film using a mixing and film-forming process which provides for uniformity of content in IUD's cut from said film the apparatus being a film-forming assembly or a sheet-forming assembly selected from the group consisting of a wet casting assembly or a hot melt extrusion assembly, and optionally further including a spray-drying assembly;
a networked control system comprising:
computing device; and
a memory storage storing executable instructions that, when executed by the computing device, cause the computing device to perform operations comprising:
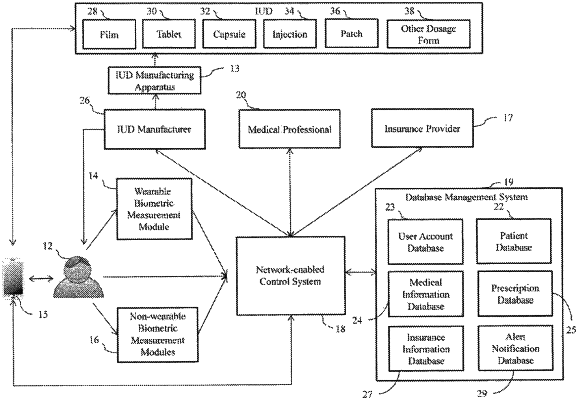
electronically communicating health information measured from the individual to a medical professional who determines a medical prescription based on the health information, the medical prescription comprising a prescribed amount of the active to be included in the personalized individual unit dose (IUD);
electronically communicating the medical prescription from the medial professional to an IUD manufacturer and directing the IUD manufacturer to make the film from the polymer carrier matrix and the active using the apparatus in accordance with the prescribed amount of the active comprised in the medical prescription associated with treatment of the individual; and
an additional apparatus for dividing or cutting the self-supporting film into a plurality of personalized IUDs, wherein each of the personalized IUDs contain an amount of the active which does not vary more than 10% from the prescribed amount of the active.
|