| CPC A61K 9/0043 (2013.01) [A61K 9/16 (2013.01); A61K 9/1617 (2013.01); A61K 9/1623 (2013.01); A61K 9/1652 (2013.01); A61K 31/00 (2013.01); A61K 31/5513 (2013.01); A61M 15/003 (2014.02); A61P 25/18 (2018.01)] | 33 Claims |
|
1. A method for acutely treating agitation in a human subject, comprising:
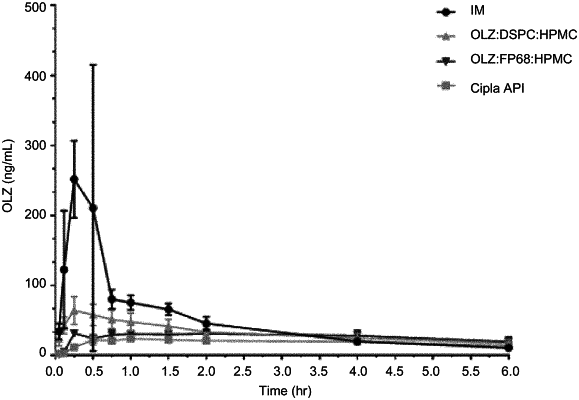
intranasally administering, using an intranasal delivery device, a single dose of a dry pharmaceutical composition comprising 2-20 mg of olanzapine as a single active ingredient to the subject exhibiting agitation, thereby reducing agitation within 30 minutes,
wherein the intranasal delivery device comprises a compound chamber containing the dry pharmaceutical composition and a separate propellant canister containing propellant;
wherein the propellant released from the canister contacts and propels dry pharmaceutical composition through a narrow, targeted delivery plume for intranasal delivery;
wherein the intranasal administration provides a shorter median Tmax compared to intramuscular or oral administration of the single dose of olanzapine, and
wherein the intranasal administration provides a mean peak plasma olanzapine concentration (Cmax) of at least 25 ng/ml.
|