| CPC C07F 5/027 (2013.01) [A61P 31/10 (2018.01); C07C 49/84 (2013.01); C07C 233/65 (2013.01); C07C 233/73 (2013.01); C07C 243/38 (2013.01); C07D 207/08 (2013.01); C07D 211/16 (2013.01); C07D 217/04 (2013.01); C07D 233/64 (2013.01); C07D 239/26 (2013.01); C07D 277/24 (2013.01); C07D 277/26 (2013.01); C07D 277/66 (2013.01); C07D 295/192 (2013.01); C07D 295/215 (2013.01); C07D 307/52 (2013.01); C07D 307/80 (2013.01); C07D 307/83 (2013.01); C07D 405/04 (2013.01); C07D 405/12 (2013.01); C07D 413/04 (2013.01)] | 12 Claims |
|
1. A compound of Formula (I), or a salt thereof;
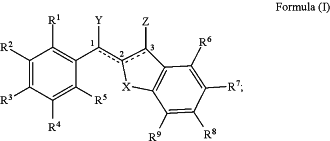 wherein:
X is O, S, or NR10;
R1 is H;
R2, R3, and R4 are each independently selected from the group consisting of —OH, —OC(═O)R11, and —OC(═O)OR11;
R5 is H;
R6 is selected from the group consisting of H, —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, F, Cl, Br, I, —CN, —NO2, —OR11, —SR11, —S(═O)R11, —S(═O)2R11, —NHS(═O)2R11, —C(═O)R11, —OC(═O)R11, —CO2R11, —OCO2R11, —CH(R11)2, —N(R11)2, —C(═O)N(R11)2, —C(═O)NHR11, —OC(═O)N(R11)2, —NHC(═O)NH(R11), —NHC(═O)R11, —NHC(═O)OR11, —C(OH)(R11)2, and —C(NH2)(R11)2,
wherein each occurrence of R11 in R6 is independently selected from the group consisting of —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, and —OH;
R7 and R8 are selected such that only one of the following conditions is met:
(i) R7 is represented by Formula (IV):
 wherein in Formula (IV):
* represents the attachment to Formula (I);
A1, A2, A3, and A4 are independently CR13 or N;
R12 is selected from the group consisting of H, C1-C6 alkyl, C1-C6 fluoroalkyl, C1-C6 perfluoroalkyl, C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, F, Cl, Br, I, —CN, —NO2, —OH, —OR14, —SR14, —S(═O)R14, —S(═O)2R14, —S(═O)2NHR14, —S(═O)2N(R14)2, —NHS(═O)2R14, —C(═O)R14, —OC(═O)R14, —CO2R14, —OCO2R14, —CH(R14)2, —N(R14)2, —C(═O)N(R14)2, —C(═O)NHR14, —OC(═O)N(R14)2, —NHC(═O)NH(R14), —NHC(═O)R14, —NHC(═O)OR14, —C(OH)(R14)2, and —C(NH2)(R14)2;
each occurrence of R13 is independently selected from the group consisting of H, C1-C6 alkyl, F, Cl, Br, I, —CN, —NO2, —OH, —CF3, C1-C6 heteroalkyl, aryl-(C1-C3)alkyl, and cycloalkyl;
or optionally two adjacent R13 are joined to form a ring;
each occurrence of R14 is independently selected from the group consisting of H, C1-C6 alkyl, F, Cl, Br, I, —CN, —NO2, —OH, —CF3, C1-C6 heteroalkyl, aryl-(C1-C3)alkyl, and cycloalkyl;
or optionally two R14 on the same atom may together form a ring; and
R8 is selected from the group consisting of H, —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, F, Cl, Br, I, —CN, —NO2, —OH, —OR11, —SR11, —S(═O)R11, —S(═O)2R11, —NHS(═O)2R11, —C(═O)R11, —OC(═O)R11, —CO2R11, —OCO2R11, —CH(R11)2, —N(R11)2, —C(═O)N(R11)2, —C(═O)NHR11, —OC(═O)N(R11)2, —NHC(═O)NH(R11), —NHC(═O)R11, —NHC(═O)OR11, —C(OH)(R11)2, and —C(NH2)(R11)2;
(ii) R7 is selected from the group consisting of H, —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, F, Cl, Br, I, —CN, —NO2, —OH, —OR11, —SR11, —S(═O)R11, —S(═O)2R11, —NHS(═O)2R11, —C(═O)R11, —OC(═O)R11, —CO2R11, —OCO2R11, —CH(R11)2, —N(R11)2, —C(═O)N(R11)2, —C(═O)NHR11, —OC(═O)N(R11)2, —NHC(═O)NH(R11), —NHC(═O)R11, —NHC(═O)OR11, —C(OH)(R11)2, and —C(NH2)(R11)2; and
R8 is selected from the group consisting of —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, —CN, —NO2, —SR11, —S(═O)R11, —S(═O)2R11, —NHS(═O)2R11, —C(═O)R11, —CO2R11, —OCO2R11, —CH(R11)2, —N(R11)2, —C(═O)N(R11)2, —C(═O)NHR11, —OC(═O)N(R11)2, —NHC(═O)NH(R11), —NHC(═O)R11, —NHC(═O)OR11, —C(OH)(R11)2, and —C(NH2)(R11)2;
R9 and R10 are each independently selected from the group consisting of H, —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, F, Cl, Br, I, —CN, —NO2, —OH, —OR11, —SR11, —S(═O)R11, —S(═O)2R11, —NHS(═O)2R11, —C(═O)R11, —OC(═O)R11, —CO2R11, —OCO2R11, —CH(R11)2, —N(R11)2, —C(═O)N(R11)2, —C(═O)NHR11, —OC(═O)N(R11)2, —NHC(═O)NH(R11), —NHC(═O)R11, —NHC(═O)OR11, —C(OH)(R11)2, and —C(NH2)(R11)2;
each occurrence of R11 in R2, R3, R4, R7, R8, R9, or R10 is independently selected from the group consisting of H, —C1-C6 alkyl, —C1-C6 fluoroalkyl, —C1-C6 heteroalkyl, aryl, heteroaryl, cycloalkyl, heterocycloalkyl, and —OH; and
the bond between carbons 1 and 2, the bond between carbons 2 and 3, Y, and Z are selected such that only one of the two following conditions is met:
(a) the bond between carbons 1 and 2 is a single bond, the bond between carbons 2 and 3 is a double bond, Y is (═O), and Z is H; or
(b) the bond between carbons 1 and 2 is a double bond, the bond between carbons 2 and 3 is a single bond, Y is H, and Z is (═O).
|