| CPC C07D 471/04 (2013.01) [A61P 35/00 (2018.01); C07D 487/04 (2013.01)] | 21 Claims |
|
1. A compound of Formula I:
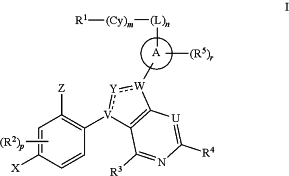 or a pharmaceutically acceptable salt thereof, wherein:
Ring A is a C6-10 aryl group, 5-14 membered heteroaryl group, C3-14 cycloalkyl group, or 4-14 membered heterocycloalkyl group;
U is N or CRU, wherein RU is H, halo, CN, OH, C1-4 alkyl, C1-4 alkoxy, amino, C1-4 alkyl amino, or C2-8 dialkylamino;
the moiety
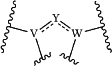 is selected from:
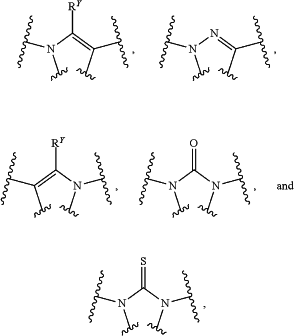 wherein RY is H, halo, CN, OH, C1-4 alkyl, C1-4 alkoxy, amino, C1-4 alkyl amino, or C2-8 dialkylamino;
X is F or C1;
L is selected from —C1-6 alkylene- and —(C1-4 alkylene)a-Q-(C1-4 alkylene)b-, wherein the C1-6 alkylene group and any C1-4 alkylene group of the —(C1-4 alkylene)a-Q-(C1-4 alkylene)b- group is optionally substituted with 1, 2, or 3 substituents independently selected from halo, CN, OH, C1-3 alkyl, C1-3 alkoxy, C1-3 hydroxyalkyl, C1-3 haloalkyl, C1-3 haloalkoxy, amino, C1-3 alkylamino, and di(C1-3 alkyl)amino;
Q is —O—, —S—, —S(═O)—, —S(═O)2—, —C(═O)—, —C(═O)NRq1—, —C(═O)O—, —OC(═O)NRq1—, —NRq1—, —NRq1C(═O)O—, —NRq1C(═O)NRq1—, —S(═O)2NRq1—, —C(═NRq2)—, or —C(═NRg2)—NRq1—, wherein each Rq is independently selected from H, C1-6 alkyl, and C1-3 hydroxyalkyl, and wherein each Rq is independently selected from H, C1-6 alkyl, and CN;
Cy is a linking C6-14 aryl, linking C3-18 cycloalkyl, linking 5-16 membered heteroaryl, or linking 4-18 membered heterocycloalkyl group, each of which is optionally substituted with 1, 2, 3, or 4 substituents independently selected from RCy;
each RCy is independently selected from halo, C1-6 alkyl, C1-4 haloalkyl, C1-4 cyanoalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, CN, NO2, ORa1, SRa1, C(O)Rb1, C(O)NRc1Rd1, C(O)ORa1, OC(O)Rb1, OC(O)NRc1Rd1, C(═NRe1)NRc1Rd1, NRc1C(═NRe1)NRc1Rd1, NRc1Rd1, NRc1C(O)Rb1, NRc1C(O)ORa1, NRc1C(O)NRc1Rd1, NRc1S(O)Rb1, NRc1S(O)2Rb1, NRc1S(O)2NRc1Rd1, S(O)Rb1, S(O)NRc1Rd1, S(O)2Rb1, and S(O)2NRc1Rd1, wherein said C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, and 4-10 membered heterocycloalkyl are each optionally substituted by 1, 2, 3, or 4 substituents independently selected from CN, NO2, ORa1, SRa1, C(O)Rb1, C(O)NRc1Rd1, C(O)ORa1, OC(O)Rb1, OC(O)NRc1Rd1, C(═NRe1)NRc1Rd1, NRc1C(═NRe1)NRc1Rd1, NRc1Rd1, NRc1C(O)Rb1, NRc1C(O)ORa1, NRc1C(O)NRc1Rd1, NRc1S(O)Rb1, NRc1S(O)2Rb1, NRc1S(O)2NRc1Rd1, S(O)Rb1, S(O)NRc1Rd1, S(O)2Rb1, and S(O)2NRc1Rd1;
R1 is H, Cy1, halo, C1-6 alkyl, C1-4 haloalkyl, C1-4 cyanoalkyl, C2-6 alkenyl, C2-6 alkynyl, CN, NO2, ORa2, SRa2, C(O)Rb2, C(O)NRc2Rd2, C(O)ORa2, OC(O)Rb2, OC(O)NRc2Rd2, C(═NRe2)NRc2Rd2, NRc2C(═NRe2)NRc2Rd2, NRc2Rd2, NRc2C(O)Rb2, NRc2C(O)ORa2, NRc2C(O)NRc2Rd2, NRc2S(O)Rb2, NRc2S(O)2Rb2, NRc2S(O)2NRc2Rd2, S(O)Rb2, S(O)NRc2Rd2, S(O)2Rb2 and S(O)2NRe2Rd2, wherein said C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl are each optionally substituted by 1, 2, 3, or 4 substituents independently selected from halo, CN, NO2, ORa2, SRa2, C(O)Rb2, C(O)NRc2Rd2, C(O)ORa2, OC(O)Rb2, OC(O)NRc2Rd2, C(═NRe2)NRc2Rd2, NRc2C(═NRe2)NRc2Rd2, NRc2Rd2, NRc2C(O)Rb2, NRc2C(O)ORa2, NRc2C(O)NRc2Rd2, NRc2S(O)Rb2, NRc2S(O)2Rb2, NRc2S(O)2NRc2Rd2, S(O)Rb2, S(O)NRc2Rd2, S(O)2Rb2, and S(O)2NRc2Rd2;
Z is Cy2, C1-4 haloalkyl, C1-4 cyanoalkyl, C2-6 alkenyl, C2-6 alkynyl, CN, NO2, ORa3, SRa3, C(O)Rb3, C(O)NRc3Rd3, C(S)NRc3Rd3, C(O)ORa3, OC(O)Rb3, OC(O)NRc3Rd3, C(═NRe3)NRc3Rd3, NRc3C(═NRe3)NRc3Rd3, NRc3Rd3, NRc3C(O)Rb3, NRc3C(O)ORa3, NRc3C(O)NRc3Rd3, NRc3S(O)Rb3, NRc3S(O)2Rb3, NRc3S(O)2NRc3Rd3, S(O)Rb3, S(O)NRc3Rd3, S(O)2Rb3, S(O)2NRc3Rd3, and P(O)Rc3Rd3 wherein said C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl are each optionally substituted by 1, 2, 3, or 4 substituents independently selected from Cy2, halo, CN, NO2, CN, NO2, ORa3, SRa3, C(O)Rb3, C(O)NRc3Rd3, C(O)ORa3, OC(O)Rb3, OC(O)NRc3Rd3, C(═NRe3)NRc3Rd3, NRc3C(═NRe3)NRc3Rd3, NRc3Rd3, NRc3C(O)Rb3, NRc3C(O)ORa3, NRc3C(O)NRc3Rd3, NRc3S(O)Rb3, NRc3S(O)2Rb3, NRc3S(O)2NRc3Rd3, S(O)Rb3, S(O)NRc3Rd3, S(O)2Rb3, and S(O)2NRc3Rd3;
each R2, R3, R4, and R5 is independently selected from H, halo, C1-6 alkyl, C1-4 haloalkyl, C1-4 cyanoalkyl, C2-6 alkenyl, C2-6 alkynyl, CN, NO2, ORa4, SRa4, C(O)Rb4, C(O)NRc4Rd4, C(O)ORa4 OC(O)Rb4, OC(O)NRc4Rd4, C(═NRe4)NRc4Rd4, NRc4C(═NRe4)NRc4Rd4, NRc4Rd4, NRc4C(O)Rb4, NRc4C(O)ORa4, NRc4C(O)NRc4Rd4, NRc4S(O)Rb4, NRc4S(O)2Rb4, NRc4S(O)2NRc4Rd4, S(O)Rb4, S(O)NRc4Rd4, S(O)2Rb4, and S(O)2NRc4Rd4, wherein said C1-6 alkyl, C2-6 alkenyl, and C2-6 alkynyl are each optionally substituted by 1, 2, 3, or 4 substituents independently selected from halo, CN, NO2, ORa4, SRa4, C(O)Rb4, C(O)NRc4Rd4, C(O)ORa4, OC(O)Rb4, OC(O)NRc4Rd4, C(═NRe4)NRc4Rd4, NRc4C(═NRe4)NRc4Rd4, NRc4Rd4, NRc4C(O)Rb4, NRc4C(O)ORa4, NRc4C(O)NRc4Rd4, NRc4S(O)Rb4, NRc4S(O)2Rb4, NRc4S(O)2NRc4Rd4, S(O)Rb4, S(O)NRc4Rd4, S(O)2Rb4, and S(O)2NRc4Rd4;
each Cy1 is independently selected from C6-14 aryl, C3-18 cycloalkyl, 5-16 membered heteroaryl, and 4-18 membered heterocycloalkyl, each of which is optionally substituted with 1, 2, 3, or 4 substituents independently selected from RCy1;
each Cy2 is independently selected from C6-14 aryl, C3-18 cycloalkyl, 5-16 membered heteroaryl, and 4-18 membered heterocycloalkyl, each of which is optionally substituted with 1, 2, 3, or 4 substituents independently selected from RCy2;
each RCy1 and RCy2 is independently selected from halo, C1-6 alkyl, C1-4 haloalkyl, C1-4 cyanoalkyl, C2-6 alkenyl, C2-6 alkynyl, phenyl, C3-7 cycloalkyl, 5-6 membered heteroaryl, and 4-7 membered heterocycloalkyl, CN, NO2, ORa5, SRa5, C(O)Rb5, C(O)NRc5Rd5, C(O)ORa5, OC(O)Rb5, OC(O)NRc5Rd5, C(═NRe5)NRc5Rd5, NRc5C(═NRe5)NRc5Rd5, NRc5Rd5, NRc5C(O)Rb5, NRc5C(O)ORa5, NRc5C(O)NRc5Rd5, NRc5S(O)Rb5, NRc5S(O)2Rb5, NRc5S(O)2NRc5Rd5, S(O)RVs, S(O)NRc5Rd5, S(O)2Rb5, and S(O)2NRc5Rd5, wherein said C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, phenyl, C3-7 cycloalkyl, 5-6 membered heteroaryl, and 4-7 membered heterocycloalkyl are each optionally substituted by 1, 2, 3, or 4 substituents independently selected from CN, NO2, ORa5, SRa5, C(O)Rb5, C(O)NRc5Rd5, C(O)ORa5, OC(O)Rb5, OC(O)NRc5Rd5, C(═NRe5)NRc5Rd5, R5C(═NRe5)NRc5Rd5, NRc5Rd5, NRc5C(O)Rb5, NRc5C(O)ORa5, NRc5C(O)NRc5Rd5, NRc5S(O)Rb5, NRc5S(O)2Rb5, NRc5S(O)2NRc5Rd5, S(O)Rb5, S(O)NRc5Rd5, S(O)2Rb5, and S(O)2NRc5Rd5;
each Ra1, Rb1, Rc1, Rd1, Ra2, Rb2, Rc2, Rd2, Ra3, Rb3, Rc3, Rd3, Ra4, Rb4, Rc4, Rd4, Ra5, Rb5, Rc5, and Rd5 is independently selected from H, C1-6 alkyl, C1-4 haloalkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl, C3-10 cycloalkyl-C1-6 alkyl, (5-10 membered heteroaryl)-C1-6 alkyl, and (4-10 membered heterocycloalkyl)-C1-6 alkyl, wherein said C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C6-10 aryl, C3-10 cycloalkyl, 5-10 membered heteroaryl, 4-10 membered heterocycloalkyl, C6-10 aryl-C1-6 alkyl, C3-10 cycloalky-C1-6 alkyl, (5-10 membered heteroaryl)-C1-6 alkyl, and (4-10 membered heterocycloalkyl)-C1-6 alkyl are each optionally substituted with 1, 2, 3, 4, or 5 substituents independently selected from Rg;
each Re1, Re2, Re3, Re4, and Re5 is independently selected from H, C1-4 alkyl, and CN;
each Rg is independently selected from the group consisting of OH, NO2, CN, halo, C1-20 alkyl, C2-6 alkenyl, C2-6 alkynyl, C1-4 haloalkyl, C1-6 alkoxy, C1-6 haloalkoxy, cyano-C1-3 alkyl, HO—C1-3 alkyl, amino, C1-6 alkylamino, di(C1-6 alkyl)amino, thiol, C1-6 alkylthio, C1-6 alkylsulfinyl, C1-6 alkylsulfonyl, carboxy, C1-6 alkylcarbonyl, and C1-6 alkoxycarbonyl;
n is 0 or 1;
m is 0 or 1;
p is 0, 1, 2, or 3;
r is 0, 1, or 2;
a is 0 or 1; and
b is 0 or 1,
wherein any cycloalkyl or heterocycloalkyl group is optionally further substituted by 1 or 2 oxo groups.
|