| CPC C12Q 1/6881 (2013.01) [C12Q 1/6883 (2013.01); C12Q 2600/156 (2013.01); C12Q 2600/158 (2013.01)] | 19 Claims |
|
1. A composition, comprising a forward nucleic acid molecule, a reverse nucleic acid molecule, and a probe nucleic acid molecule, wherein the forward, reverse and probe nucleic acid molecules are complementary to SE-LR (DERAA (SEQ ID NO:89)), and wherein the forward nucleic acid molecule has a length of up to 23 nucleotides and comprises the sequence as set forth in SEQ ID NO.:64, the reverse nucleic acid molecule has a length of up to 26 nucleotides and comprises the sequence as set forth in SEQ ID NO.:65, and the probe nucleic acid molecule comprises a nucleic acid sequence, a fluorophore and a quencher, wherein the nucleic acid sequence has a length of up to 18 nucleotides and comprises the sequence as set forth in SEQ ID NO.:66.
|
|
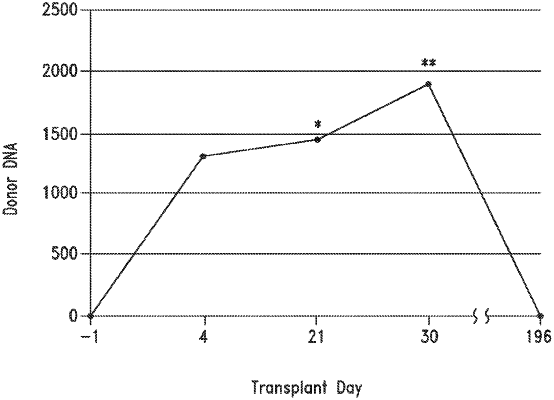
8. A process for detecting microchimerism, comprising:
(a) amplifying target nucleic acid molecules of a test biological sample using one or more nucleic acid compositions according to claim 1, wherein the test biological sample comprises a known HLA genotype;
(b) amplifying nucleic acid molecules of a control biological sample using the one or more nucleic acid molecule compositions of (a), wherein the control biological sample comprises a known HLA genotype that is different from the test biological sample; and
(c) detecting the presence of microchimerism when the amplification from the test biological sample and the control biological sample identifies HLA markers in the test biological sample that are also present in the control biological sample.
|