| CPC A61K 9/0036 (2013.01) [A61K 31/04 (2013.01); A61K 31/216 (2013.01); A61K 31/428 (2013.01); A61K 31/465 (2013.01); A61K 31/485 (2013.01); A61K 31/5513 (2013.01); A61K 38/09 (2013.01); A61K 38/24 (2013.01); A61K 38/28 (2013.01); A61P 15/08 (2018.01); A61K 9/08 (2013.01)] | 19 Claims |
|
1. A method of administering a therapeutically active compound for the treatment of a medical condition, the method comprising:
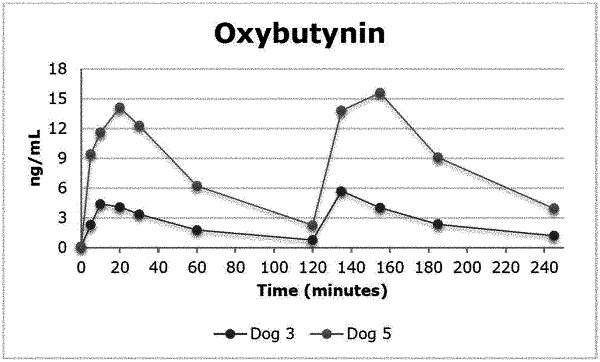
administering the therapeutically active compound in liquid formulation via the vagina by using an intravaginal ring configured to provide a detectable level in blood or plasma in 1 hour or less; wherein the intravaginal ring comprises a first rigid member having a first and second end, a second rigid member having a third and fourth end, a first flexible member coupled between the first and third ends, and a flexible part coupled between the second and fourth ends;
wherein at least one of the first flexible member and the flexible part is at least partially elastic, wherein the elasticity of the at least one of the first flexible member and the flexible part is such that:
the ring can be squeezed to transform a shape of the device from an extended shape to a collapsed shape for allowing the ring to be inserted into a vagina of a user;
the device is pre-biased to assume the extended shape when little to no external force is being applied thereto, said extended shape corresponding to a substantially oval or annular ring shape;
the device assumes a shape substantially corresponding to the extended shape when the device is placed and released at or near the fornix posterior vaginae of a user;
wherein the first rigid member and/or second rigid member comprises a reservoir holding the therapeutically active compound to be delivered, an opening, and a pump for pumping the therapeutically active compound out of said opening; and a diagnostic mechanism for performing an intravaginal diagnosis or measurement therefor.
|