| CPC C07D 513/04 (2013.01) [A61P 37/04 (2018.01); C07D 495/04 (2013.01)] | 21 Claims |
|
1. A compound of formula (I):
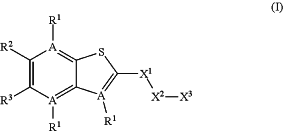 or a pharmaceutically acceptable salt thereof, wherein
each A-R1 is selected independently from the group consisting of C—R1 and N, and
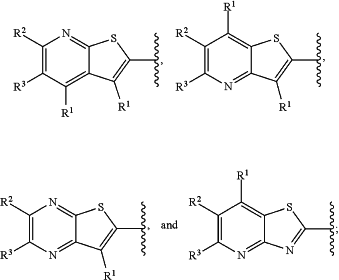
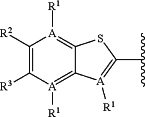 is selected from the group consisting of
 each R1 is selected independently from the group consisting of H, halogen, OR6, N(R6)2, SR6, C1-C6 alkyl, C1-C6 haloalkyl, C1-C6 alkyl substituted by OR6, C1-C6 alkyl substituted by SR6, C1-C6 alkyl substituted by N(R6)2, C1-C6 haloalkyl substituted by OR6, C1-C6 haloalkyl substituted by SR6, and C1-C6 haloalkyl substituted by N(R6)2;
R2 is selected from the group consisting of halogen, CN, OR6, N(R6)2, COOR6, C(O)N(R6)2, SR6, SO2R6, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, and a 3- to 6-membered heterocyclic ring including 1 to 2 ring members selected from the group consisting of O, S, N, and N(R6), wherein said C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, and 3- to 6-membered heterocyclic ring groups are optionally substituted by one or more substituents independently selected from the group consisting of CN, OR6, N(R6)2, and SR6, and wherein said C3-C6 cycloalkyl and 3- to 6-membered heterocyclic ring are each further optionally substituted with a member of the group consisting of C1-C3 alkyl and C1-C3 haloalkyl;
R3 is selected from the group consisting of H, halogen, CN, OR6, N(R6)2, COOR6, C(O)N(R6)2, SR6, SO2R6, C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, and a 3- to 6-membered heterocyclic ring including 1 to 2 ring members selected from the group consisting of O, S, N, and N(R6), wherein said C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, and 3- to 6-membered heterocyclic ring groups are optionally substituted by one or more substituents independently selected from the group consisting of CN, OR6, N(R6)2, and SR6, and wherein said C3-C6 cycloalkyl and 3- to 6-membered heterocyclic ring are each further optionally substituted with a member of the group consisting of C1-C3 alkyl and C1-C3 haloalkyl;
optionally R3 and an adjacent A-R1 may be taken together with the atoms to which they are attached form a fused 5- or 6-membered heterocyclic ring including 1 to 2 ring members selected from the group consisting of O, S, N, and N(R6) wherein said heterocyclic ring is optionally substituted with or more members of the group consisting of C1-C3 alkyl and C1-C3 haloalkyl;
each R6 is independently selected from the group consisting of H, C1-C6 alkyl, and C1-C6 haloalkyl, wherein said C1-C6 alkyl, and C1-C6 haloalkyl are optionally substituted with OH, O(C1-C3 alkyl), and O(C1-C3 haloalkyl);
X1 is selected from the group consisting of CH2 and C(O);
X2 is (C(R8)2)(1-3);
each R8 is independently selected from the group consisting of H, halogen, CN, OR6, N(R6)2, SR6, C1-C6 alkyl, C1-C6 haloalkyl, C3-C6 cycloalkyl, and a 3- to 6-membered heterocyclic ring including 1 to 2 ring members selected from the group consisting of O, S, N, and N(R6), wherein said C1-C6 alkyl, C1-C6 haloalkyl, C2-C6 alkenyl, C2-C6 haloalkenyl, C2-C6 alkynyl, C2-C6 haloalkynyl, C3-C6 cycloalkyl, and 3- to 6-membered heterocyclic ring groups are optionally substituted by one or more substituents independently selected from the group consisting of CN, OR6, N(R6)2, and SR6, and wherein said C3-C6 cycloalkyl and 3- to 6-membered heterocyclic ring are each further optionally substituted with a member of the group consisting of C1-C3 alkyl and C1-C3 haloalkyl;
optionally 2 R8 on different carbon atoms may be taken together, along with the atoms to which they are attached, to form a 3- to 6-membered fused ring;
optionally 2 R8 on a single carbon atom may be taken together, along with the atom to which they are attached, to form a 3- to 6-membered spirocycle;
X3 is selected from the group consisting of COOR6,
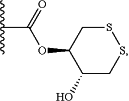 C(O)SR6, C(S)OR6, SO2R6, and C(O)N(R9)2; and
each R9 is independently selected from the group consisting of H, COOR6, and SO2R6.
|