| CPC C07D 493/04 (2013.01) [C07D 215/02 (2013.01); C07D 221/04 (2013.01); C07D 471/04 (2013.01); C07D 493/14 (2013.01)] | 41 Claims |
|
1. A compound of formula (III), or a salt, solvate, geometric isomer, stereoisomer, tautomer, and any mixtures thereof:
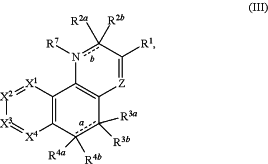 wherein:
Z is CR12;
R1 is selected from the group consisting of H, halogen, —C(═O)OR8, —C(═O)NHR8, —C(═O)NH—OR8, —C(═O)NHNHR8, —C(═O)NHNHC(═O)R8, —C(═O)NHS(═O)2R8, —CN, and 1H-1,2,4-triazol-5-yl;
R2a and R2b combine to form ═O;
bond b is a single bond;
R7 is selected from the group consisting of H, optionally substituted C1-C6 alkyl, and optionally substituted C3-C8 cycloalkyl;
R3a, R3b, R4a, and R4b are each independently selected from the group consisting of H, optionally substituted C1-C6 alkyl, and optionally substituted C3-C8 cycloalkyl;
or one pair selected from the group consisting of R3a/R3b, R4a/R4b, and R3a/R4a combine to form a divalent group selected from the group consisting of C1-C6 alkanediyl, —(CH2)nO(CH2)n—, —(CH2)nNR9(CH2)n—, —(CH2)nS(CH2)n—, —(CH2)nS(═O)(CH2)n—, and —(CH2)nS(═O)2(CH2)n—, wherein each occurrence of n is independently selected from the group consisting of 1 and 2 and wherein each divalent group is optionally substituted with at least one C1-C6 alkyl or halo;
bond a is a single bond;
X1 is selected from the group consisting of CR6I and N;
X2 is selected from the group consisting of CR6II and N;
X3 is selected from the group consisting of CR6III and N;
X4 is selected from the group consisting of CR6IV and N;
or either X3 and X4, or X1 and X2, combine to form —S—;
wherein 0-2 substituents selected from the group consisting of X1, X2, X3 and X4 are N, each of which, if present, is optionally substituted with C1-C6 alkyl if the adjacent carbon atom in the ring is substituted with —OH;
R6I, R6II, R6III, and R6IV are independently selected from the group consisting of H, halogen, —CN, optionally substituted C1-C6 alkyl, optionally substituted C3-C8 cycloalkyl, —OR, —N(R)(R), optionally substituted heterocyclyl, and C1-C6 haloalkoxy,
wherein each occurrence of R is independently selected from the group consisting of H, C1-C6 alkyl, R′-substituted C1-C6 alkyl, C1-C6 hydroxyalkyl, optionally substituted (C1-C6 alkoxy)-C1-C6 alkyl, and optionally substituted C3-C8 cycloalkyl,
wherein each occurrence of R′ is selected from the group consisting of —NH2, —NH(C1-C6 alkyl), —N(C1-C6 alkyl)(C1-C6 alkyl), —NHC(═O)OtBu, —N(C1-C6 alkyl)C(═O)OtBu, and a 5- or 6-membered heterocyclic group, which is optionally N-linked;
or X2 is CR6II, X3 is CR6III, and R6II and R6III combine to form a divalent group selected from the group consisting of —O(CR9R11)O—, —O(CR9R11)(CR9R11)O—, —O(CR9R11)(CR9R11)— and —O(CR9R11)(CR9R11)(CR9R11)—;
or X3 and R6III, X4 is CR6IV, and R6III and R6IV combine to form a divalent group selected from the group consisting of —O(CR9R11)O—, —O(CR9R11)(CR9R11)O—, —O(CR9R11)(CR9R11)—, and —O(CR9R11)(CR9R11)(CR9R11)—;
each occurrence of R8 is independently selected from the group consisting of H, optionally substituted C1-C6 alkyl, and optionally substituted C3-C8 cycloalkyl;
each occurrence of R9 is independently selected from the group consisting of H and C1-C6 alkyl;
each occurrence of is independently selected from the group consisting of H, OH, C1-C6 alkyl, C1-C6 alkoxy, alkoxy-C1-C6 alkyl and alkoxy-C1-C6 alkoxy, wherein two R11 groups bound to the same carbon atom are not simultaneously OH; and wherein R11 is not OH if it is bound to a carbon that is further bound to an oxygen atom;
or two R11 groups combine with the carbon atom to which they are bound to form a moiety selected from the group consisting of C═O, C═CH2 and oxetane-3,3-diyl;
R12 is selected from the group consisting of H, OH, halogen, optionally substituted C1-C6 alkoxy, optionally substituted C1-C6 alkyl, and optionally substituted C3-C8 cycloalkyl;
wherein each occurrence of optionally substituted alkyl and optionally substituted cycloalkyl is independently optionally substituted with at least one substituent selected from the group consisting of C1-C6 alkyl, halogen, —OR″, tetrahydro-2-H-pyranyl, —N(R″)(R″), 1-methyl-imidazol-2-yl, pyridin-2-yl, pyridin-3-yl, pyridin-4-yl, —C(═O)OH, —C(═O)O(C1-C6)alkyl, trifluoromethyl, —C≡N, —C(═O)NH2, —C(═O)NH(C1-C6)alkyl, —C(═O)N((C1-C6)alkyl)2, —SO2NH2, —SO2NH(C1-C6 alkyl), —SO2N(C1-C6 alkyl)2, —C(═NH)NH2, and —NO2,
wherein each occurrence of R″ is independently H, C1-C6 alkyl, or C3-C8 cycloalkyl;
wherein each occurrence of optionally substituted heterocyclyl is independently optionally substituted with at least one substituent selected from the group consisting of C1-C6 alkyl, OH, C1-C6 alkoxy, halogen, amino, acetamido, and NO2; and
wherein at least one of the following applies:
(a) at least one of X1, X2, X3, and X4 is N; and
(b) at least one of R3a or R3b is independently selected from the group consisting of n-propyl, isopropyl, n-butyl, isobutyl, sec-butyl, and tert-butyl.
|