| CPC C07D 487/14 (2013.01) [C07D 519/00 (2013.01)] | 18 Claims |
|
1. A compound of Formula IA-7 or IA-8:
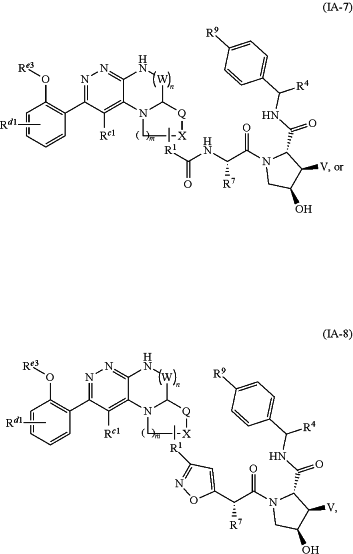 or a pharmaceutically acceptable salt thereof,
wherein:
R1 is a chemical moiety represented by the formula:
-(A)q-,
wherein:
q is an integer from 1 to 14;
each A is independently selected from the group consisting of CR1aR1b, O, S, SO, SO2, NR1c, SO2NR1c, SONR1c, SO(═NR1c), SO(═NR1c)NR1d, CONR1c, NR1cCONR1d, NR1cC(O)O, NR1cSO2NR1d, CO, CR1a═CR1b, C≡C, SiR1aR1b, P(O)R1a, P(O)OR1a, (CR1aR1b)1-4, —(CR1aR1b)1-4O(CR1aR1b)1-4—(CR1aR1b)1-4S(CR1aR1b)1-4, —(CR1aR1b)1-4NR(CR1aR1b)1-4, NR1cC(═NCN)NR1dNR1cC(═NCN), NR1cC(═CNO2)NR1d, 3-11 membered cycloalkyl, optionally substituted with 0-6 R1a and/or R1b groups, 3-11 membered heterocyclyl optionally substituted with 0-6 R1a and/or R1b groups, aryl optionally substituted with 0-6 R1a and/or R1b groups, or heteroaryl optionally substituted with 0-6 R1a and/or R1b groups,
wherein R1a, R1b, R1c, R1d and R1e are each independently, —H, D, -halo, —C1-C8alkyl, —O—C1-C8alkyl, —C1-C6haloalkyl, —S—C1-C8alkyl, —NHC1-C8alkyl, —N(C1-C8alkyl)2, 3-11 membered cycloalkyl, aryl, heteroaryl, 3-11 membered heterocyclyl, —O-(3-11 membered cycloalkyl), —S-(3-11 membered cycloalkyl), NH-(3-11 membered cycloalkyl), N(3-11 membered cycloalkyl)2, N-(3-11 membered cycloalkyl)(C1-C8alkyl), —OH, —NH2, —SH, —SO2C1-C8alkyl, SO(NH)C1-C8alkyl, P(O)(OC1-C8alkyl)(C1-C8alkyl), —P(O)(OC1-C8alkyl)2, —C≡C—C1-C8alkyl, —C≡CH, —CH═CH(C1-C8alkyl), —C(C1-C8alkyl)═CH(C1-C8alkyl), —C(C1-C8alkyl)═C(C1-C8alkyl)2, —Si(OH)3, —Si(C1-C8alkyl)3, —Si(OH)(C1-C8alkyl)2, —C(O)C1-C8alkyl, —CO2H, —CN, —CF3, —CHF2, —CH2F, —NO2, —SF5, —SO2NHC1-C8alkyl, —SO2N(C1-C8alkyl)2, —SO(NH)NHC1-C8alkyl, —SO(NH)N(C1-C8alkyl)2, —SONHC1-C8alkyl, —SON(C1-C8alkyl)2, —CONHC1-C8alkyl, —CON(C1-C8alkyl)2, —N(C1-C8alkyl)CONH(C1-C8alkyl), —N(C1-C8alkyl)CON(C1-C8alkyl)2, —NHCONH(C1-C8alkyl), —NHCON(C1-C8alkyl)2, —NHCONH2, —N(C1-C8alkyl)SO2NH(C1-C8alkyl), —N(C1-C8alkyl)SO2N(C1-C8alkyl)2, —NHSO2NH(C1-C8alkyl), —NHSO2N(C1-C8alkyl)2, or —NHSO2NH2; and where R1a or R1b, each independently may be optionally linked to other groups to form cycloalkyl and/or heterocyclyl moiety, optionally substituted with 0-4 R1e groups;
m=1 to 3;
n=0 to 3;
W is —CH2—, —C(O)—, —S(O)—, or —S(O)2—; wherein when n=2 or 3, only one W may be —C(O)—, —S(O)—, or —S(O)2—;
Rc1 and Rd1 are independently H, D, Halo, C1-3 alkyl, C1-3 haloalkyl, or C1-4 alkoxyl;
Re3 is H, —C(O)Rf, or —P(O)(ORg)2; wherein Rf and Rg are independently H, C1-4 alkyl, C3-8 cyclcoalkyl, or C3-8 heterocyclcoalkyl;
X is —CH2—, or NH; or, if R1 is attached to X, then X is —CH— or N; and Q is —CH2—, —(CH2)2—, —C(O)—, —CH2C(O)—, —S(O)—, —S(O)2—, —CH2S(O)2—, or —CH2S(O)—;
R9 is —CN or
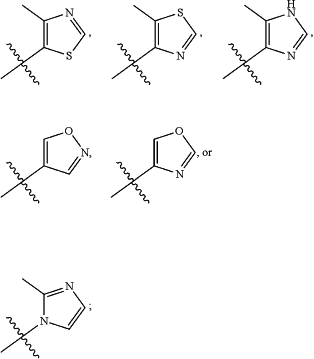 V is H or F;
R4 is H, D, haloalkyl, alkyl, cycloalkyl, heterocycloalkyl, —CORd, or —CONRe1Re2;
R7 is H, D, alkyl, cycloalkyl, or haloalkyl, Rd is H, or alkyl;
each Re1 and Re2 is independently H, D, alkyl,
or Re1 and Re2 together with the nitrogen atom to which they are attached form a 4-7 membered heterocyclyl.
|