| CPC C07D 471/04 (2013.01) [A61K 31/546 (2013.01)] | 19 Claims |
|
1. A method of treating an autoimmune or inflammatory disease, comprising administering to a patient in need of such treatment a therapeutically effective amount of a compound of Formula IIIa:
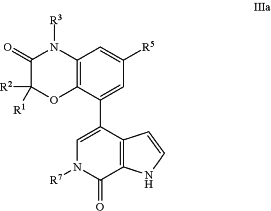 or a pharmaceutically acceptable salt thereof, wherein:
R1 and R2 are each independently selected from H, methyl, ethyl, propyl, cyclopropyl, cyclopentyl, pyran-4-yl, phenyl, pyridin-2-yl, 2-chloro-4-phenyl, 2-chloro-4-fluorophenyl, and 2-hydroxyethyl;
or R1 and R2 together with the carbon atom to which they are attached form cyclopropyl;
R3 is H, methyl, ethyl, or propyl, wherein said methyl is optionally substituted with cyclopropyl, pyridinyl, —C(═O)NH2, —C(═O)NHCH3, —C(═O)(4-methylpiperazin-1-yl), —C(═O)NH(4-methylpiperazin-1-yl), or —C(═O)OH;
R5 is H, 1-methyl-1H-pyrazol-4-yl, 2-furyl, CN, NO2, methoxy, —C(═O)NH2, —C(═O)NH(CH3), —C(═O)N(CH3)2, —C(═O)-(morpholin-4-yl), —C(═O)CH3, —CH2OH, —CH2OCH3, —CH2NH2, —CH2NHSO2(CH2CH3), —CH2NHC(═O)CH3, —CH(OH)CH3, —SO2CH3, —SO2CH2CH3, —SO2-(isopropyl), —SO2N(CH3)2, —SO2NH(CH3), —SO2—NH(isopropyl), or —SO2-(piperidin-1-yl); and
R7 is methyl.
|