| CPC C01G 45/06 (2013.01) [C01G 45/006 (2013.01); C09K 11/616 (2013.01); C01P 2006/80 (2013.01)] | 3 Claims |
|
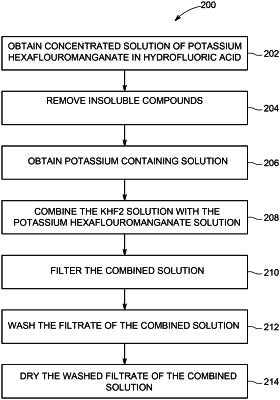
1. A method comprising:
obtaining a first solution comprising potassium hexafluoromanganate (K2MnF6) and one or more impurities, wherein the first solution is prepared at an elevated temperature in a range of between sixty-five and seventy-five degrees Celsius;
filtering the first solution of the potassium hexafluoromanganate and the one or more impurities to form a filtrate; and
separating the potassium hexafluoromanganate from the one or more impurities by crystallizing the filtrate, wherein separating the potassium hexafluoromanganate from the one or more impurities comprises crystallizing the filtrate by:
adding a potassium containing solution to the filtrate to form a second solution; and
cooling the second solution by exposing the second solution to a temperature less than the elevated temperature;
and
wherein the crystallized filtrate of potassium hexafluoromanganate has no more than forty parts per million of calcium.
|