|
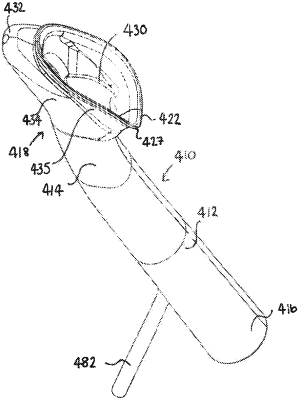
1. An airway device for human or animal use, the device comprising an airway tube having a distal end and a proximal end, the distal end of the airway tube is provided with a pre-formed and non-inflatable peri-pharyngeal bowl, the peri-pharyngeal bowl comprising a posterior bowl portion having a back dorsal portion and a side wall extending around and depending from the periphery of the back dorsal portion to define an internal space, the peri-pharyngeal bowl further comprising a resiliently deformable flange extending lateral and perpendicular to the side wall of the back dorsal portion which defines an extended internal space, the resiliently deformable flange having inner and outer surfaces that extend to a circumferential edge wherein the circumferential edge is provided with a circumferential lip and wherein the resiliently deformable flange is configured to extend substantially around the entire circumference of the peri-pharyngeal bowl and wherein the resiliently deformable flange is configured to form a seal with the peri-larynx in the hypopharynx within and against the mucosa of the pharyngeal and hypo-pharyngeal walls of the human or animal patient by enveloping the glottis within the peri-pharyngeal bowl when in situ in a human or animal patient and wherein the internal space and the extended internal space together comprises a combined internal space and wherein the combined internal space is configured to contain and envelope 50% to 100% of the body of the larynx of the human or animal patient without making contact therewith once the airway device is in situ in a human or animal patient.
|