| CPC A61K 31/4162 (2013.01) [A61K 31/337 (2013.01); A61K 31/501 (2013.01); A61K 31/675 (2013.01); A61K 31/704 (2013.01); A61K 45/06 (2013.01); A61P 35/00 (2018.01); C07K 16/2818 (2013.01)] | 11 Claims |
|
1. A method of increasing a response to a chemotherapeutic agent or an immunotherapeutic agent in a patient in need thereof, comprising:
administering to the patient a compound A represented by formula:
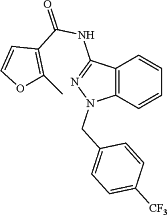 or tautomer thereof, and/or a pharmaceutically acceptable salt thereof, wherein the patient is undergoing or about to undergo chemotherapy; and wherein the chemotherapeutic agent is paclitaxel, cyclophosphamide, or doxorubicin.
|