| CPC A61K 31/18 (2013.01) [A61K 9/0053 (2013.01); A61P 25/28 (2018.01)] | 21 Claims |
|
1. A method of treating one or more symptoms of Alzheimer's disease in a subject, comprising administering to the subject a pharmaceutical composition comprising a compound of Formula I
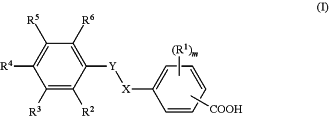 or an enantiomer, a mixture of enantiomers, a mixture of two or more diastereomers, a tautomer, a mixture of two or more tautomers, or an isotopic variant thereof; or a pharmaceutically acceptable salt, solvate, or hydrate thereof; and a pharmaceutically acceptable excipient;
wherein:
X is —SO2— and Y is —NRX—; or X is —NRX— and Y is —SO2—;
RX is hydrogen;
each R1 is independently halo or C1-6 alkyl;
R2 and R4 are each hydrogen;
R3 is nitro, C1-6 alkyl, C1-6 alkoxy, or C1-6 alkylsulfonamido;
R5 is hydrogen or C1-6 alkoxy;
R6 is hydrogen, C1-6 alkyl, or C1-6 alkoxy;
m is an integer of 0, 1, or 2,
wherein each alkyl and alkoxy is optionally substituted with one, two, three, or four substituents Q, where each Q is independently selected from (a) deuterium, cyano, halo, and nitro; (b) C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, C6-14 aryl, C7-15 aralkyl, heteroaryl, and heterocyclyl, each of which is further optionally substituted with one, two, three, or four substituents Qa; and (c) —C(O)Ra, —C(O)ORa, —C(O)NRbRc, —C(O)SRa, —C(NRa)NRbRc, —C(S)Ra, —C(S)ORa, —C(S)NRbRc, —ORa, —OC(O)Ra, —OC(O)ORa, —OC(O)NRbRc, —OC(O)SRa, —OC(═NRa)NRbRc, —OC(S)Ra, —OC(S)ORa, —OC(S)NRbRc, —OS(O)Ra, —OS(O)2Ra, —OS(O)NRbRc, —OS(O)2NRbRc, —NRbRc, —NRa C(O)Rd, —NRa C(O)ORd, —NRaC(O)NRbRc, —NRa C(O)SRd, —NRa C(═NRd)NRbRc, —NRaC(S)Rd, —NRaC(S)ORd, —NRaC(S)NRbRc, —NRaS(O)Rd, —NRa S(O)2Rd, —NRaS(O)NRbRc, —NRaS(O)2NRbRc, —SRa, —S(O)Ra, —S(O)2Ra, —S(O)NRbRc, and —S(O)2NRbRc, wherein each Ra, Rb, Rc, and Rd is independently (i) hydrogen or deuterium; (ii) C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, C6-14 aryl, C7-15 aralkyl, heteroaryl, or heterocyclyl, each of which is optionally substituted with one, two, three, or four substituents Qa; or (iii) Rb and Rc together with the N atom to which they are attached form heterocyclyl, optionally substituted with one, two, three, or four substituents Qa; and
wherein each Qa is independently selected from the group consisting of (a) deuterium, cyano, halo, and nitro; (b) C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, C6-14 aryl, C7-15 aralkyl, heteroaryl, and heterocyclyl; and (c) —C(O)Re, —C(O)ORe, —C(O)NRfRg, —C(O)SRe, —C(NRe)NRfRg, —C(S)Re, —C(S)ORe, —C(S)NRfRg, —ORe, —OC(O)Re, —OC(O)ORe, —OC(O)NRfRg, —OC(O)SRe, —OC(═NRe)NRfRg, —OC(S)Re, —OC(S)ORe, —OC(S)NRfRg, —OS(O)Re, —OS(O)2Re, —OS(O)NRfRg, —OS(O)2NRfRg, —NRfRg, —NRC(O)Rh, —NReC(O)ORf, —NReC(O)NRfRg, —NReC(O)SRf, —NReC(═NRh)NRfRg, —NReC(S)Rh, —NReC(S)ORf, —NReC(S)NRfRg, —NReS(O)Rh, —NReS(O)2Rh, —NReS(O)NRfRg, —NReS(O)2NRfRg, —SRe, —S(O)Re, —S(O)2Re, —S(O)NRfRg, and —S(O)2NRfRg; wherein each Re, Rf, Rg, and Rh is independently (i) hydrogen or deuterium; (ii) C1-6 alkyl, C2-6 alkenyl, C2-6 alkynyl, C3-10 cycloalkyl, C6-14 aryl, C7-15 aralkyl, heteroaryl, or heterocyclyl; or (iii) Rf and Rg together with the N atom to which they are attached form heterocyclyl.
|