| CPC C07D 403/14 (2013.01) [C07B 2200/13 (2013.01)] | 14 Claims |
|
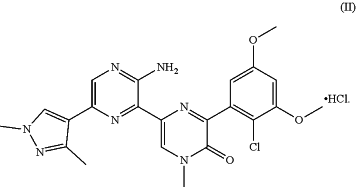
1. A crystal form C of the hydrochloride salt as shown in formula (II), wherein the crystal form C has an X-ray powder diffraction pattern comprising characteristic diffraction peaks at the following 2θ angles: 5.24±0.20°, 9.58±0.20°, 10.45±0.20°, 14.26±0.20°, 20.86±0.20°, 24.99±0.20°, 26.21±0.20° and 27.71±0.20°,
 |