| CPC A61K 31/198 (2013.01) [A61K 9/0053 (2013.01); A61K 9/14 (2013.01); A61K 31/197 (2013.01)] | 15 Claims |
|
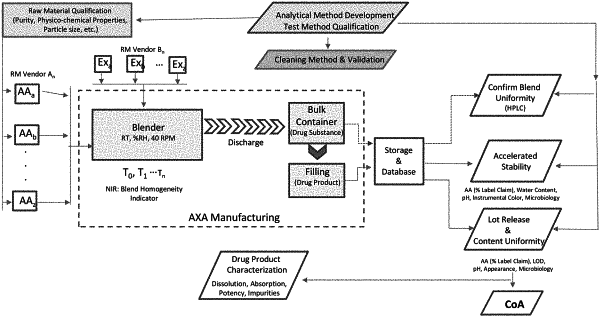
1. A method of manufacturing a pharmaceutical grade dry blended preparation (PGDBP) comprising at least four pharmaceutical grade amino acid entities, said method comprising:
forming a combination of at least four pharmaceutical grade amino acid entities and blending the combination at a temperature lower than 40° C. for a time sufficient to achieve a PGDBP having a mass of at least 10 kg, the combination being blended substantially lacking solvent,
wherein:
the PGDBP comprises a level of a contaminant that is less than 0.15% (w/w), and
less than 20%, by weight, of at least one of the pharmaceutical grade amino acid entities transforms from a crystalline state to an amorphous state during blending and wherein at least 50% of the pharmaceutical grade amino acid entities are in a crystalline state during blending.
|