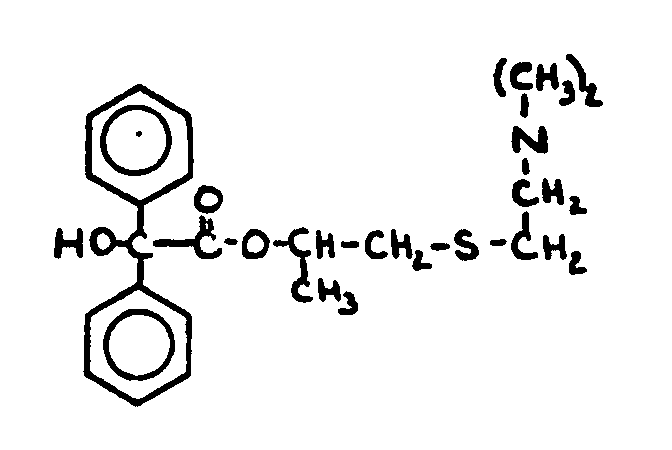
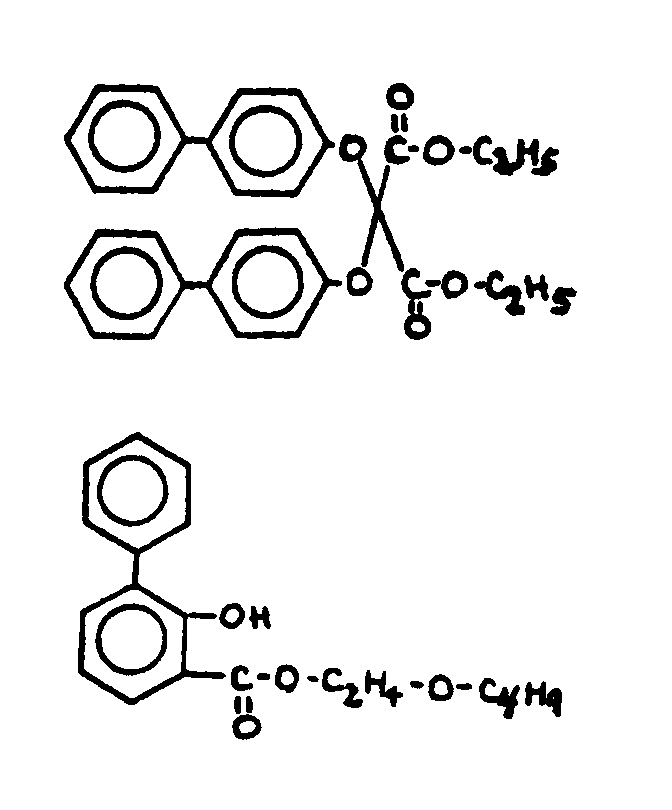
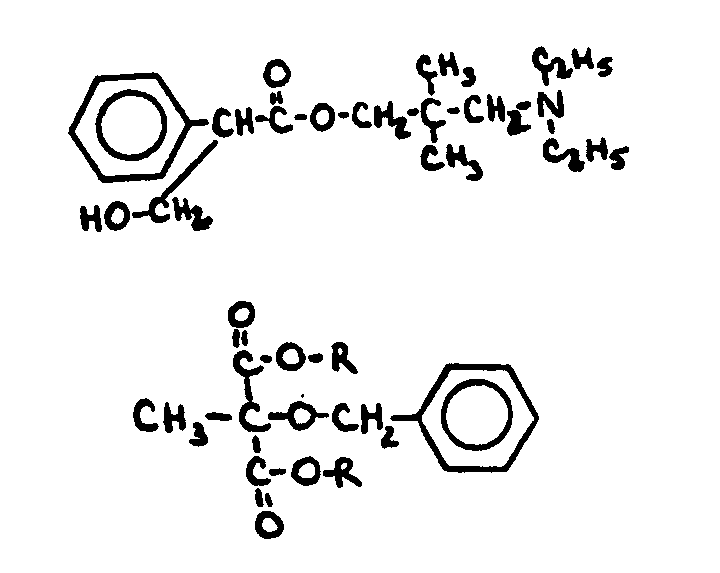
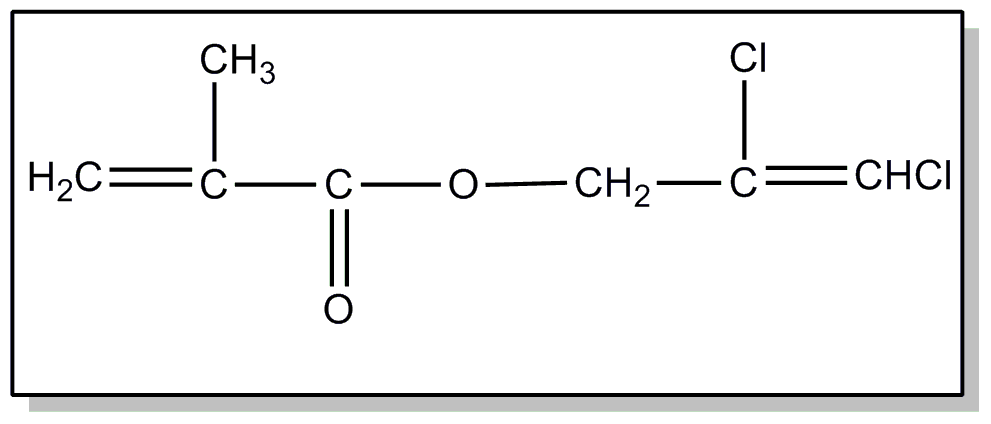
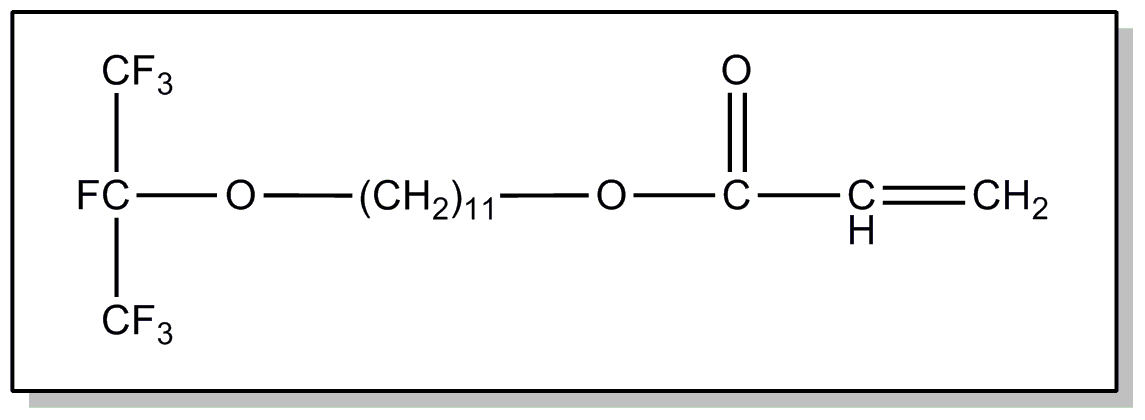
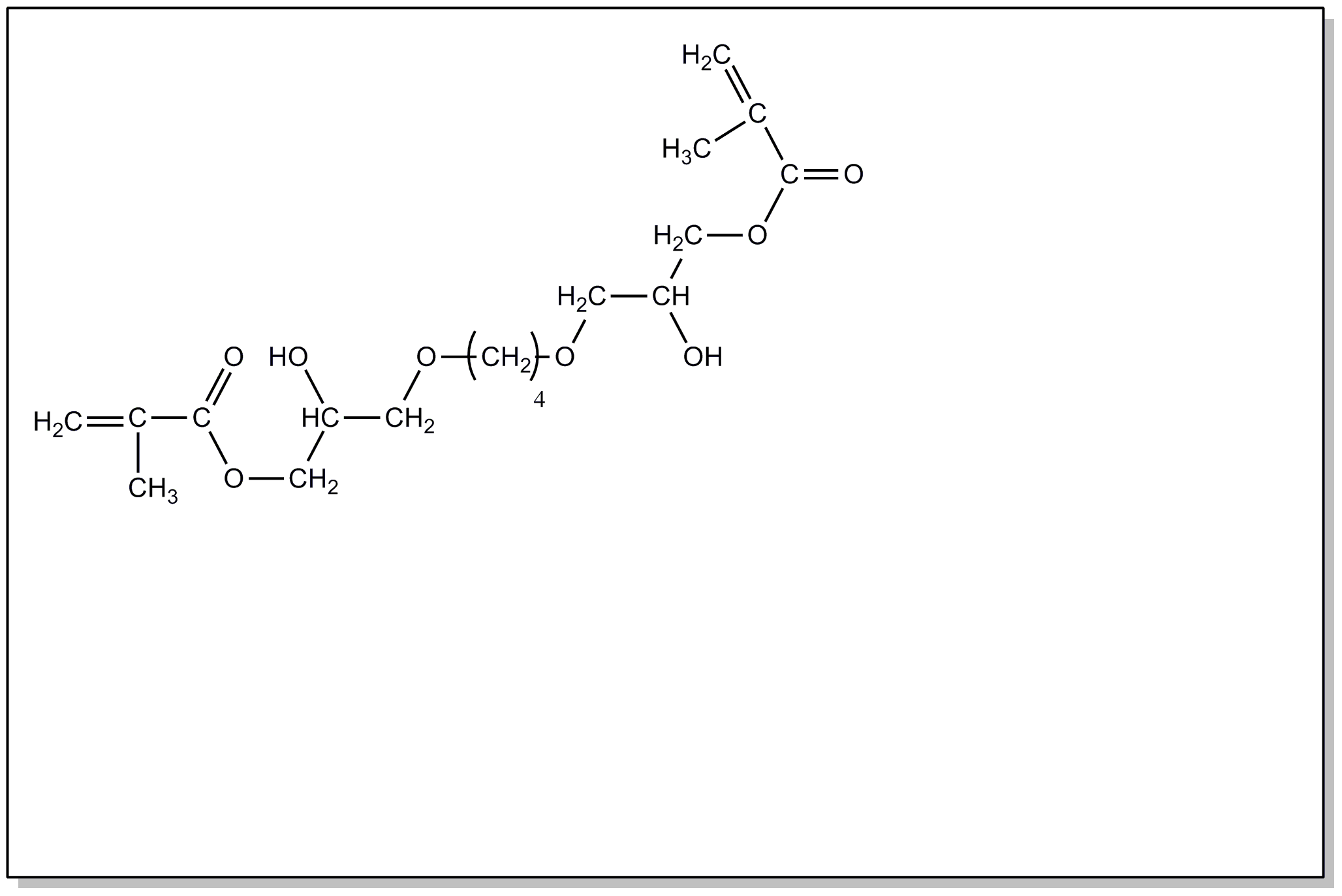
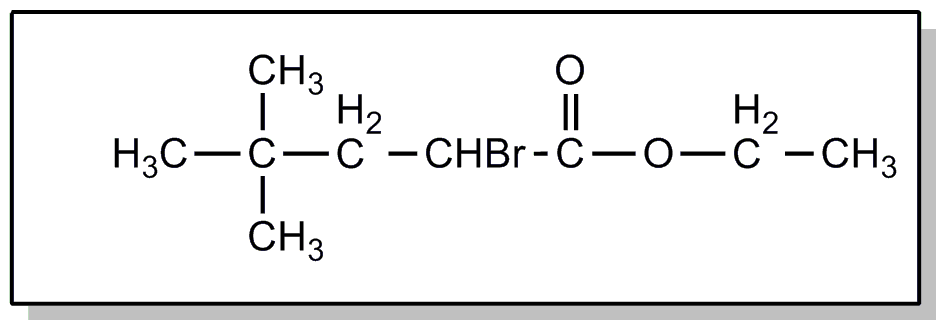
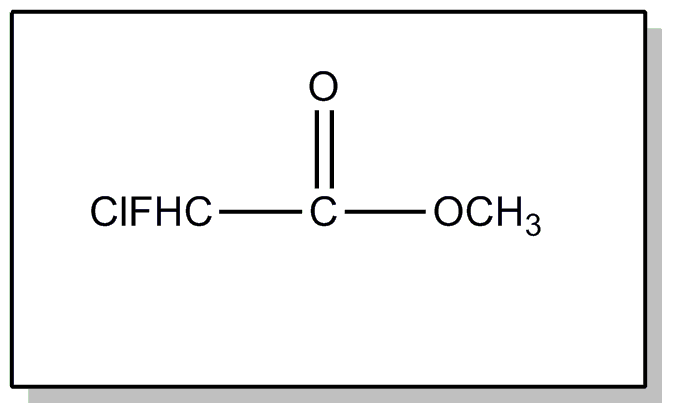
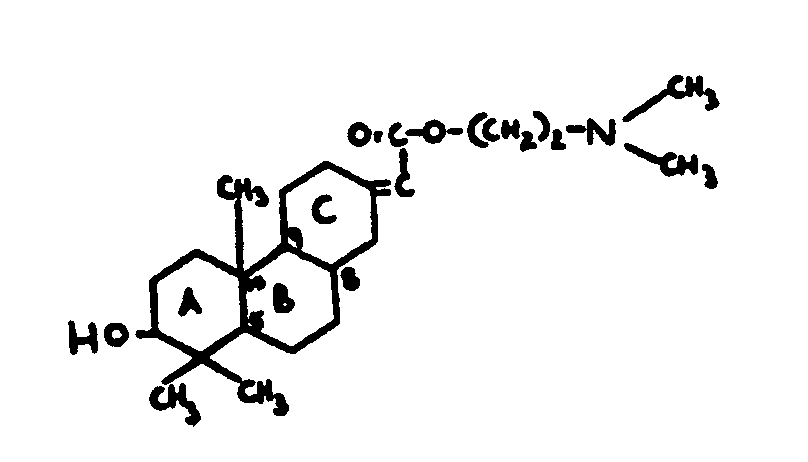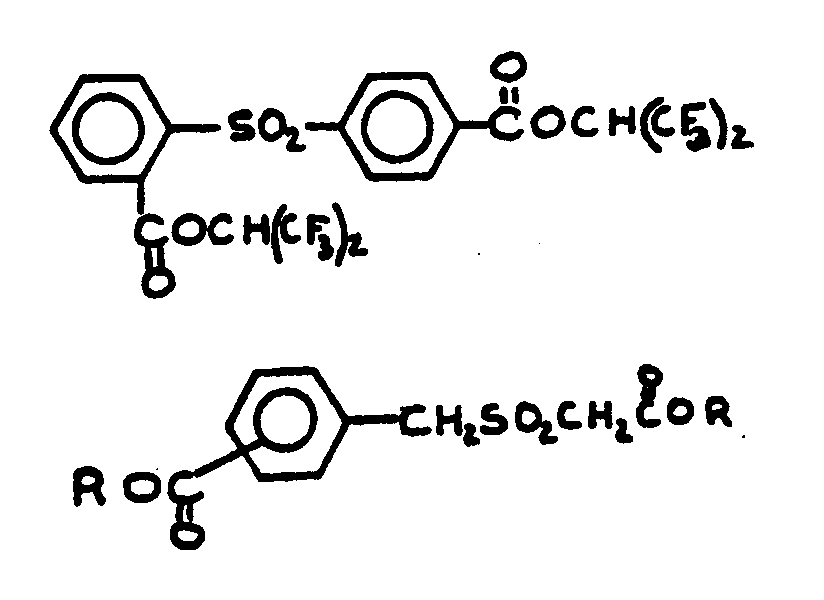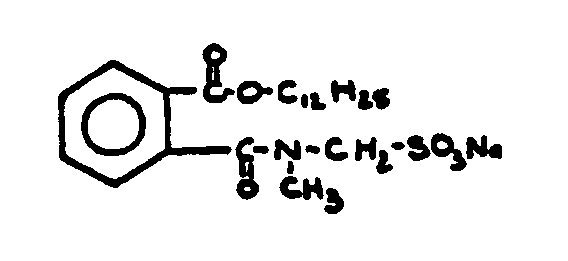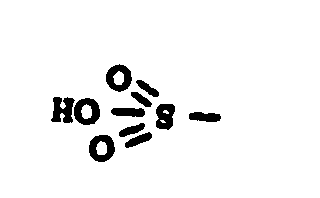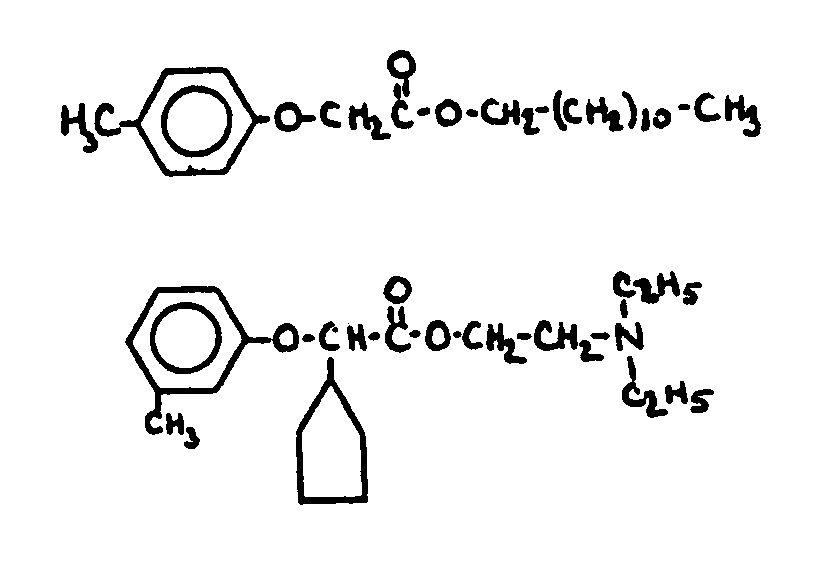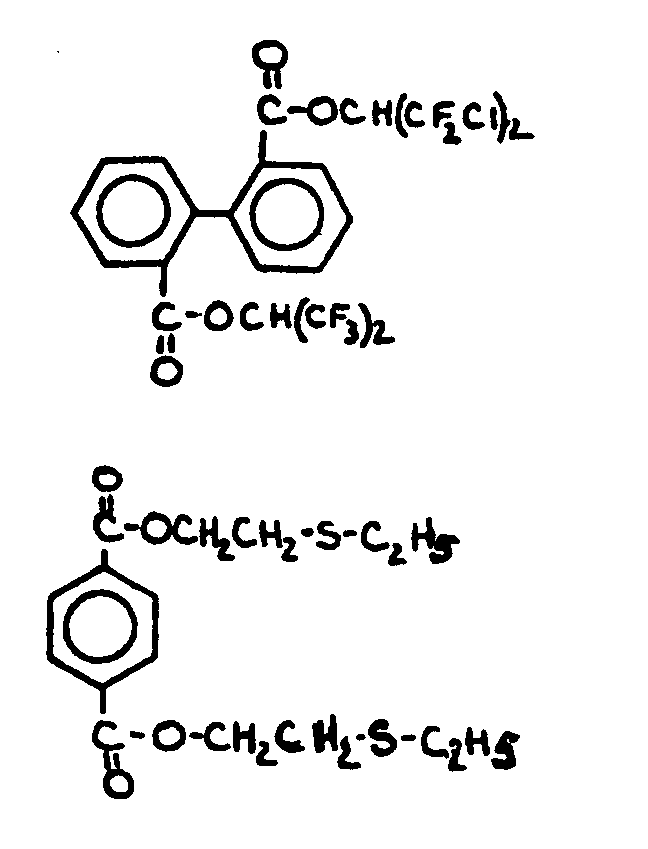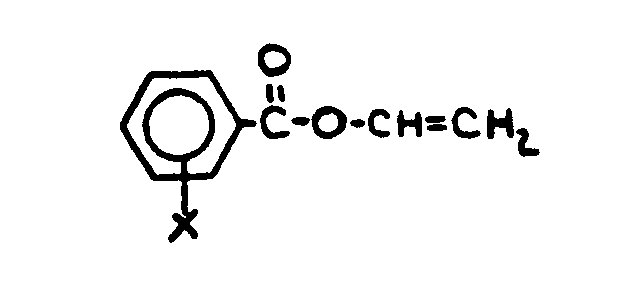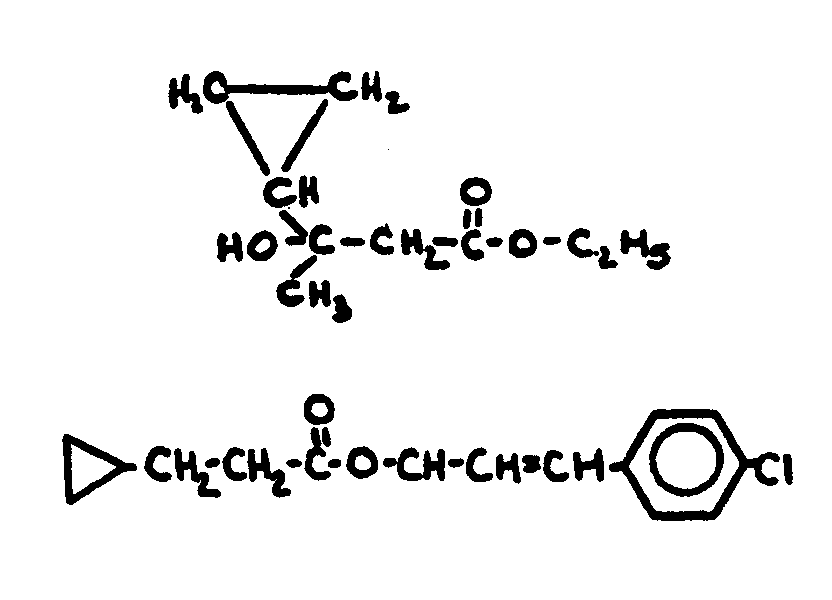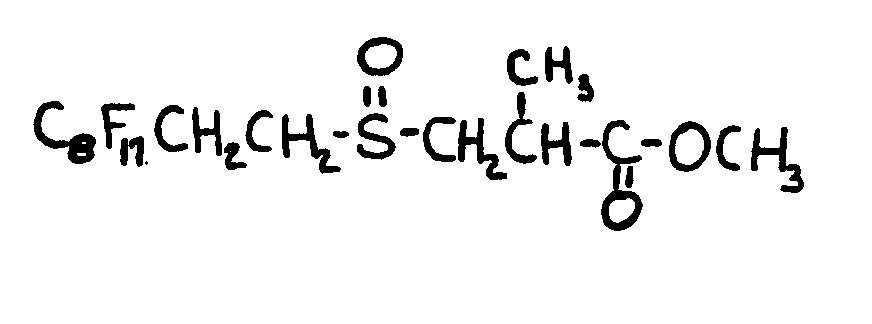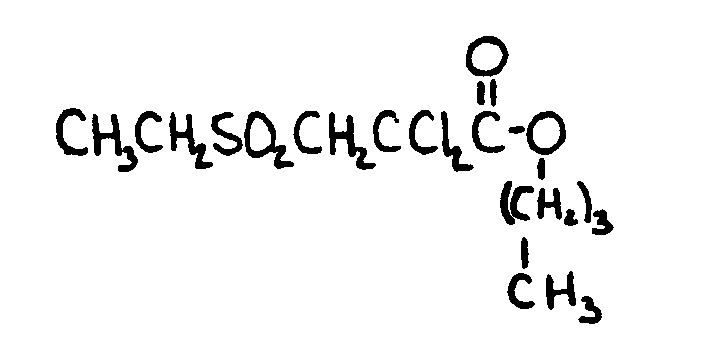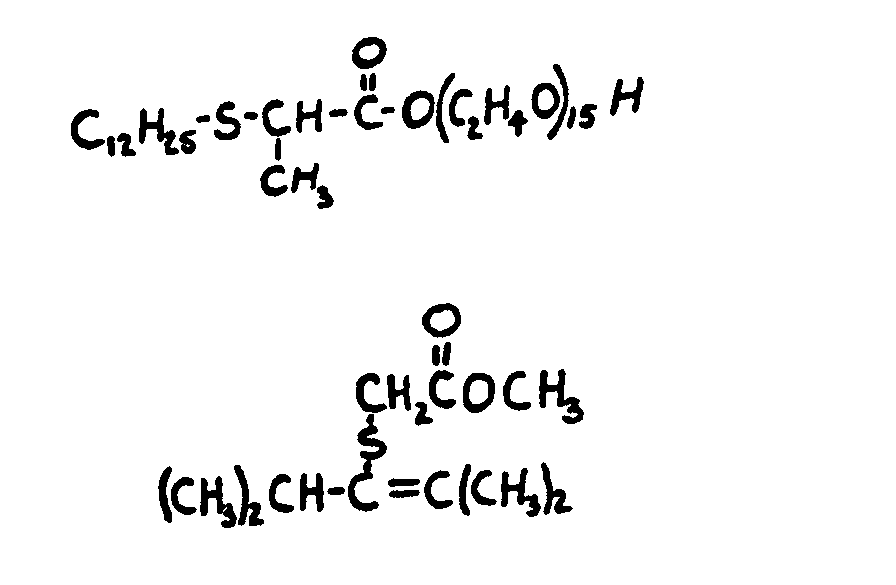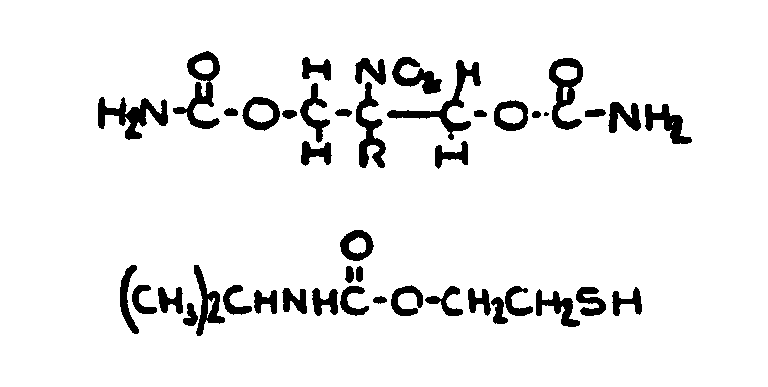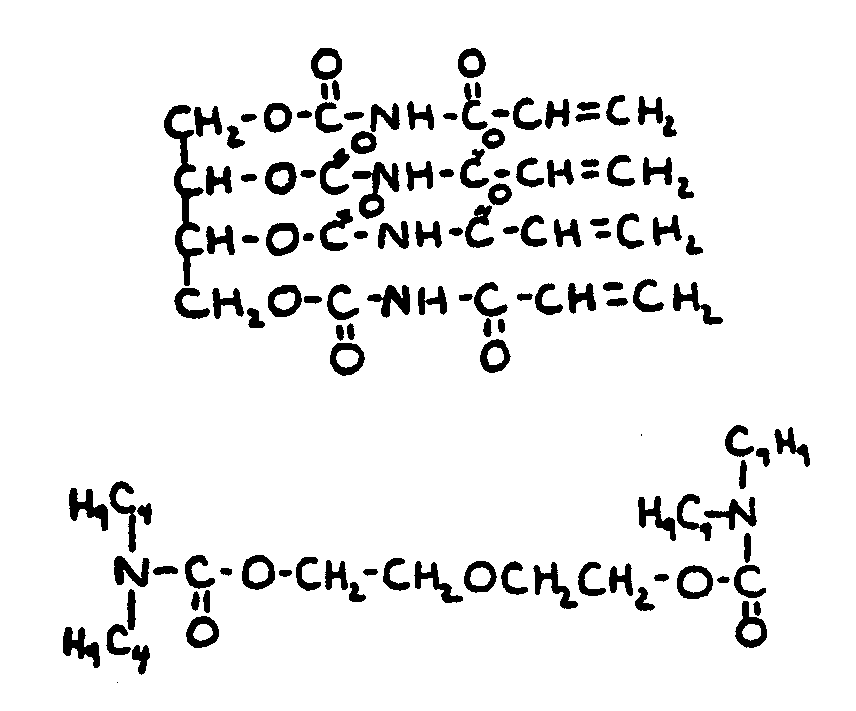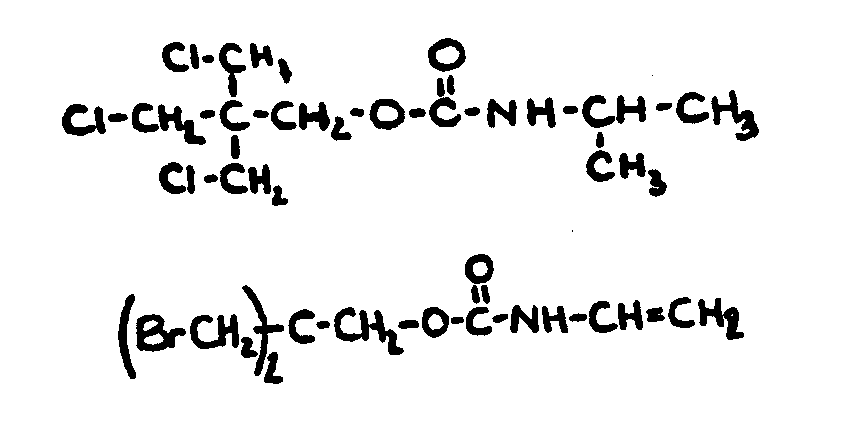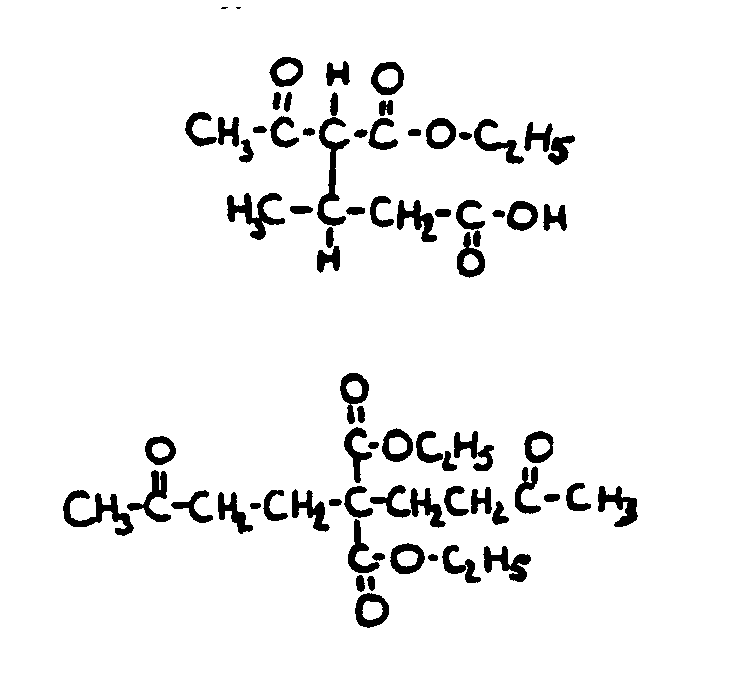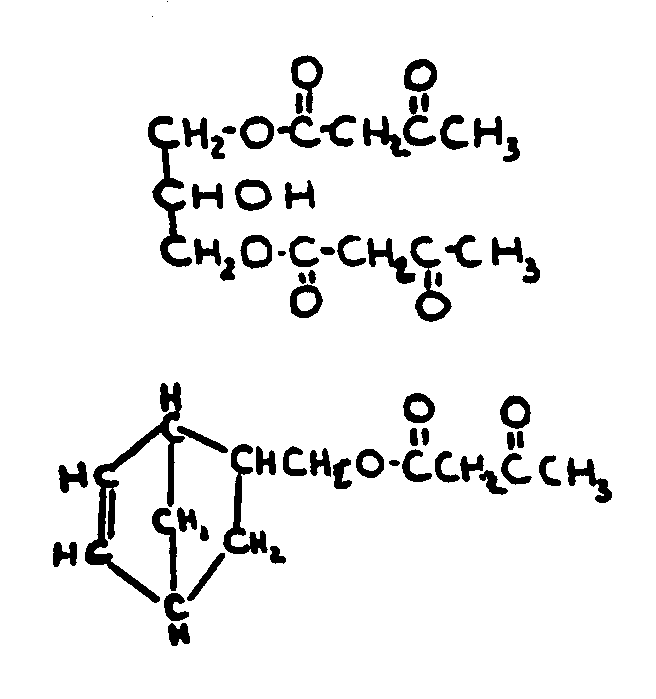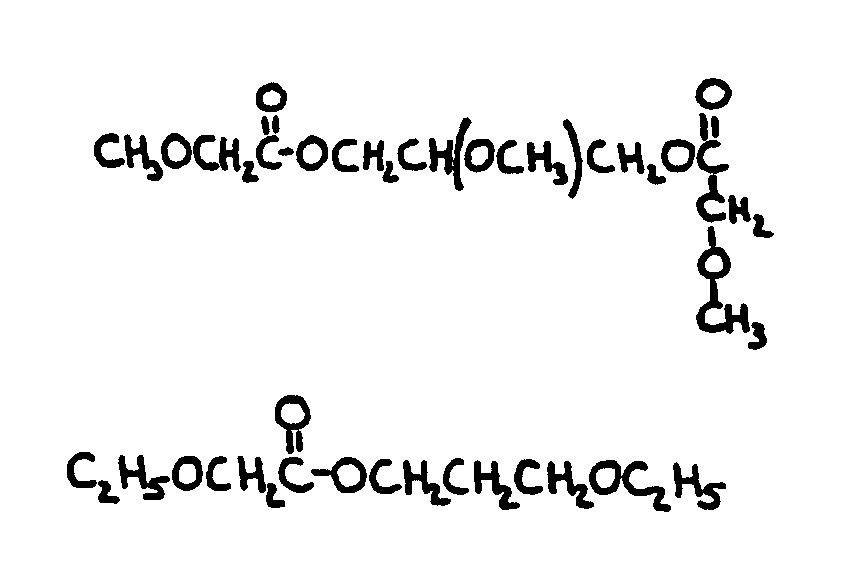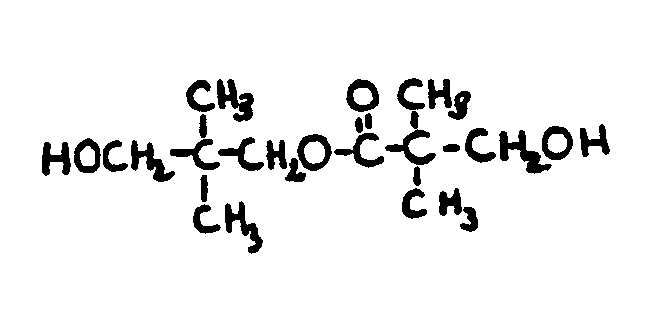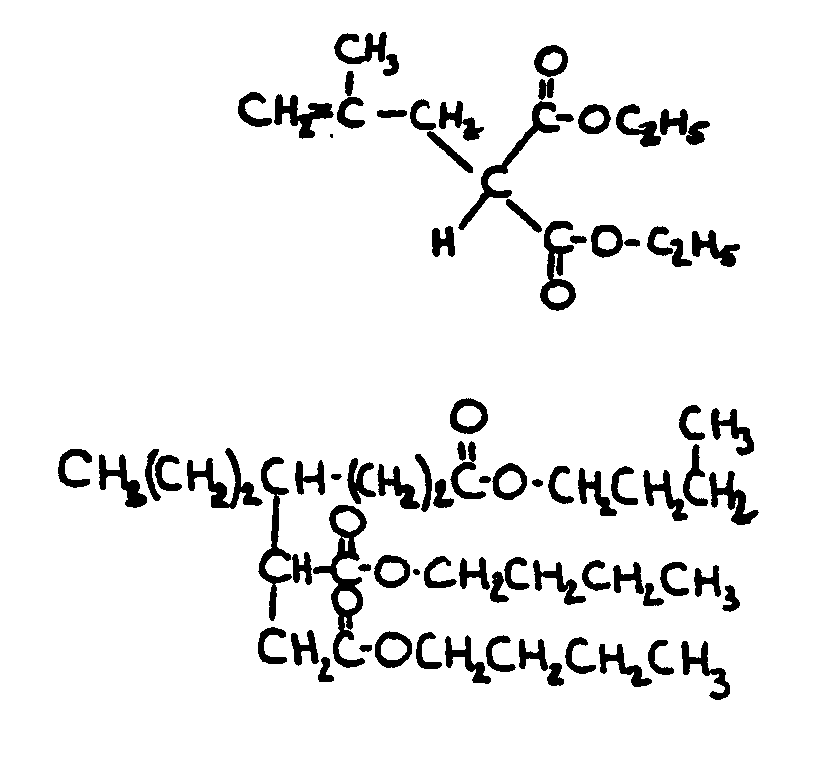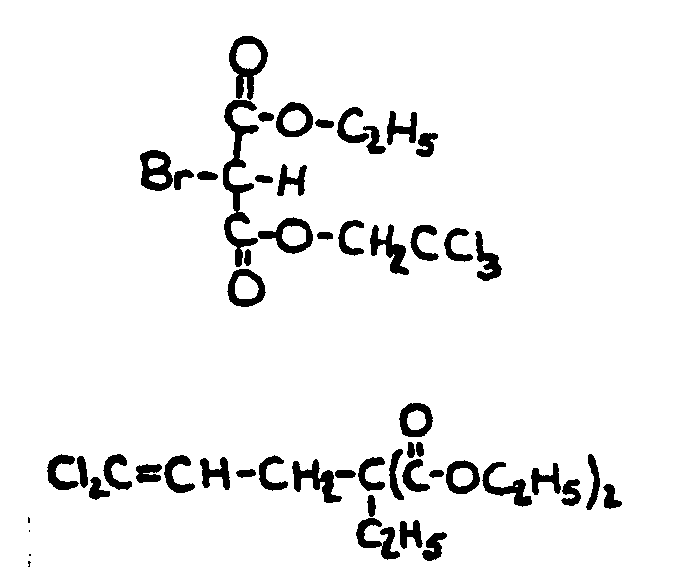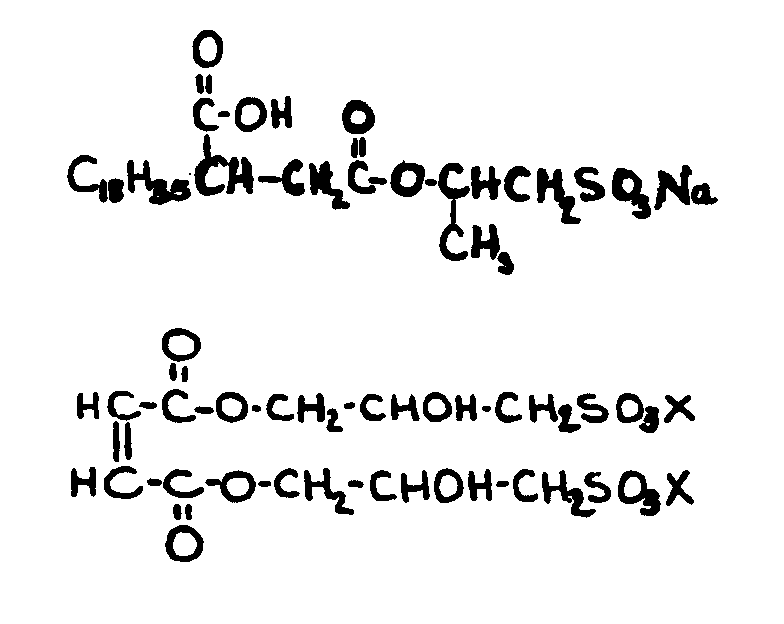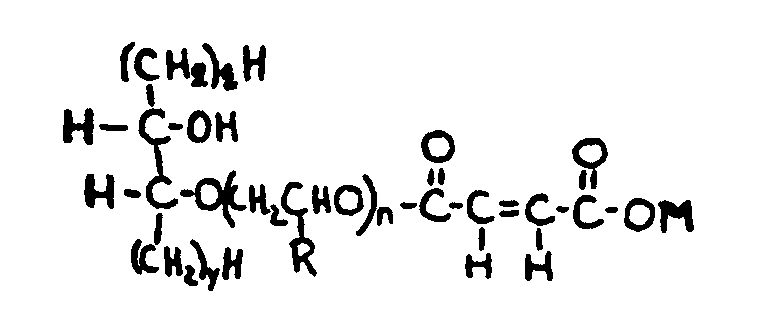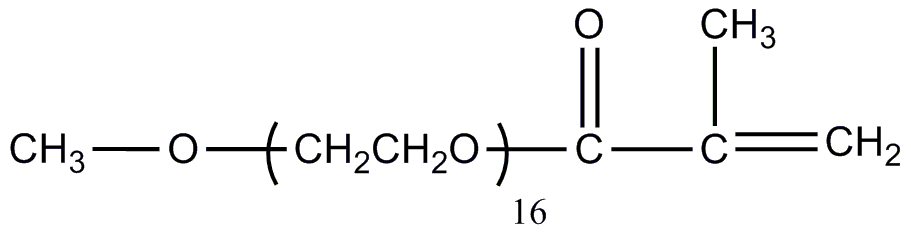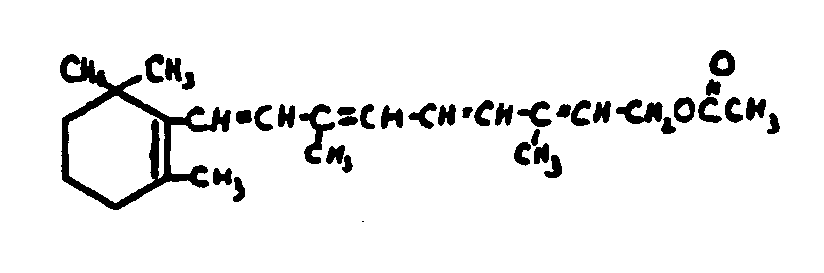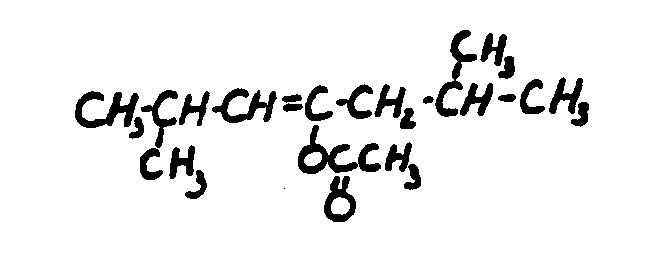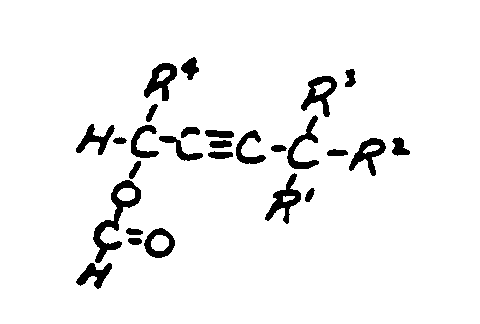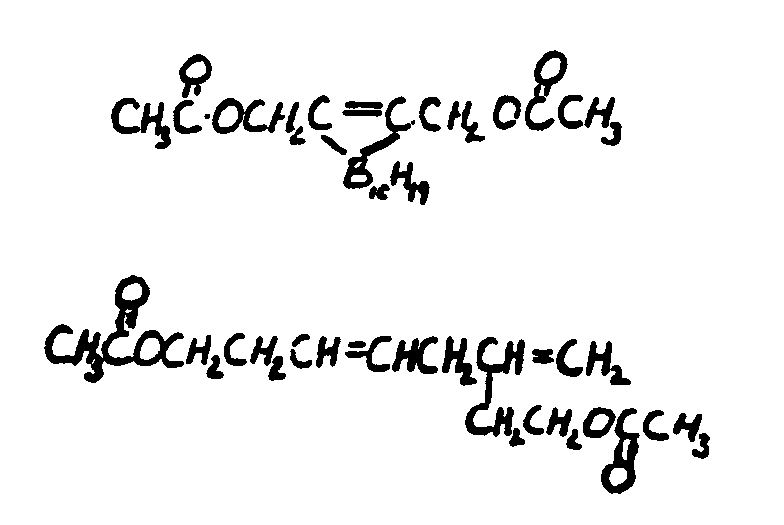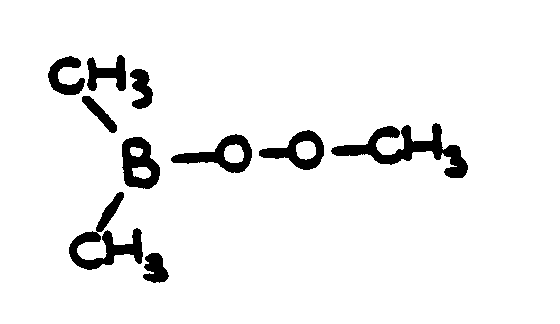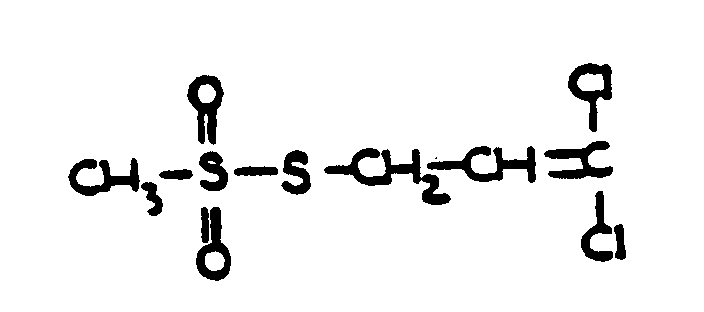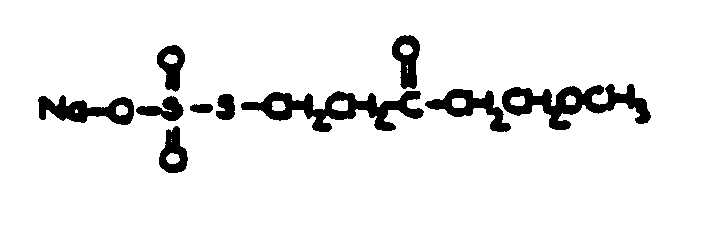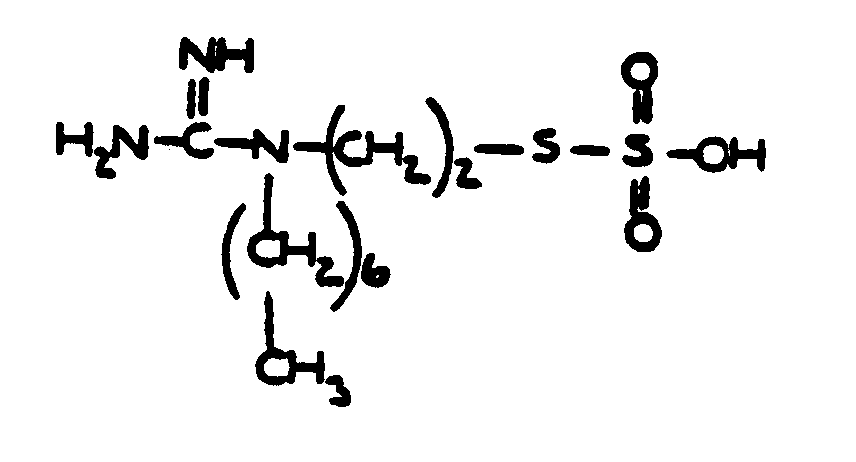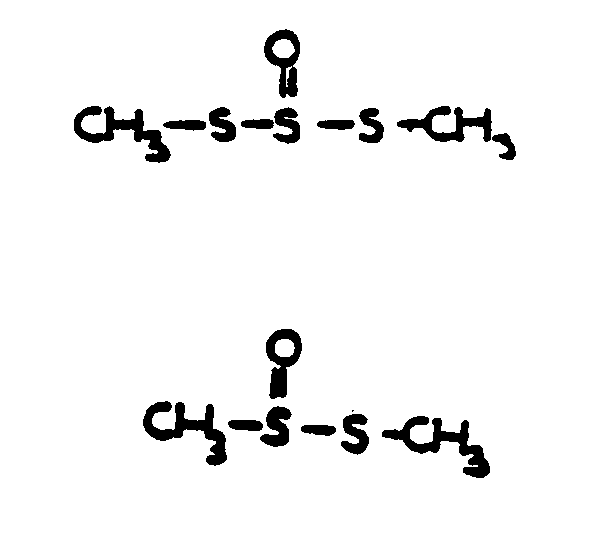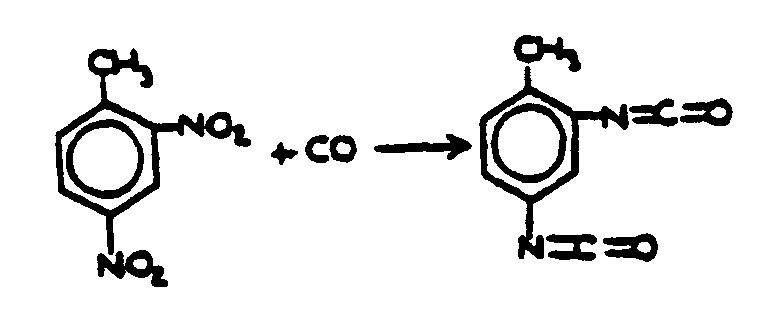![[Search a list of Patent Appplications for class 560]](../as.gif) CLASS 560, CLASS 560, | ORGANIC COMPOUNDS -- PART OF THE CLASS 532-570 SERIES |
| Click here for a printable version of this file | |
SUBCLASSES
![[List of Patents for class 560 subclass 1]](../ps.gif) 1 1 | Carboxylic acid esters: | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the acid function
entering into the formation of the esters is a carboxyl group.
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
![[List of Patents for class 560 subclass 2]](../ps.gif) 2 2 | With preservative: |
| This subclass is indented under subclass 1. Products wherein the ester is mixed with a preserving agent whose sole function is to prevent physical or chemical change. | |
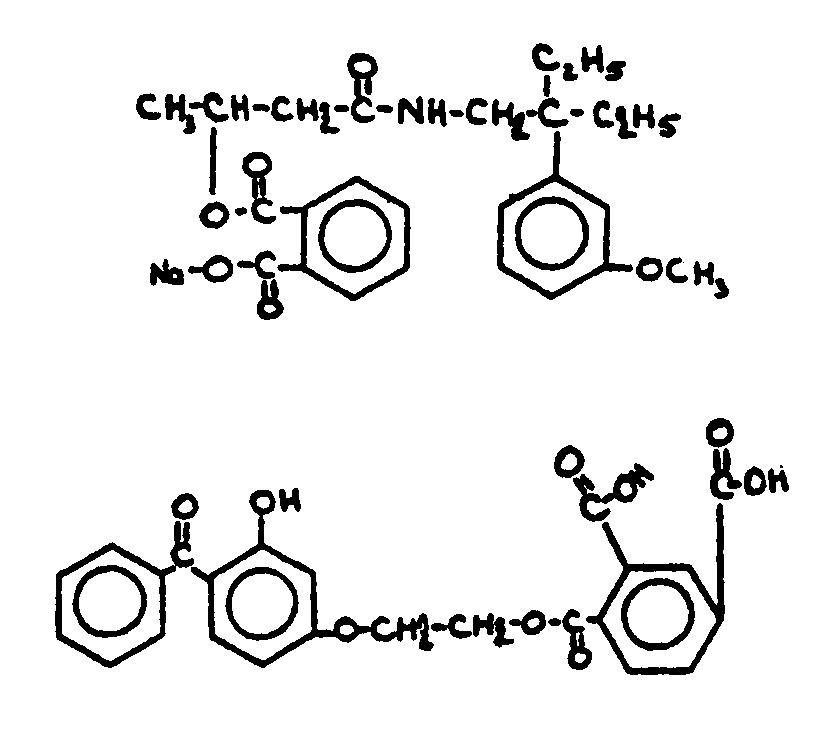
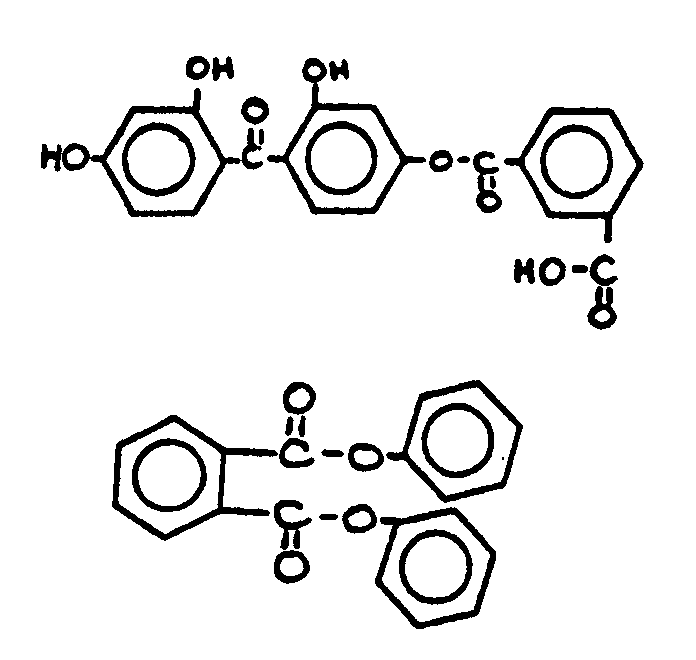
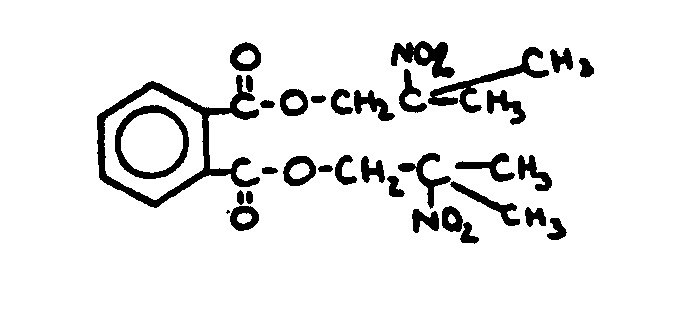
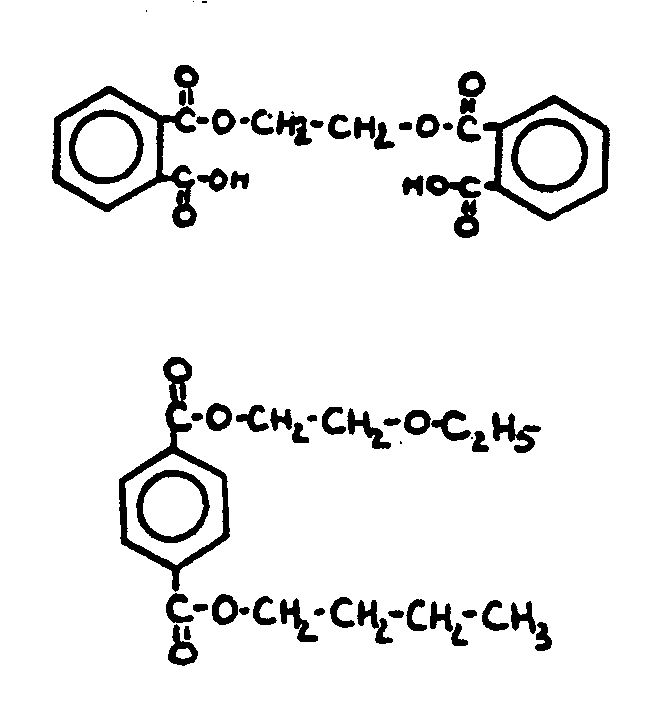
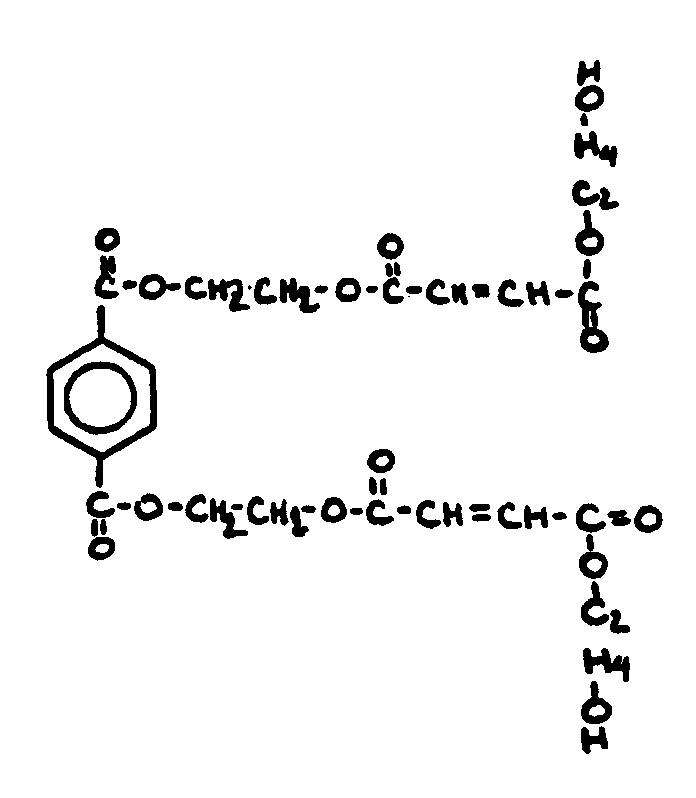
![[List of Patents for class 560 subclass 3]](../ps.gif) 3 3 | Aromatic polycarboxylic acid esters: |
| This subclass is indented under subclass 2. Products wherein the ester is an aromatic polycarboxylic acid ester. | |
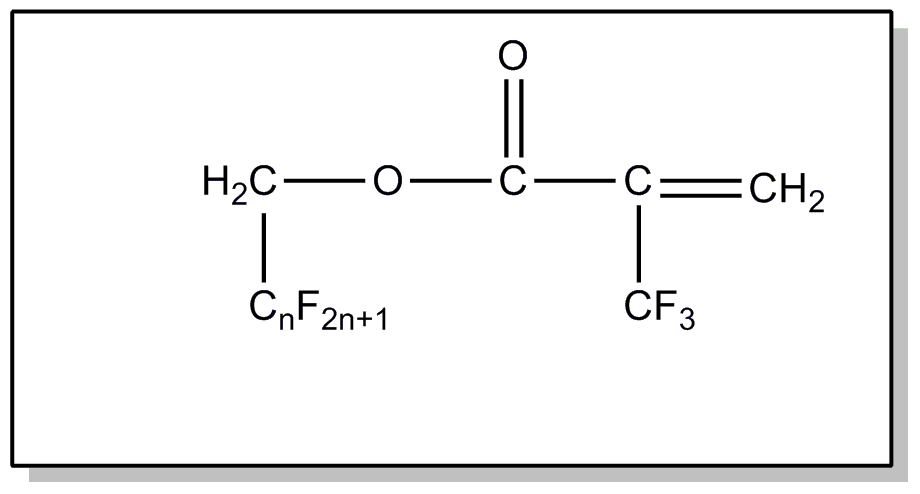
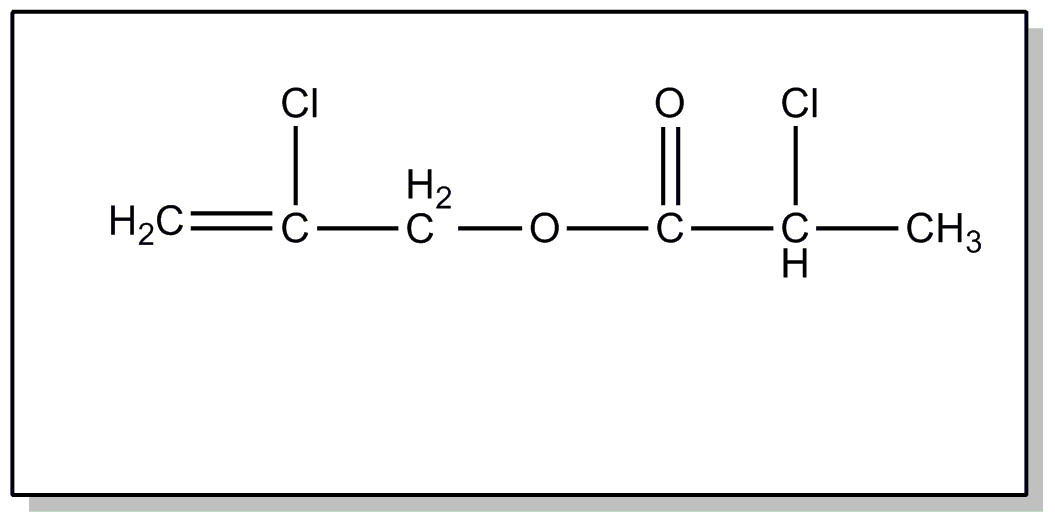
![[List of Patents for class 560 subclass 4]](../ps.gif) 4 4 | Acyclic unsaturated monocarboxylic acid esters: |
| This subclass is indented under subclass 2. Products wherein the ester is an acyclic unsaturated monocarboxylic acid ester. | |
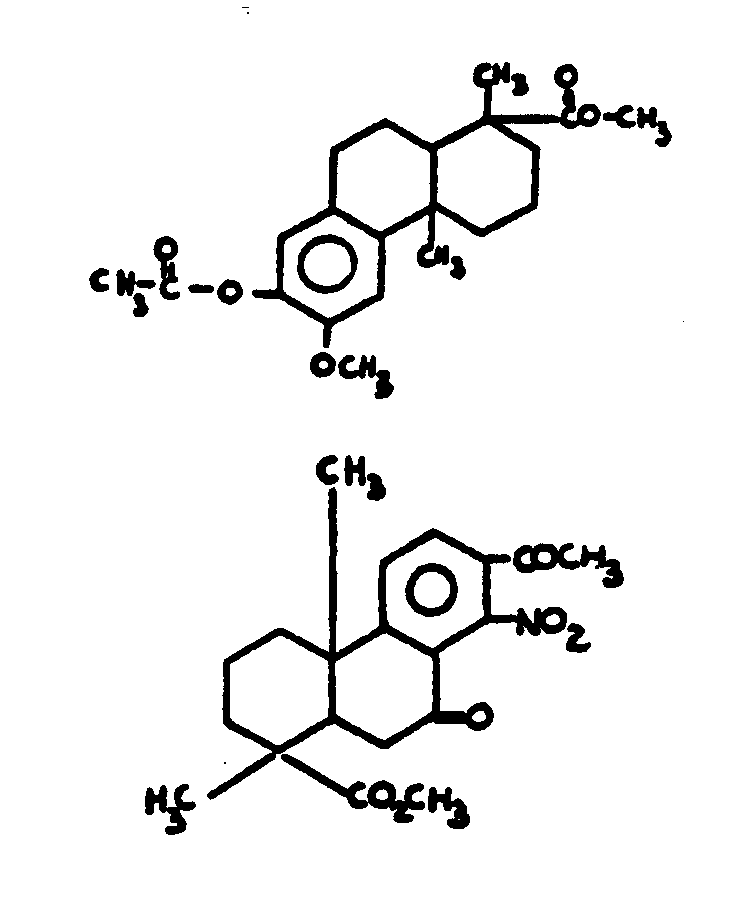
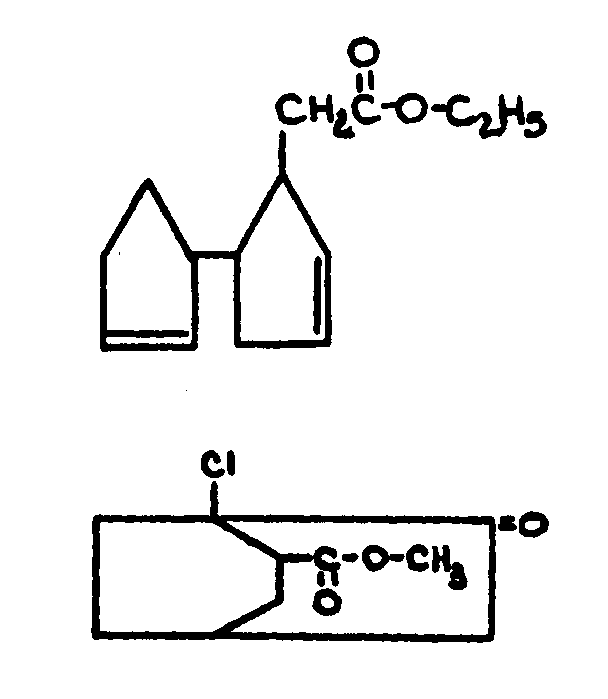
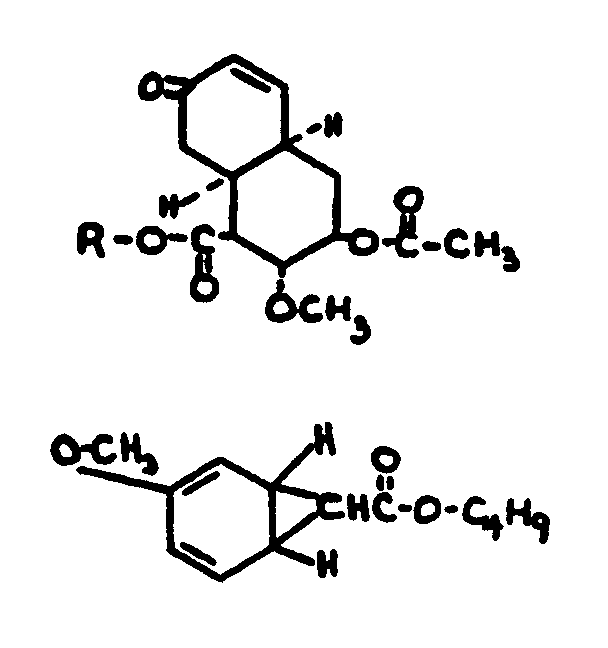
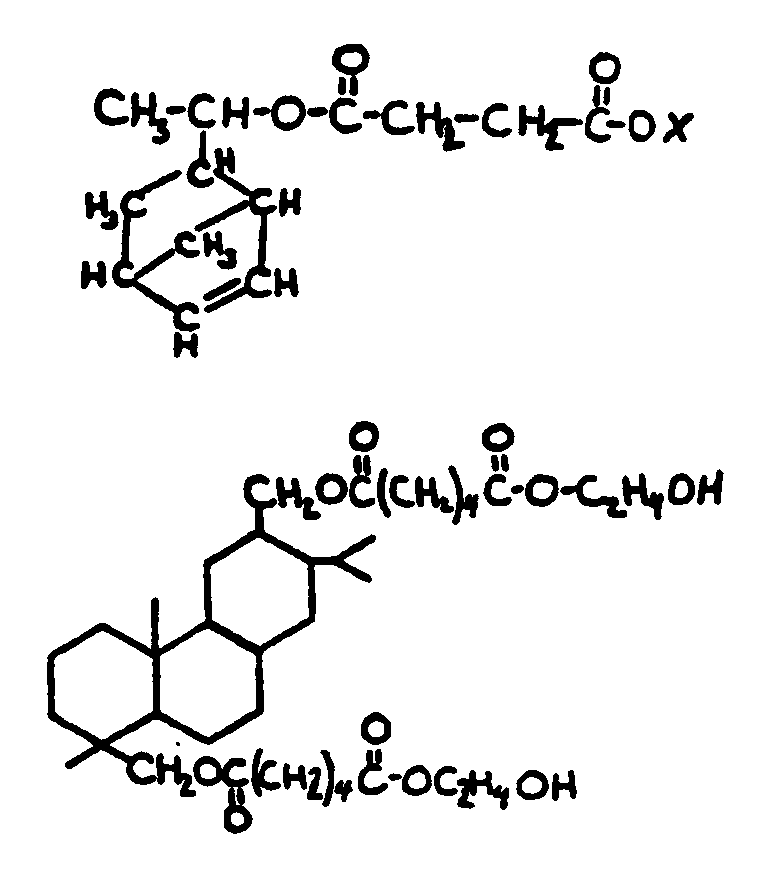
![[List of Patents for class 560 subclass 5]](../ps.gif) 5 5 | Hydrophenanthrene in acid moiety: | ||||||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains a hydrophenanthrene
nucleus not provided for above.
SEE OR SEARCH CLASS:
| |||||||
![[List of Patents for class 560 subclass 6]](../ps.gif) 6 6 | Polycyclo ring system having the hydrophenanthrene and at least one additional ring as cyclos: | ||
This subclass is indented under subclass 5. Compounds wherein the hydrophenanthrene nucleus contains
additional rings formed by ortho fusion or by a bridge.
| |||
![[List of Patents for class 560 subclass 7]](../ps.gif) 7 7 | 1, 4a-dimethylhydrophenanthrene - 1 carboxylic acid: | ||
This subclass is indented under subclass 5. Compounds which contain the nucleus 1, 4a-dimethlhydrophenanthrene
-1 carboxylic acid.
| |||
![[List of Patents for class 560 subclass 8]](../ps.gif) 8 8 | Aromatic acid moiety: | ||||||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains a benzene or
other carbocyclic aromatic group.
SEE OR SEARCH THIS CLASS, SUBCLASS:
SEE OR SEARCH CLASS:
| |||||||
![[List of Patents for class 560 subclass 9]](../ps.gif) 9 9 | Sulfur in acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains sulfur covalently
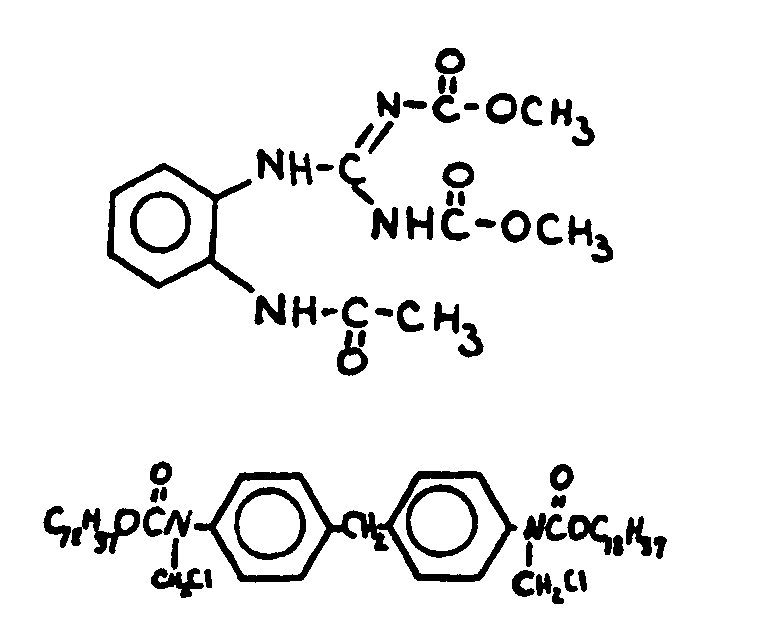
bonded.
| |||
![[List of Patents for class 560 subclass 10]](../ps.gif) 10 10 | Ortho fused rings in acid moiety: | ||
This subclass is indented under subclass 9. Compounds wherein the acid radical contains two or more
carbocyclic nuclei joined throught two ortho positioned nuclear
carbon atoms.
| |||
![[List of Patents for class 560 subclass 11]](../ps.gif) 11 11 | Sulfoxy in acid moiety: | ||
This subclass is indented under subclass 9. Compounds wherein the acid radical contains sulfur bonded
to oxygen.
| |||
![[List of Patents for class 560 subclass 12]](../ps.gif) 12 12 | Nitrogen in acid moiety: | ||||
This subclass is indented under subclass 11. Compounds wherein the acid radical contains nitrogen covalently
bonded.
| |||||
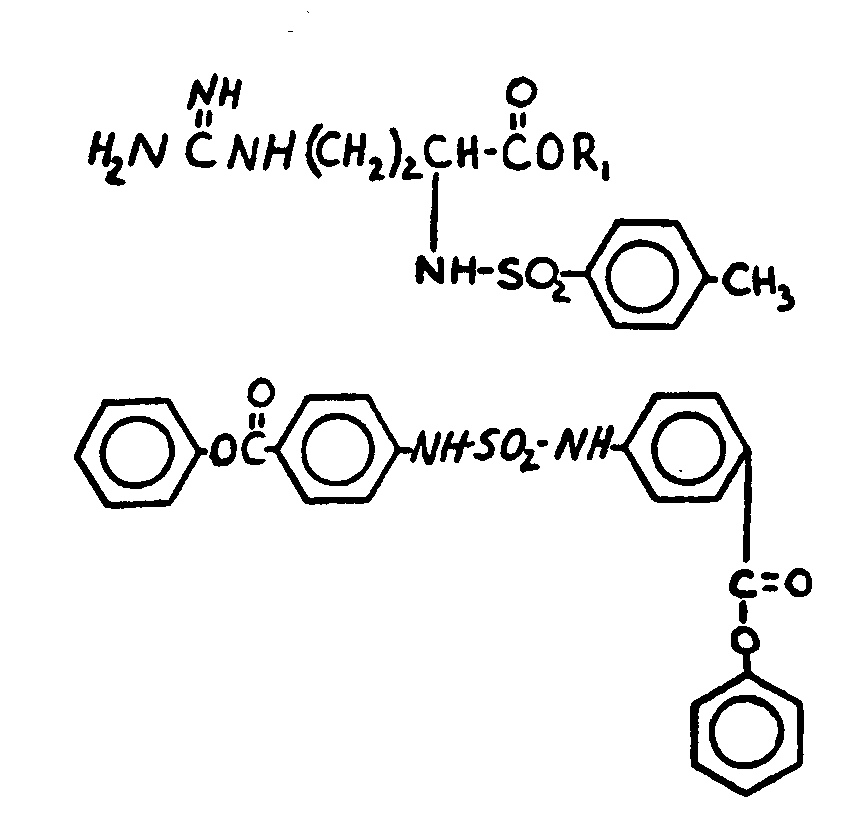
![[List of Patents for class 560 subclass 13]](../ps.gif) 13 13 | Plural nitrogens in acid moiety: | ||
This subclass is indented under subclass 12. Compounds wherein the acid radical contains more than one
nitrogen.
| |||
![[List of Patents for class 560 subclass 14]](../ps.gif) 14 14 | Sulfonic acids, salts or acid halides: | ||
| This subclass is indented under subclass 11. Compounds wherein the acid radical contains the group, shown
below, or its salts or acid halides.
| |||
![[List of Patents for class 560 subclass 15]](../ps.gif) 15 15 | Sulfur, not bonded directly to a ring, in same side chain as ester function: | ||||||
This subclass is indented under subclass 9. Compounds wherein the esterified carboxylic acid function
is on a side chain containing sulfur in or attached to the chain,
but not directly bonded to a carbon of a carbocyclic nucleus.
| |||||||
![[List of Patents for class 560 subclass 16]](../ps.gif) 16 16 | Nitrogen in acid moiety: | ||
This subclass is indented under subclass 15. Compounds wherein the acid radical also contains nitrogen
covalently bonded.
| |||
![[List of Patents for class 560 subclass 17]](../ps.gif) 17 17 | Sulfur, bonded directly to a ring, in same side chain as ester function: | ||
This subclass is indented under subclass 9. Compounds wherein the esterified carboxylic acid function
is on a side chain which contains sulfur directly bonded to a carbon
of a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 18]](../ps.gif) 18 18 | Ester function attached directly to a ring: | ||
This subclass is indented under subclass 9. Compounds wherein the esterified carboxylic acid function
is directly bonded to a carbon of a carbocyclic nucleus.
| |||
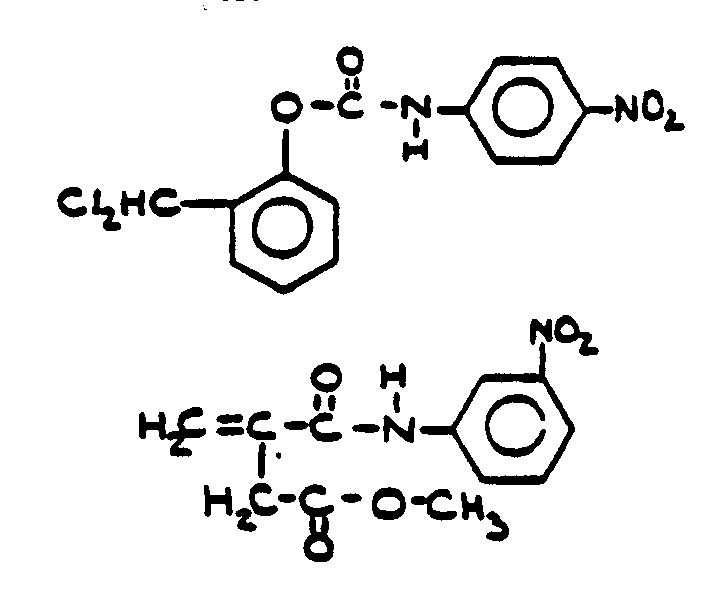
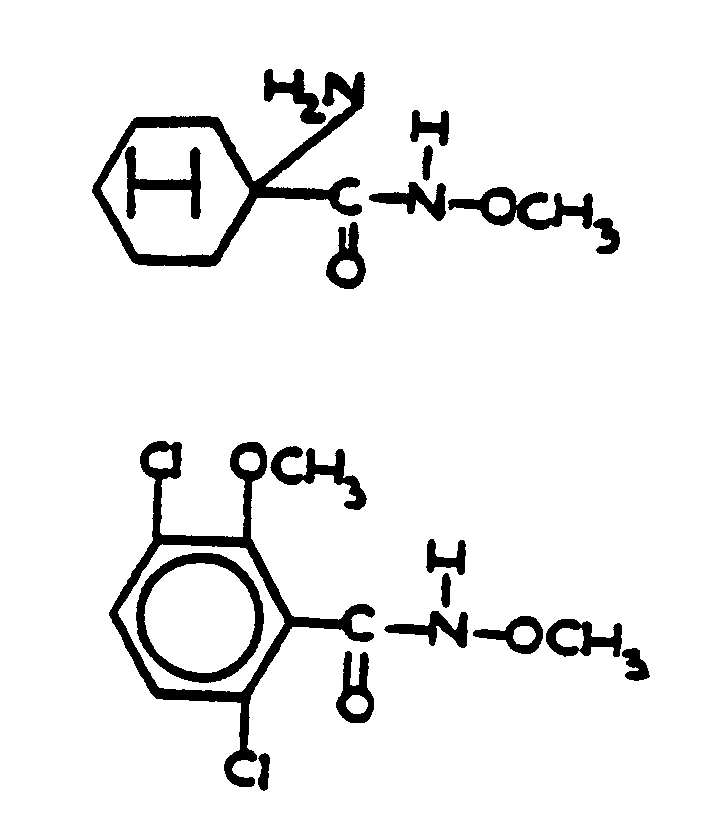
![[List of Patents for class 560 subclass 19]](../ps.gif) 19 19 | Nitrogen in acid moiety other than as nitroso or isocyanate (e.g., amino acid esters, etc.): | ||||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains covalently bonded
nitrogen other than in the form of an isocyanate or nitroso group.
| |||||
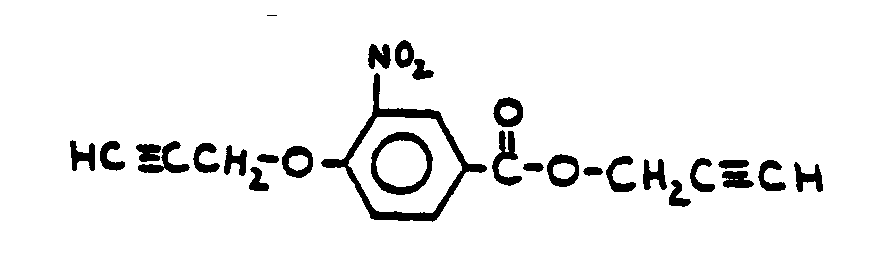
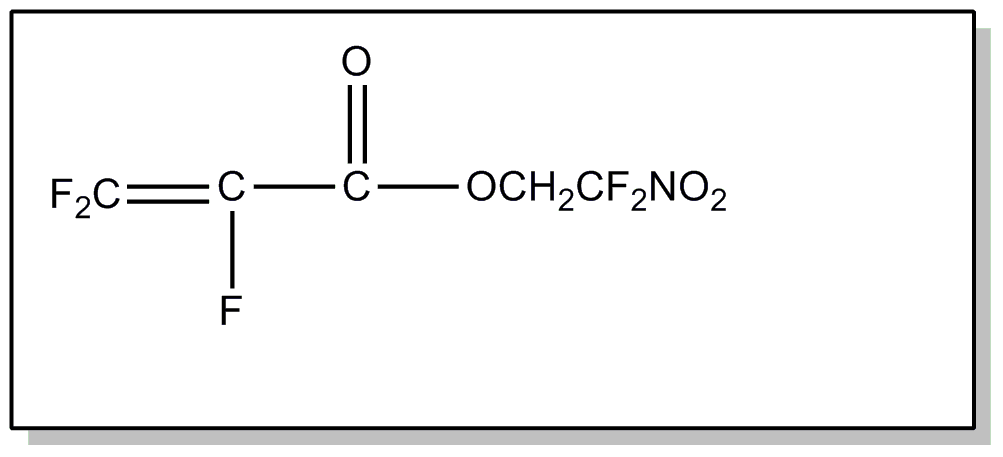
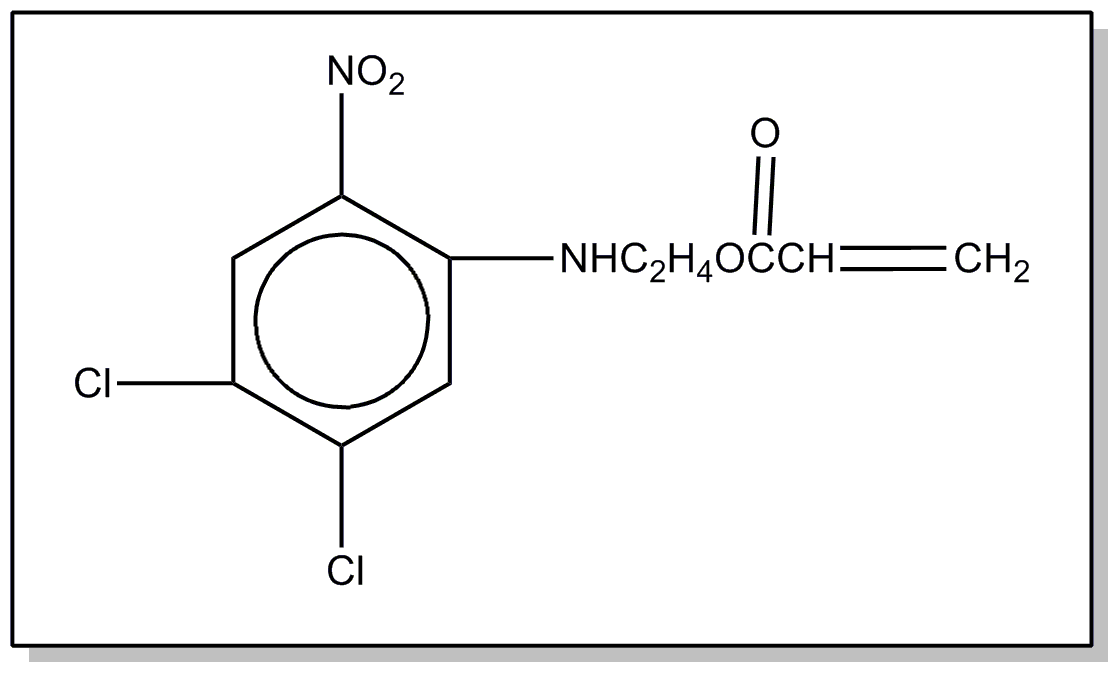
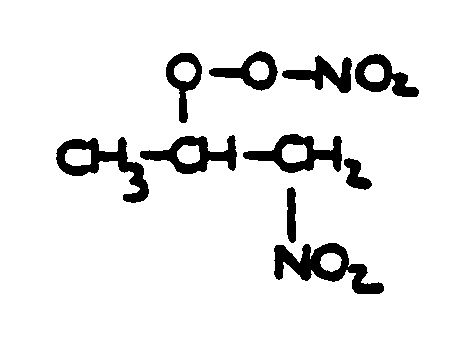
![[List of Patents for class 560 subclass 20]](../ps.gif) 20 20 | Nitro bonded to carbon in acid moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains the group -N(=O)2
bonded to carbon.
| |||
![[List of Patents for class 560 subclass 21]](../ps.gif) 21 21 | Plural rings in acid moiety: | ||
This subclass is indented under subclass 20. Compounds wherein the acid radical contains more than one
carbocyclic group.
| |||
![[List of Patents for class 560 subclass 22]](../ps.gif) 22 22 | Additional nitrogen in acid moiety: | ||||
This subclass is indented under subclass 20. Compounds wherein the acid radical contains an additional
nitrogen covalently bonded.
| |||||
![[List of Patents for class 560 subclass 23]](../ps.gif) 23 23 | Oxy, aldehyde or ketone group in acid moiety: | ||
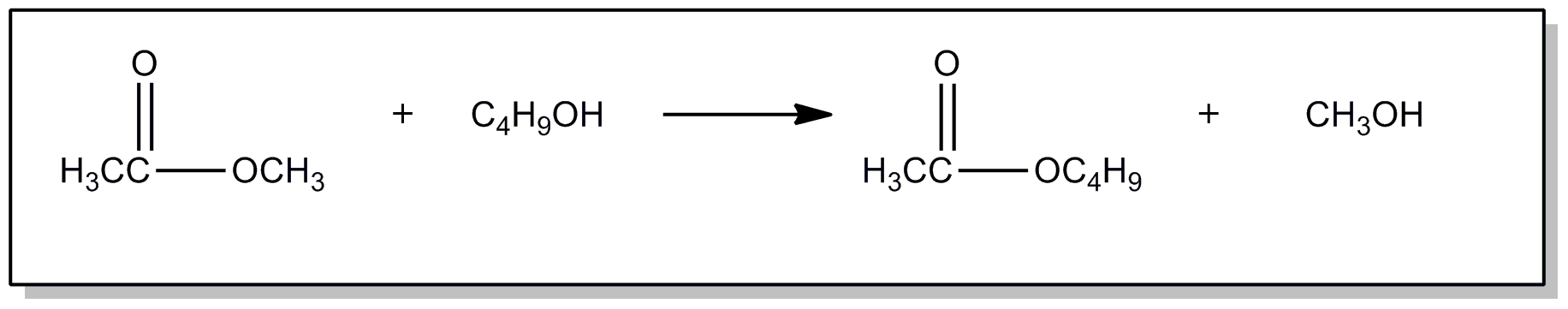
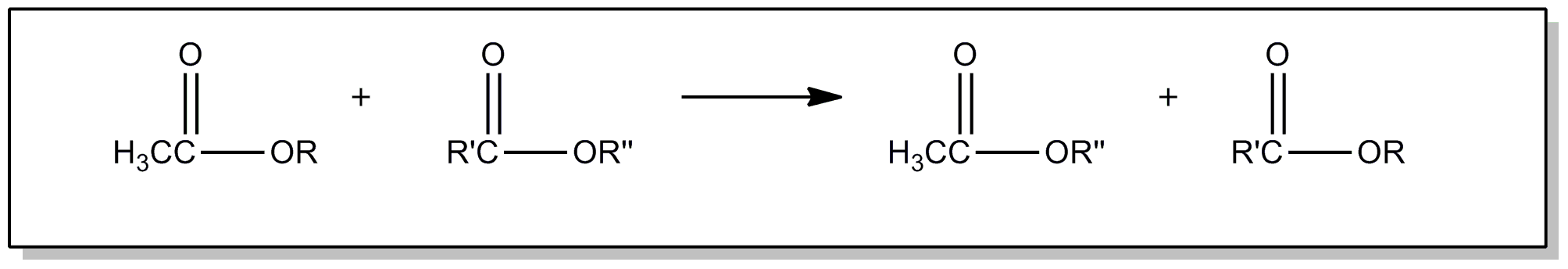
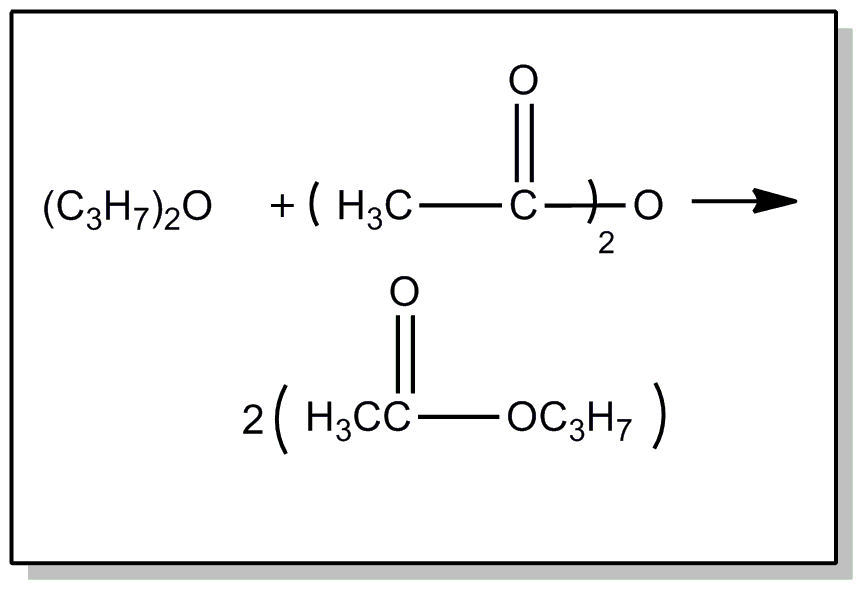
This subclass is indented under subclass 20. Compounds wherein the acid radical contains a carbonyl group
bonded to C and X where X is carbon or hydrogen; or an -OX group
bonded to a noncarbonylic C where X is C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
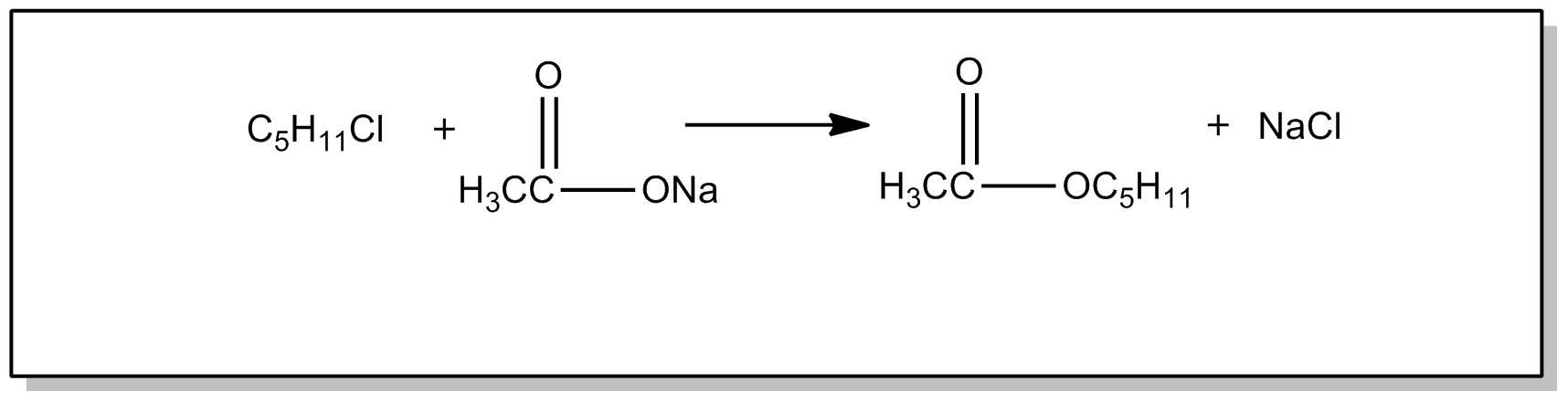
![[List of Patents for class 560 subclass 24]](../ps.gif) 24 24 | Carbamic acid: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains nitrogen directly
bonded to the carbon of the esterified carboxyl group.
| |||
![[List of Patents for class 560 subclass 25]](../ps.gif) 25 25 | Polycarbamic: | ||||
This subclass is indented under subclass 24. Compounds wherein the acid radical contains more than one
carbamic acid group, at least one of which is esterfied.
| |||||
![[List of Patents for class 560 subclass 26]](../ps.gif) 26 26 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 25. Compounds wherein the alcohol moiety contains in addition
to an esterified hydroxyl, another -OX group attached to a noncarbonylic C
where X is H, C, an alcoholate forming group not provided for above,
or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 27]](../ps.gif) 27 27 | Plural rings in acid moiety: | ||
This subclass is indented under subclass 24. Compounds wherein the acid radical contains more than one
carbocyclic group.
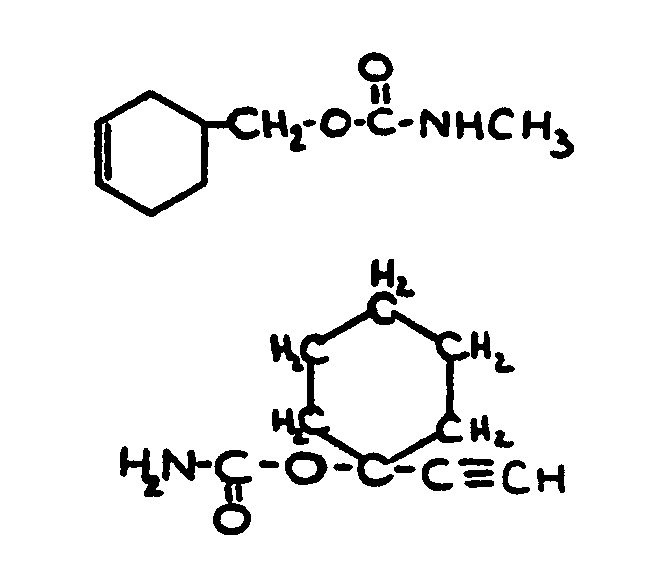
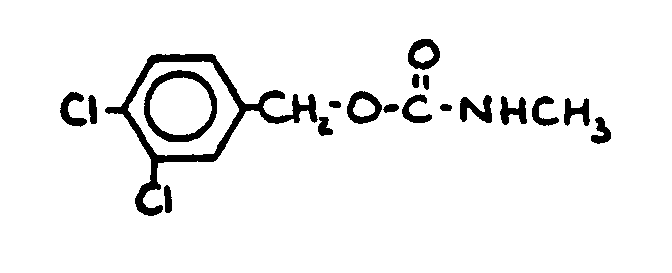
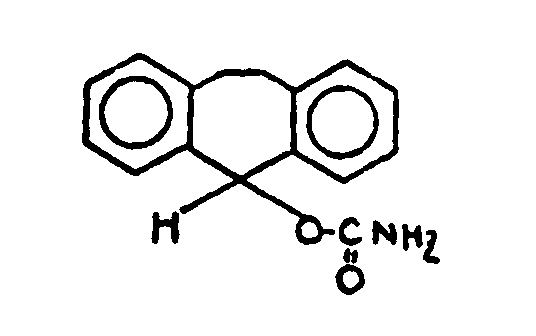
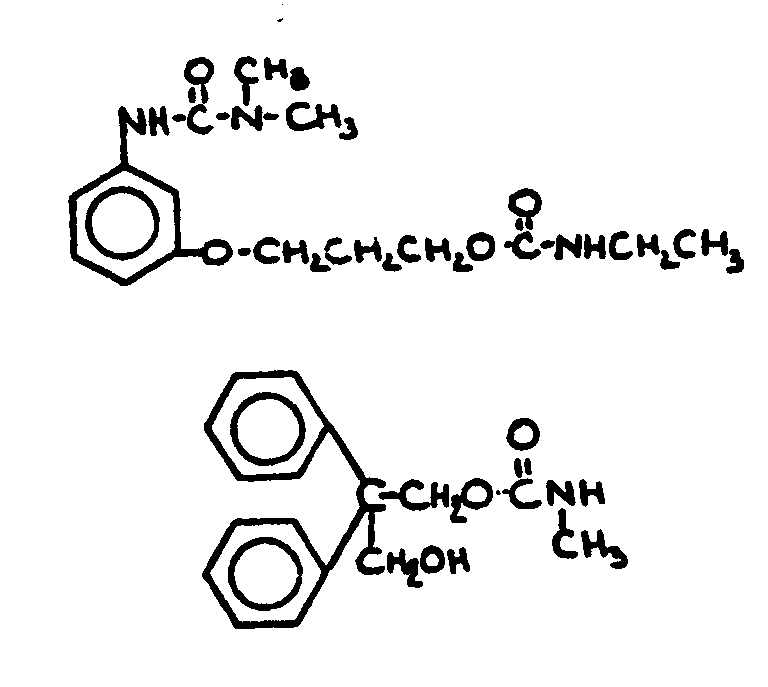
| |||
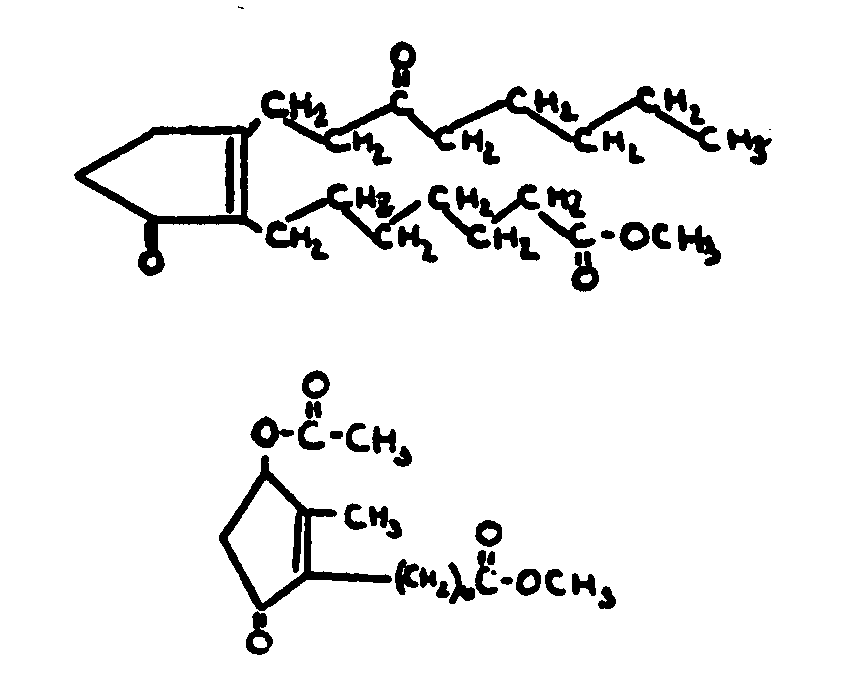
![[List of Patents for class 560 subclass 28]](../ps.gif) 28 28 | Ortho fused: | ||
This subclass is indented under subclass 27. Compounds wherein at least two carbocyclic groups are joined
through two ortho positioned carbon atoms.
| |||
![[List of Patents for class 560 subclass 29]](../ps.gif) 29 29 | Oxy in acid moiety: | ||
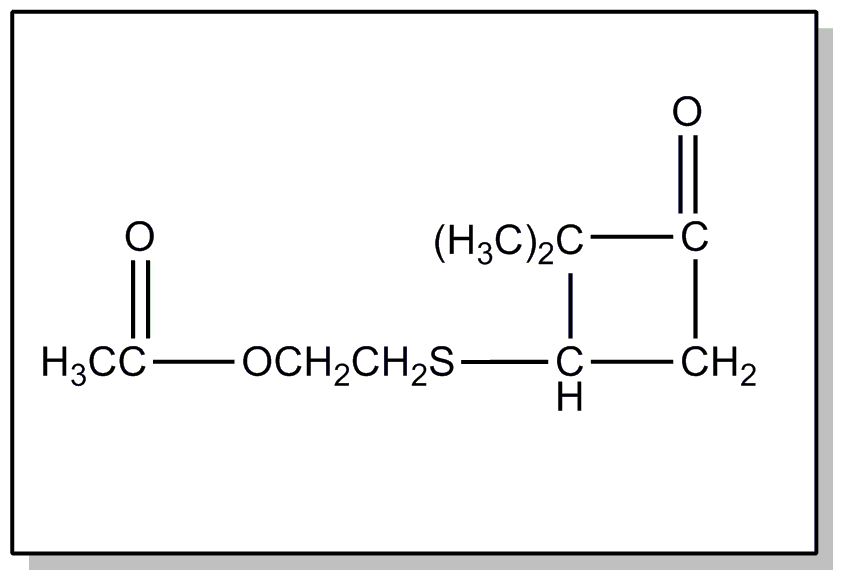
This subclass is indented under subclass 24. Compounds wherein the acid radical contains the -OX group
attached to a noncarbonylic C where X is C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 30]](../ps.gif) 30 30 | Halogen in acid moiety: | ||
This subclass is indented under subclass 24. Compounds wherein the acid radical contains a covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 31]](../ps.gif) 31 31 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 30. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 32]](../ps.gif) 32 32 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 24. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 33]](../ps.gif) 33 33 | Sulfur, nitrogen, halogen or additional oxy in alcohol moiety: | ||
This subclass is indented under subclass 24. Compounds wherein the alcohol moiety contains sulfur, nitrogen
or halogen covalently bonded or in addition to the esterified hydroxyl,
an -OX group attached to a noncarbonylic C where X is C, H, an alcoholate
forming group not provided for above, or an acyl group not provided
for above.
| |||
![[List of Patents for class 560 subclass 34]](../ps.gif) 34 34 | Ureido, guanido or hydrazino in acid moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains a ureido group >NC(=O)N<< or
a guanido group >NC(=N-)N<< or
a hydrazo group>NN<<.
| |||
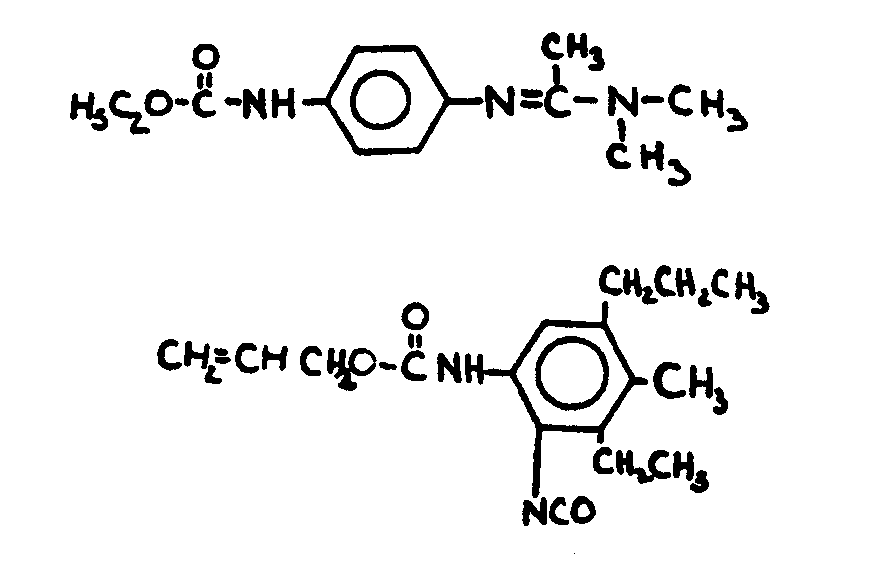
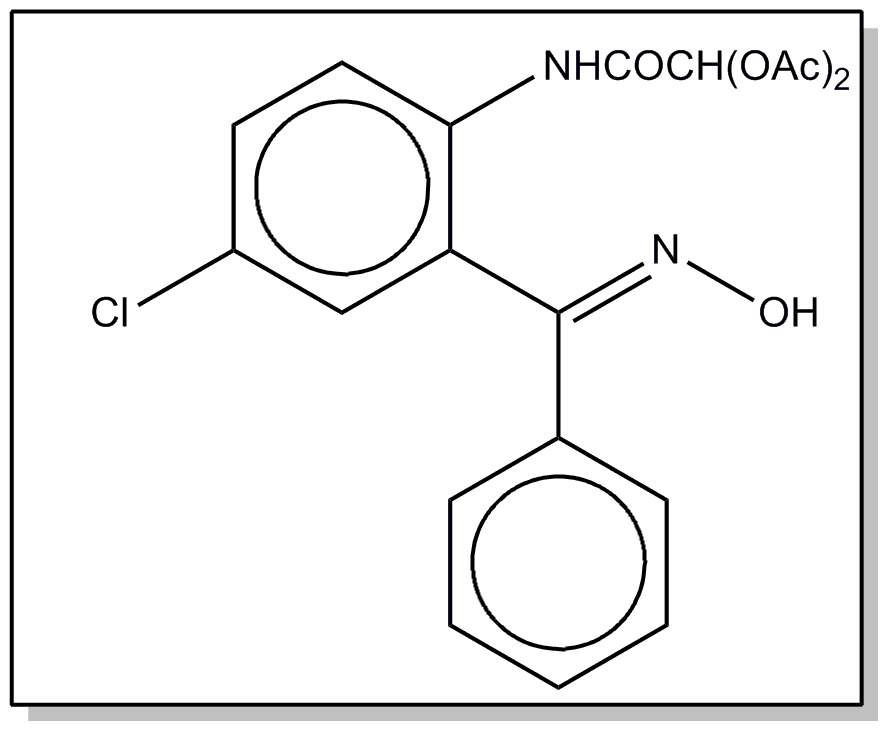
![[List of Patents for class 560 subclass 35]](../ps.gif) 35 35 | Amidine, azomethine, ketimine or oxime in acid moiety: | ||
This subclass is indented under subclass 19. Compounds containing the grouping -C=N-, including
amidines not provided for above or compounds equivalent in structure
to those formed by reacting an alde-hyde or a ketone with ammonia
or an amine.
| |||
![[List of Patents for class 560 subclass 36]](../ps.gif) 36 36 | Plural rings bonded directly to the same carbon in acid moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains two carbocyclic
nuclei attached to a methylene or carbonyl group.
| |||
![[List of Patents for class 560 subclass 37]](../ps.gif) 37 37 | The nitrogen is not bonded directly to a ring: | ||||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains nitrogen which
is not directly attached to a carbocyclic nucleus.
| |||||
![[List of Patents for class 560 subclass 38]](../ps.gif) 38 38 | The nitrogen is in same side chain as ester function: | ||
This subclass is indented under subclass 37. Compounds wherein the acid radical contains nitrogen in
or attached to the same side chain which contains the carboxylic
acid ester function.
| |||
![[List of Patents for class 560 subclass 39]](../ps.gif) 39 39 | Oxy in acid moiety: | ||
This subclass is indented under subclass 38. Compounds wherein the acid radical also contains the group
-OX attached to a noncarbonylic C where X is C, H, an alcoholate
forming group not provided for above, or an acyl group not provided
for above.
| |||
![[List of Patents for class 560 subclass 40]](../ps.gif) 40 40 | Phenylalanines: | ||
This subclass is indented under subclass 39. Compounds wherein the acid radical contains a phenylalanine
group.
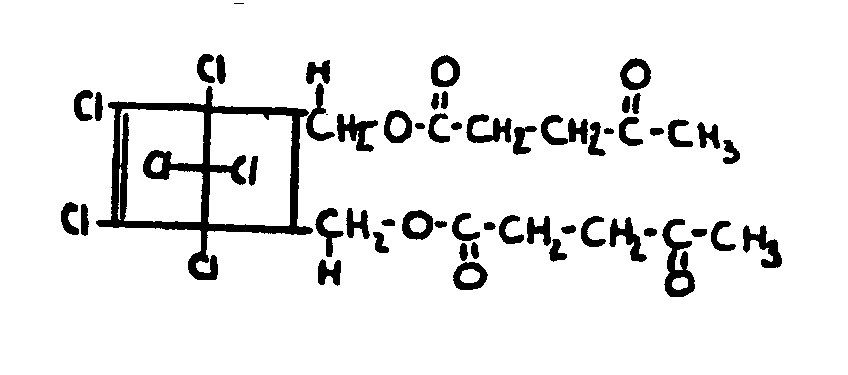
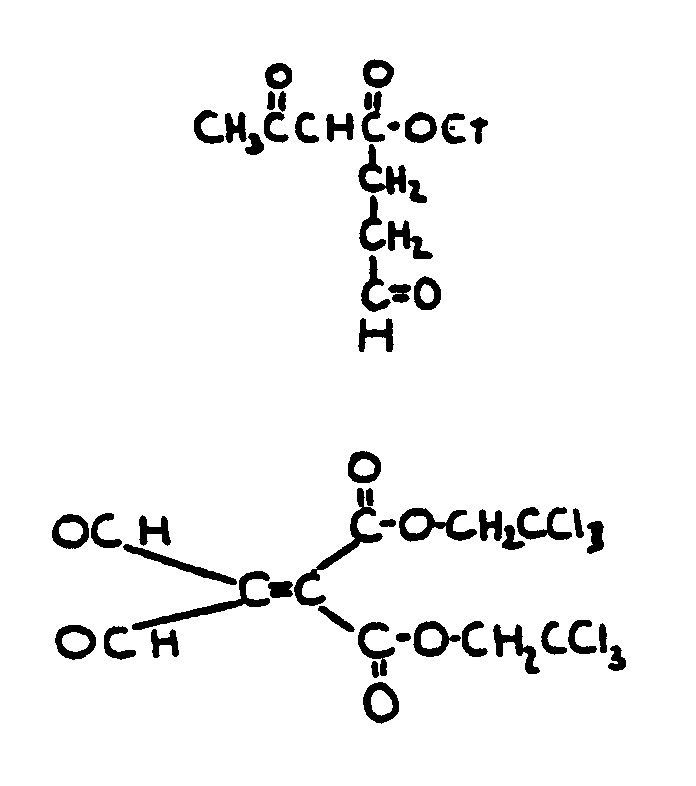
| |||
![[List of Patents for class 560 subclass 41]](../ps.gif) 41 41 | Amide in acid moiety: | ||
This subclass is indented under subclass 38. Compounds wherein the acid radical contains an acyl group,
not provided for above, attached to the nitrogen to form an amide.
| |||
![[List of Patents for class 560 subclass 42]](../ps.gif) 42 42 | Oxy in acid moiety: | ||
Compounds under subclass 37 wherein the acid radical also
contains the group -OX attached to a noncarbonylic C where X is
C, H, an alcoholate forming group not provided for above, or an
acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 43]](../ps.gif) 43 43 | The nitrogen is bonded directly to a ring and is in same side chain as ester function: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical has the ester function
on a side chain attached to nitrogen, which is directly attached
to a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 44]](../ps.gif) 44 44 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 43. Compounds wherein the acid radical has more than one carboxyl
group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 45]](../ps.gif) 45 45 | Oxy in acid moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains an -OX group
attached to a noncarbonylic C where X is C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 46]](../ps.gif) 46 46 | Benzoic acid substituted on ring with oxy and nitrogen: | ||
This subclass is indented under subclass 45. Compounds wherein the acid radical has an esterified carboxyl
group, nitrogen an an -OX group all directly attached to the benzene
ring, wherein X is C, H, and alcoholate forming group not provided
for above, or an acyl group not provided for above.
| |||
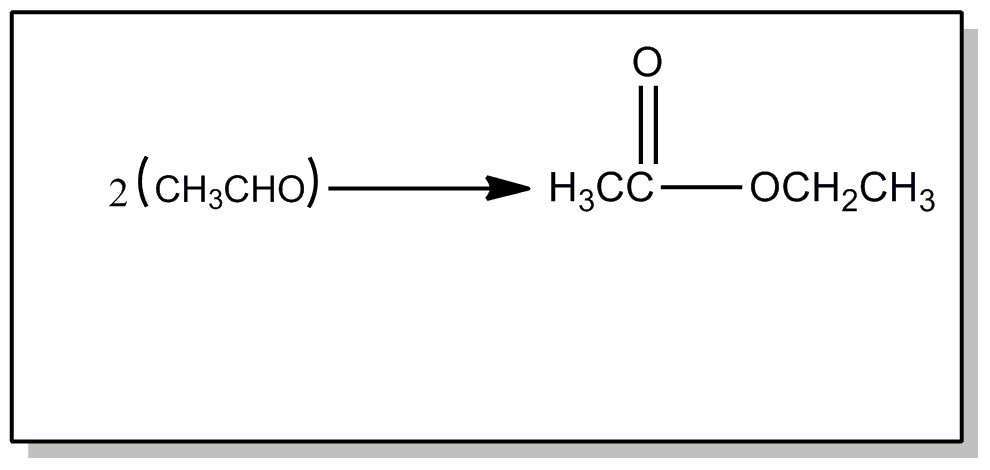
![[List of Patents for class 560 subclass 47]](../ps.gif) 47 47 | Halogen in acid moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains a covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 48]](../ps.gif) 48 48 | Plural rings in acid moiety with nitrogen bonded directly to at least one of the rings: | ||
This subclass is indented under subclass 19. Compounds wherein the acid radical contains more than one
carbocyclic group with nitrogen directly attached to at least one
carbocyclic group.
| |||
![[List of Patents for class 560 subclass 49]](../ps.gif) 49 49 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the alcohol moiety contains nitrogen covalently
bonded.
| |||
![[List of Patents for class 560 subclass 50]](../ps.gif) 50 50 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 19. Compounds wherein the alcohol moiety contains in addition
to the esterified hydroxyl group, an -OX group attached to a noncarbonylic
C where X is C, H, an alcoholate forming group not provided for
above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 51]](../ps.gif) 51 51 | Aldehyde or ketone group in acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains the group -C(=O)X
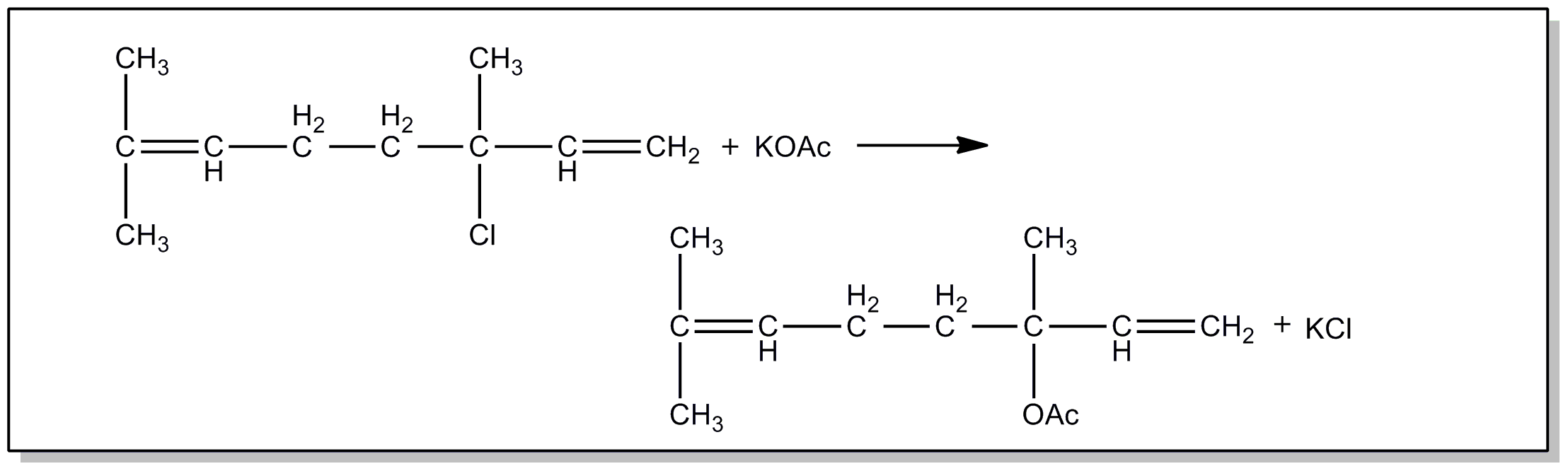
bonded to carbon and X is C or H, i.e., aldehyde or ketone group
containing esters.
| |||
![[List of Patents for class 560 subclass 52]](../ps.gif) 52 52 | Plural rings bonded directly to the same carbonyl in acid moiety: | ||
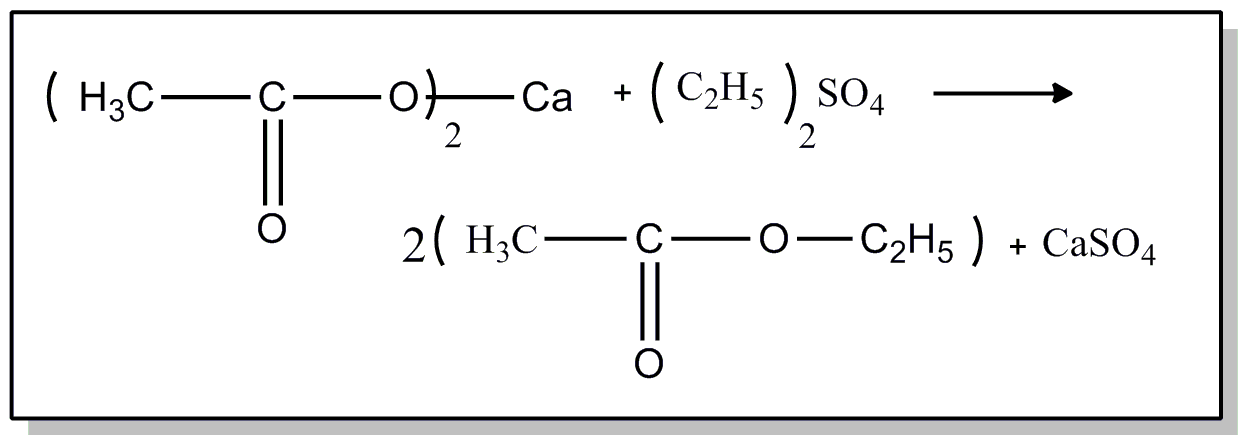
This subclass is indented under subclass 51. Compounds wherein the acid radical contains two carbocyclic
nuclei directly attached to a carbonyl group.
| |||
![[List of Patents for class 560 subclass 53]](../ps.gif) 53 53 | Oxy in acid moiety: | ||
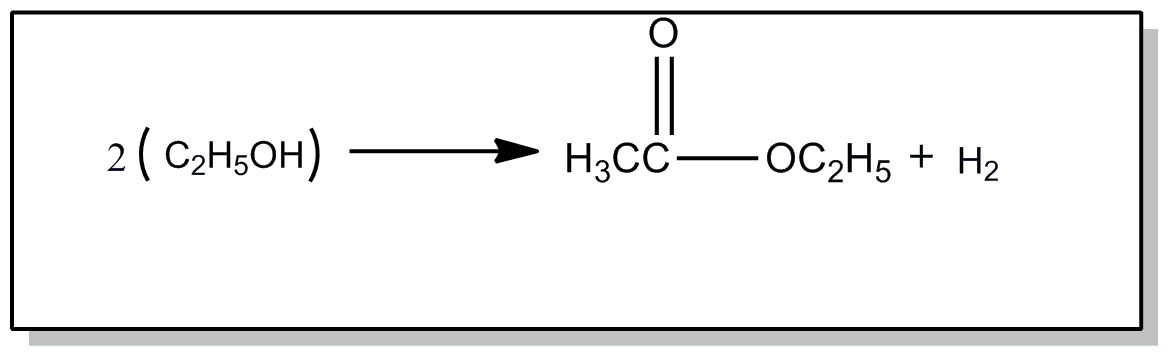
This subclass is indented under subclass 51. Compounds wherein the acid radical also contains the group
-OX attached to a noncarbonylic C and X is C, H, an alcoholate forming group
not provided for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 54]](../ps.gif) 54 54 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 51. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 55]](../ps.gif) 55 55 | Oxy in acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains the -OX group
attached to a noncarbonylic C where X may be C, H, an alcoholate
forming group not provided for above, or an acyl group not provided
for above.
| |||
![[List of Patents for class 560 subclass 56]](../ps.gif) 56 56 | Ortho fused rings in acid moiety: | ||
This subclass is indented under subclass 55. Compounds wherein the acid radical contains two or more
carbocyclic nuclei joined through a pair of ortho positioned carbon
atoms.
| |||
![[List of Patents for class 560 subclass 57]](../ps.gif) 57 57 | Plural rings bonded directly to the same carbon in acid moiety: | ||
This subclass is indented under subclass 55. Compounds wherein the acid radical contains two carbocyclic
nuclei attached to a methylene group.
| |||
![[List of Patents for class 560 subclass 58]](../ps.gif) 58 58 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 57. Compounds wherein the alcohol moiety contains nitrogen covalently
bonded.
| |||
![[List of Patents for class 560 subclass 59]](../ps.gif) 59 59 | Rings bonded directly to each other in acid moiety: | ||
This subclass is indented under subclass 55. Compounds wherein the acid radical contains two carbocyclic
groups joined through a covalent bond.
| |||
![[List of Patents for class 560 subclass 60]](../ps.gif) 60 60 | Oxy, not bonded directly to a ring, in same side chain as ester function: | ||||
This subclass is indented under subclass 55. Compounds wherein the esterified acid function is on a side
chain containing an oxy group attached to or in the chain, but not
attached to a carbocyclic nucleus.
| |||||
![[List of Patents for class 560 subclass 61]](../ps.gif) 61 61 | Oxy, bonded directly to a ring, in same side chain as ester function: | ||
This subclass is indented under subclass 55. Compounds wherein the esterified acid function is on side
chain containing an oxy group which is directly attached to a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 62]](../ps.gif) 62 62 | Halogen in acid moiety: | ||
This subclass is indented under subclass 61. Compounds wherein the acid radical also contains covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 63]](../ps.gif) 63 63 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 62. Compounds wherein the alcohol moiety contains in addition
to the esterified hydroxyl, an -OX group attached to a noncarbonylic
C where X may be C, H, an alcoholate forming group not provided
for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 64]](../ps.gif) 64 64 | Ester function attached directly to a ring: | ||
This subclass is indented under subclass 55. Compounds wherein the esterified acid function is directly
attached to a carbocyclic nucleus.
| |||
![[List of Patents for class 560 subclass 65]](../ps.gif) 65 65 | Halogen in acid moiety: | ||
This subclass is indented under subclass 64. Compounds wherein the acid function contains a covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 66]](../ps.gif) 66 66 | Acylated: | ||
This subclass is indented under subclass 64. Compounds wherein a hydroxy group of the esterified acid
radical has been esterified by an acyl group not provided for above.
| |||
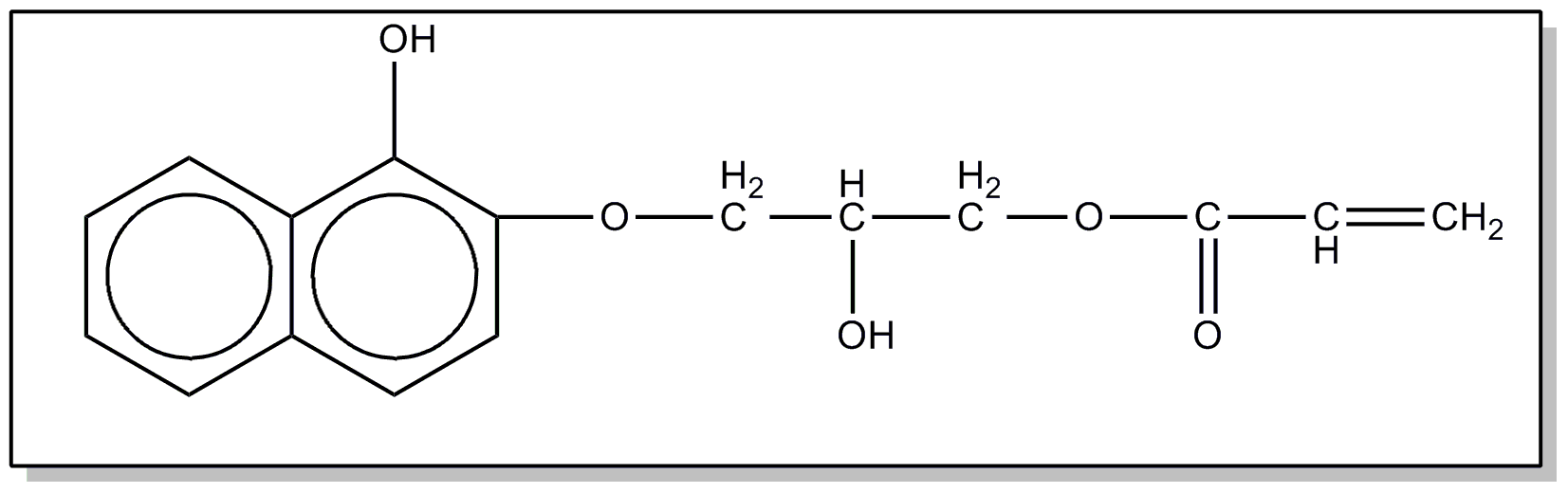
![[List of Patents for class 560 subclass 67]](../ps.gif) 67 67 | Phenolic hydroxy or metallate: | ||
This subclass is indented under subclass 64. Compounds wherein the acid function contains an -OX group,
where X is H or an alcoholate forming group not provided for above,
directly attached to the carbon of a benzene ring.
| |||
![[List of Patents for class 560 subclass 68]](../ps.gif) 68 68 | Tannins and reaction products thereof: | ||
This subclass is indented under subclass 67. Products and their processes which are known as tannins
or tannic acids. Chemically they appear to be esters of gallic
acid in which the carboxyl group thereof is esterified by the hydroxyl
group of a second molecule of gallic acid, or glucosides thereof.
| |||
![[List of Patents for class 560 subclass 69]](../ps.gif) 69 69 | Extraction from bark or vegetable material: |
| This subclass is indented under subclass 68. Processes which are directed to or include the treatment of bark, shell galls, or other vegetable material to remove tannins therefrom. | |
![[List of Patents for class 560 subclass 70]](../ps.gif) 70 70 | Polyphenolic hydroxy or metallate: | ||
This subclass is indented under subclass 67. Compounds wherein the acid function contains more than one
-OX group attached to a carbon of a benzene ring, where X is H or
an alcoholate forming group not provided for above.
| |||
![[List of Patents for class 560 subclass 71]](../ps.gif) 71 71 | Salicyclic acid: | ||||
This subclass is indented under subclass 67. Compounds wherein the acid radical is derived from the compound
known as salicyclic acid, e.g., salol, oil of wintergreen.
| |||||
![[List of Patents for class 560 subclass 72]](../ps.gif) 72 72 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 67. Compounds wherein the alcohol moiety contains a carbocyclic
nucleus.
| |||
![[List of Patents for class 560 subclass 73]](../ps.gif) 73 73 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 64. Compounds wherein the alcohol moiety contains a carbocyclic
nucleus.
| |||
![[List of Patents for class 560 subclass 74]](../ps.gif) 74 74 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 64. Compounds wherein the alcohol moiety contains nitrogen covalently
bonded.
| |||
![[List of Patents for class 560 subclass 75]](../ps.gif) 75 75 | Phenolic hydroxy or metallate: | ||
This subclass is indented under subclass 55. Compounds wherein the acid function contains an -OX group,
where X is H or an alcoholate forming group not provided for above,
directly attached to a benzene ring.
| |||
![[List of Patents for class 560 subclass 76]](../ps.gif) 76 76 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 77]](../ps.gif) 77 77 | Producing carboxyl group by oxidation: | ||
This subclass is indented under subclass 76. Processes wherein at least one of the carboxyl groups of
the acid is formed by oxidizing an aromatic material.
| |||
![[List of Patents for class 560 subclass 78]](../ps.gif) 78 78 | Purification or recovery: |
| This subclass is indented under subclass 76. Processes which are directed to the purification, separation or recovery of aromatic polycarboxylic acid esters. | |
![[List of Patents for class 560 subclass 79]](../ps.gif) 79 79 | Of esters of polyoxy alcohols: |
| This subclass is indented under subclass 78. Processes wherein the compounds treated are polyoxy alcohol esters. | |
![[List of Patents for class 560 subclass 80]](../ps.gif) 80 80 | Ortho fused rings in acid moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the acid radical contains two or more
carbocyclic nuclei joined through ortho positioned nuclear carbons.
| |||
![[List of Patents for class 560 subclass 81]](../ps.gif) 81 81 | Esterified carboxy not bonded directly to a ring: | ||
This subclass is indented under subclass 76. Compounds wherein an esterified carboxyl group of the acid
radical is not directly bonded to a nuclear carbon of a carbocyclic
group.
| |||
![[List of Patents for class 560 subclass 82]](../ps.gif) 82 82 | Malonates: | ||
This subclass is indented under subclass 81. Compounds in which the methylene group of a malonic acid
ester contains an aromatic substituent.
| |||
![[List of Patents for class 560 subclass 83]](../ps.gif) 83 83 | Halogen in acid moiety: | ||||
This subclass is indented under subclass 76. Compounds wherein the acid radical contains covalently bonded
halogen.
| |||||
![[List of Patents for class 560 subclass 84]](../ps.gif) 84 84 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the alcohol moiety contains a carbocyclic
nucleus.
| |||
![[List of Patents for class 560 subclass 85]](../ps.gif) 85 85 | Aromatic alcohol moiety: | ||
This subclass is indented under subclass 84. Compounds wherein the carbocyclic nucleus is aromatic.
| |||
![[List of Patents for class 560 subclass 86]](../ps.gif) 86 86 | Esterified phenolic hydroxy: | ||
This subclass is indented under subclass 85. Compounds wherein the ester is formed with a phenolic hydroxyl
group.
| |||
![[List of Patents for class 560 subclass 87]](../ps.gif) 87 87 | Sulfur or halogen in alcohol moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the alcohol moiety contains sulfur or
a halogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 88]](../ps.gif) 88 88 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the alcohol moiety contains nitrogen covalently
bonded.
| |||
![[List of Patents for class 560 subclass 89]](../ps.gif) 89 89 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the alcohol moiety contains in addition
to the esterified hydroxyl group, an -OX group attached to a noncarbonylic
C where X may be H, C, an acyl radical not provided for above, or
an alcoholate forming group not provided for above.
| |||
![[List of Patents for class 560 subclass 90]](../ps.gif) 90 90 | Additional esterifying acid: | ||
This subclass is indented under subclass 89. Compounds wherein the polyoxy alcohol is additionally esterified
by a different carboxylic acid not provided for above.
| |||
![[List of Patents for class 560 subclass 91]](../ps.gif) 91 91 | Polyoxyalkylene alcohol moiety: | ||
This subclass is indented under subclass 89. Compounds wherein the polyoxy alcohol moiety has the structure-O-(CnH2
n )m, where n and m are positive integers and m>1.
| |||
![[List of Patents for class 560 subclass 92]](../ps.gif) 92 92 | Preparing esters by ester interchange: |
| This subclass is indented under subclass 89. Processes wherein the ester is prepared by reacting an ester with another ester, acid or alcohol, to produce a different ester. | |
![[List of Patents for class 560 subclass 93]](../ps.gif) 93 93 | Preparing esters from alkylene oxides: |
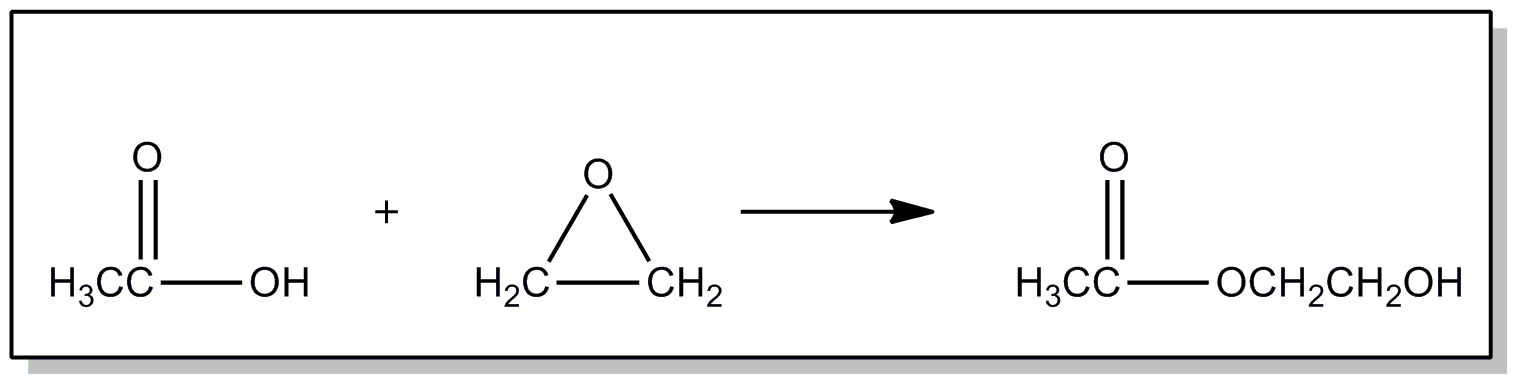
| This subclass is indented under subclass 89. Processes wherein the esters are prepared from alkylene oxides. | |
![[List of Patents for class 560 subclass 94]](../ps.gif) 94 94 | Preparing esters from acid or from nitrile and diol: |
| This subclass is indented under subclass 89. Processes wherein the esters are prepared by reaction of a carboxylic acid or a nitrile with a diol. | |
![[List of Patents for class 560 subclass 95]](../ps.gif) 95 95 | Unsaturation in alcohol moiety: | ||
This subclass is indented under subclass 76. Compounds wherein the alcohol moiety is acyclic and contains
an ethylenic double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 96]](../ps.gif) 96 96 | Processes: | ||
This subclass is indented under subclass 76. Processes for preparing compounds classifiable in that subclass.
| |||
![[List of Patents for class 560 subclass 97]](../ps.gif) 97 97 | Carbonylation: |
| This subclass is indented under subclass 96. Processes wherein the ester is prepared through formation of a carboxyl group on a starting material by reacting with a carbonylating agent such as carbon monoxide in the presence of an alcohol or by subsequent esterification. | |
![[List of Patents for class 560 subclass 98]](../ps.gif) 98 98 | Esterification of acid, salt, acid halide or anhydride with alcohol: |
| This subclass is indented under subclass 96. Processes in which the compounds are prepared by reacting an alcohol with a carboxylic acid or its salt, acid halide or anhydride. | |
![[List of Patents for class 560 subclass 99]](../ps.gif) 99 99 | Metal containing catalyst utilized: |
| This subclass is indented under subclass 98. Processes wherein the esterification reaction is carried out in the presence of a metal containing catalyst. | |
![[List of Patents for class 560 subclass 100]](../ps.gif) 100 100 | Naphthyl in acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains the naphthyl
group.
| |||
![[List of Patents for class 560 subclass 101]](../ps.gif) 101 101 | Plural rings bonded directly to the same carbon in acid moiety: | ||||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains two carbocyclic
nuclei attached to a noncarbonylic methylene group.
| |||||
![[List of Patents for class 560 subclass 102]](../ps.gif) 102 102 | Rings bonded directly to each other in acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains a carbocyclic
nucleus directly linked to another carbocyclic nucleus through a
covalent bond.
| |||
![[List of Patents for class 560 subclass 103]](../ps.gif) 103 103 | Monocyclic acid moiety: | ||
This subclass is indented under subclass 8. Compounds wherein the acid radical contains only one carbocyclic
group.
| |||
![[List of Patents for class 560 subclass 104]](../ps.gif) 104 104 | Additional unsaturation in acid moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the acid radical contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 105]](../ps.gif) 105 105 | Carboxyl, not bonded directly to a ring, in acid moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the carboxyl group of the acid radical
is not directly attached to the carbocyclic group.
| |||
![[List of Patents for class 560 subclass 106]](../ps.gif) 106 106 | Ring in alcohol moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the alcohol moiety contains a carbocyclic
group.
| |||
![[List of Patents for class 560 subclass 107]](../ps.gif) 107 107 | Plural rings in alcohol moiety: | ||||
This subclass is indented under subclass 106. Compounds wherein the alcohol moiety contains more than
one carbocyclic group.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 108]](../ps.gif) 108 108 | Esterified phenolic hydroxy: | ||
This subclass is indented under subclass 107. Compounds wherein the ester function is formed with a phenolic
OH group.
| |||
![[List of Patents for class 560 subclass 109]](../ps.gif) 109 109 | Esterified phenolic hydroxy: | ||
This subclass is indented under subclass 106. Compounds in which the ester function is formed with a phenolic
OH group.
| |||
![[List of Patents for class 560 subclass 110]](../ps.gif) 110 110 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the alcohol moiety contains nitrogen covalently
bonded.
| |||
![[List of Patents for class 560 subclass 111]](../ps.gif) 111 111 | Halogen in alcohol moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the alcohol moiety contains a covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 112]](../ps.gif) 112 112 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the alcohol moiety is an acyclic polyoxy
alcohol, which in addition to the esterified PH, contains at least
one other oxy group, -OX wherein X may be C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 113]](../ps.gif) 113 113 | Unsaturation in alcohol moiety: | ||
This subclass is indented under subclass 103. Compounds wherein the alcohol moiety contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 114]](../ps.gif) 114 114 | Preparing alicyclic acid esters by carbonylation: |
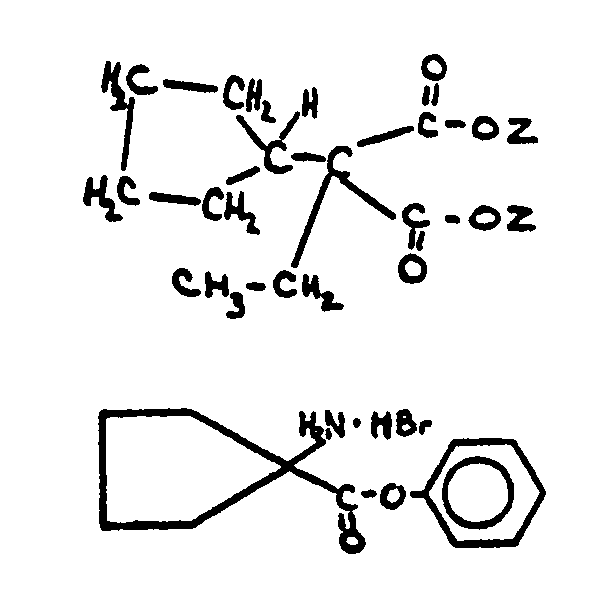
| This subclass is indented under subclass 1. Processes wherein an alicyclic acid ester is prepared through formation of a carboxyl group on a starting material by reaction with a carbonylating agent such as carbon monoxide in the presence of an alcohol or through subsequent esterification. | |
![[List of Patents for class 560 subclass 115]](../ps.gif) 115 115 | Alicyclic carbamates: | ||
This subclass is indented under subclass 1. Compounds in which the acid radical contains an alicyclic
nucleus and a nitrogen directly attached to the carbon of an esterified
carboxyl group.
| |||
![[List of Patents for class 560 subclass 116]](../ps.gif) 116 116 | Plural alicyclic rings in acid moiety: | ||||
This subclass is indented under subclass 1. Compounds in which the acid radical contains more than one
alicyclic group.
| |||||
![[List of Patents for class 560 subclass 117]](../ps.gif) 117 117 | Tricyclo ring system in acid moiety: | ||
This subclass is indented under subclass 116. Compounds in which the acid radical contains three alicyclic
groups which are joined to each other either through ortho fusion
or by a bridge.
| |||
![[List of Patents for class 560 subclass 118]](../ps.gif) 118 118 | Two rings only in acid moiety: | ||
This subclass is indented under subclass 116. Compounds in which the acid radical contains two alicyclic
groups.
| |||
![[List of Patents for class 560 subclass 119]](../ps.gif) 119 119 | Ortho fused: | ||
This subclass is indented under subclass 118. Compounds in which the acid radical contains two alicyclic
groups joined through two ortho positioned carbon atoms.
| |||
![[List of Patents for class 560 subclass 120]](../ps.gif) 120 120 | 2,2,1-bicyclo: | ||
This subclass is indented under subclass 118. Compounds in which the acid radical contains a 2,2,1-bicyclo
nucleus.
| |||
![[List of Patents for class 560 subclass 121]](../ps.gif) 121 121 | Cyclopentyl in acid moiety (e.g., prostaglandins, etc.): | ||||
This subclass is indented under subclass 1. Compounds in which the acid radical contains a cyclopentyl
group.
| |||||
![[List of Patents for class 560 subclass 122]](../ps.gif) 122 122 | Cyclopentyl - (C)x - COOR; x=0-2: | ||
This subclass is indented under subclass 121. Compounds wherein the carboxylic acid ester function is
directly attached to the cyclopentyl nucleus or is attached thereto
by a chain of no more than two carbon atoms.
| |||
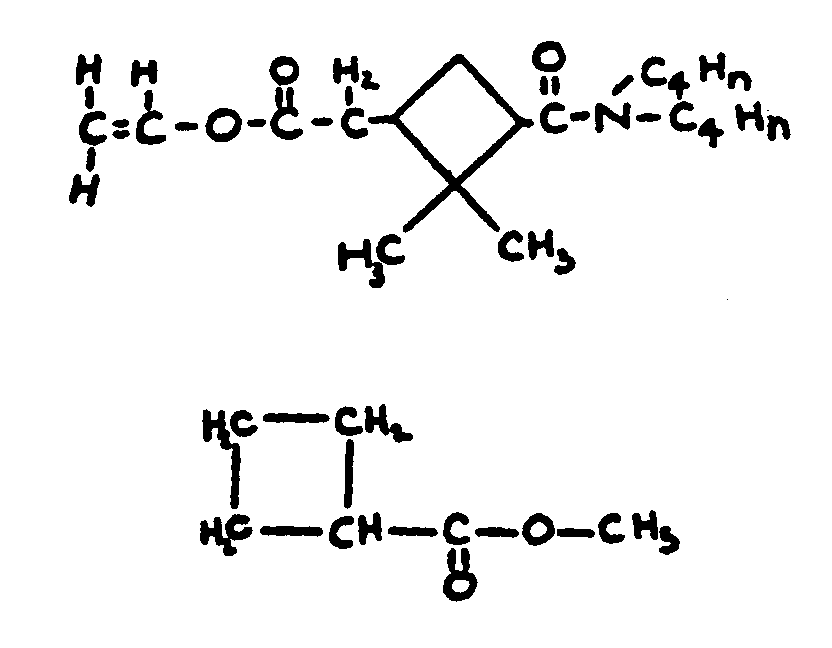
![[List of Patents for class 560 subclass 123]](../ps.gif) 123 123 | Cyclobutyl in acid moiety: | ||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains a cyclobutyl
group.
| |||
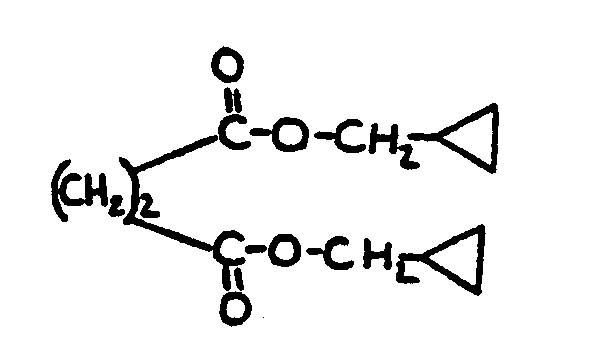
![[List of Patents for class 560 subclass 124]](../ps.gif) 124 124 | Cyclopropyl in acid moiety: | ||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains a cyclopropyl
group.
| |||
![[List of Patents for class 560 subclass 125]](../ps.gif) 125 125 | Alicyclic acid moiety containing N, S, P, B or halogen: | ||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains an alicyclic
nucleus not provided for above and contains nitrogen, sulfur, phosphorus,
boron or halogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 126]](../ps.gif) 126 126 | Alicyclic acid moiety containing oxy, aldehyde or ketone group: | ||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains an alicyclic
nucleus not provided for above and contains the group-C(=O)X
bonded to carbon, where X is C or H; or the group -OX attached to
a noncarbonylic carbon where X is C, H, an alcoholate forming group
not provided for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 127]](../ps.gif) 127 127 | Alicyclic polycarboxylic acid moiety: | ||||
This subclass is indented under subclass 1. Compounds which contain an alicyclic nucleus not provided
for above and contain more than one carboxyl group, at least one
of which is esterified.
| |||||
![[List of Patents for class 560 subclass 128]](../ps.gif) 128 128 | Alicyclic acid moiety containing unsaturation: | ||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains an alicyclic
nucleus not provided for above and contains an ethylenic double
bond or a triple bond.
| |||
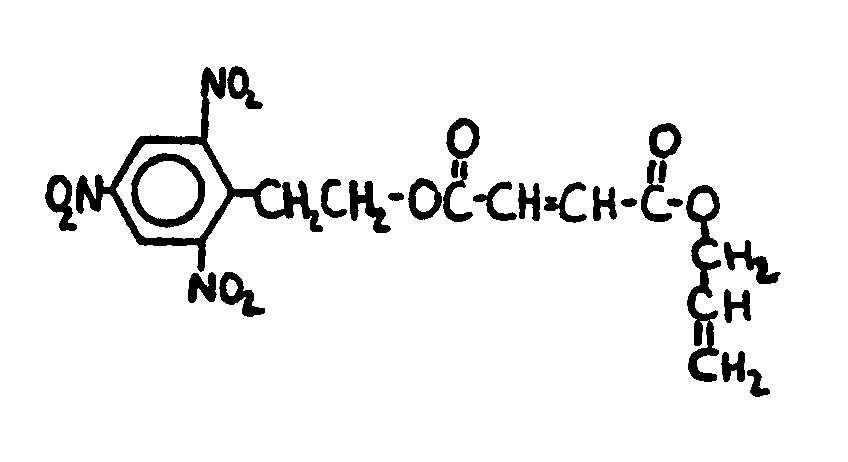
![[List of Patents for class 560 subclass 129]](../ps.gif) 129 129 | Acyclic acid moiety: | ||||||
This subclass is indented under subclass 1. Compounds wherein the acid radical contains no carbocyclic
nucleus.
| |||||||
![[List of Patents for class 560 subclass 130]](../ps.gif) 130 130 | Esterified phenolic hydroxy: | ||
This subclass is indented under subclass 129. Compounds wherein the carboxyl group is esterified by a
phenolic hydroxyl group.
| |||
![[List of Patents for class 560 subclass 131]](../ps.gif) 131 131 | Preparing esters by oxidation: |
| This subclass is indented under subclass 130. Processes whereby phenolic esters are produced by the reaction of a starting material with oxygen or an oxygen producing material. | |
![[List of Patents for class 560 subclass 132]](../ps.gif) 132 132 | Carbamic acid: | ||
This subclass is indented under subclass 130. Compounds wherein the acid radical contains nitrogen directly
attached to the carbon of an esterified carboxyl group.
| |||
![[List of Patents for class 560 subclass 133]](../ps.gif) 133 133 | Plural rings in phenolic moiety: | ||
This subclass is indented under subclass 132. Compounds wherein the phenolic moiety contains more than
one carbocyclic group.
| |||
![[List of Patents for class 560 subclass 134]](../ps.gif) 134 134 | Ortho fused: | ||
This subclass is indented under subclass 133. Compounds wherein the carbocyclic groups are joined through
two ortho positioned carbon atoms.
| |||
![[List of Patents for class 560 subclass 135]](../ps.gif) 135 135 | Sulfur in phenolic moiety: | ||
This subclass is indented under subclass 132. Compounds wherein the phenolic moiety contains sulfur covalently
bonded.
| |||
![[List of Patents for class 560 subclass 136]](../ps.gif) 136 136 | Nitrogen in phenolic moiety: | ||
This subclass is indented under subclass 132. Compounds wherein the phenolic moiety contains nitrogen
covalently bonded.
| |||
![[List of Patents for class 560 subclass 137]](../ps.gif) 137 137 | Sulfur, halogen or additional nitrogen or oxygen in carbamic acid moiety: | ||
This subclass is indented under subclass 132. Compounds wherein the acid radical contains sulfur, halogen
or nitrogen or oxygen in addition to that present in the carbamic
acid group.
| |||
![[List of Patents for class 560 subclass 138]](../ps.gif) 138 138 | Plural rings in phenolic moiety: | ||||
This subclass is indented under subclass 130. Compounds wherein the phenolic moiety contains more than
one carbocyclic group.
| |||||
![[List of Patents for class 560 subclass 139]](../ps.gif) 139 139 | Ortho fused: | ||
This subclass is indented under subclass 138. Compounds wherein the carbocyclic groups of the phenolic
moiety are joined through two ortho positioned carbon atoms.
| |||
![[List of Patents for class 560 subclass 140]](../ps.gif) 140 140 | Plural rings bonded directly to the same carbon in phenolic moiety: | ||
This subclass is indented under subclass 138. Compounds wherein the phenolic moiety contains two carbocyclic
groups attached to a methylene or carbonyl group.
| |||
![[List of Patents for class 560 subclass 141]](../ps.gif) 141 141 | Rings bonded directly to each other in phenolic moiety: | ||
This subclass is indented under subclass 138. Compounds wherein the phenolic moiety contains two carbocyclic
groups joined to each other through a covalent bond.
| |||
![[List of Patents for class 560 subclass 142]](../ps.gif) 142 142 | Nitrogen or sulfur in phenolic moiety: | ||
This subclass is indented under subclass 130. Compounds wherein the phenolic moiety contains sulfur or
nitrogen covalently bonded.
| |||
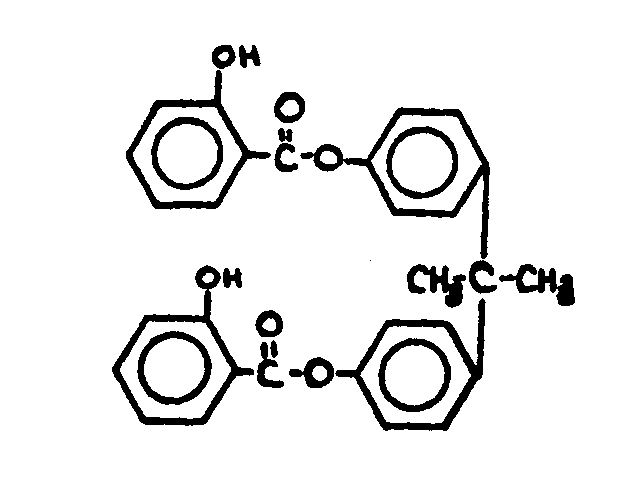
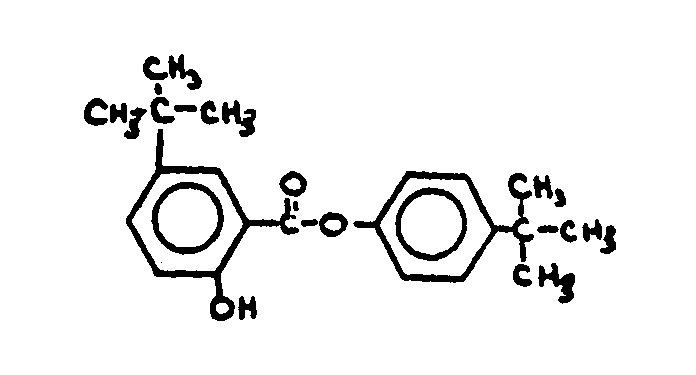
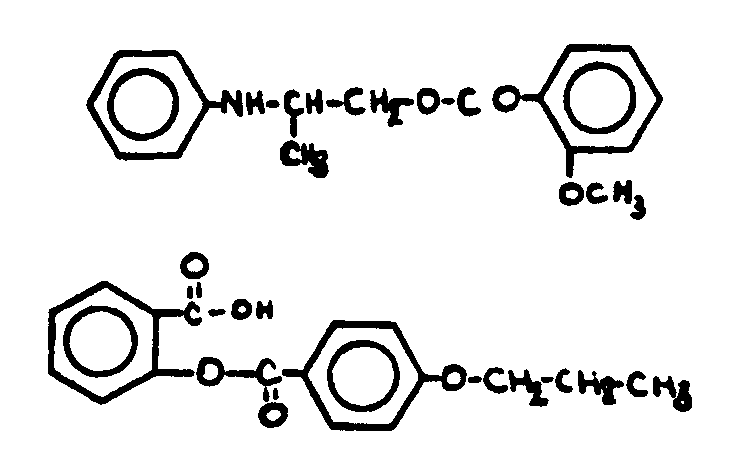
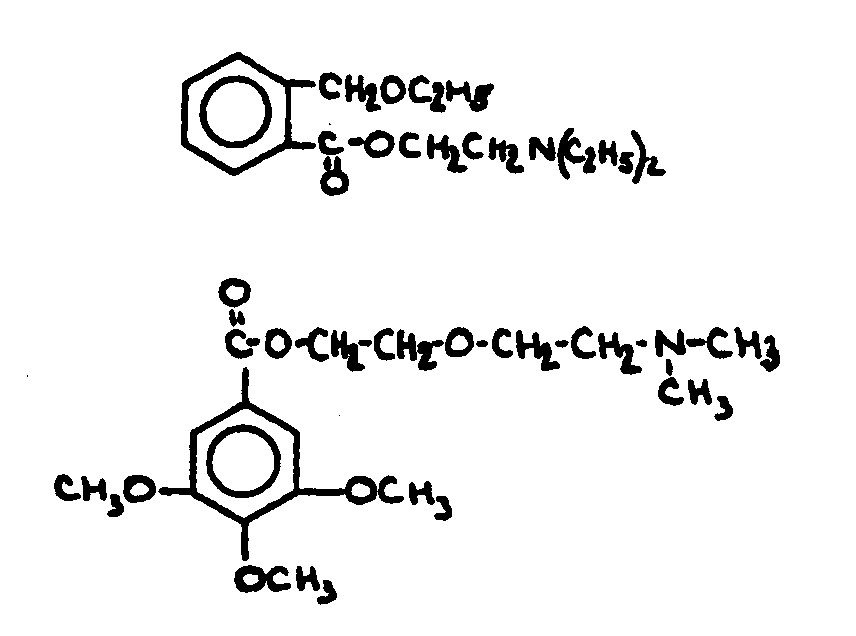
![[List of Patents for class 560 subclass 143]](../ps.gif) 143 143 | Salicylic acid or functional derivative: | ||||||||
This subclass is indented under subclass 130. Compounds wherein the phenolic moiety is salicyclic acid,
its salts, acid halides or anhydrides not provided for above.
| |||||||||
![[List of Patents for class 560 subclass 144]](../ps.gif) 144 144 | Polyoxy phenolic moiety: | ||
This subclass is indented under subclass 130. Compounds wherein the phenolic moiety contains, in addition
to the esterified hydroxyl group, an -OX group attached to a noncarbonylic
carbon, where X is C, H, an alcoholate forming group not provided
for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 145]](../ps.gif) 145 145 | Sulfur, nitrogen, halogen, oxy, or aldehyde or ketone group in acid moiety: | ||
This subclass is indented under subclass 130. Compounds wherein the acid radical contains sulfur, nitrogen
or halogen covalently bonded; or an -OX group attached to noncarbonylic
carbon, where X is C, H, an alcoholate forming group not provided
for above, or an acyl group not provided for above; or a -C(=O)X
group bonded to carbon, where X is C or H.
| |||
![[List of Patents for class 560 subclass 146]](../ps.gif) 146 146 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 130. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 147]](../ps.gif) 147 147 | Sulfur in acid moiety: | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains sulfur covalently
bonded.
| |||
![[List of Patents for class 560 subclass 148]](../ps.gif) 148 148 | Carbamic acid: | ||
This subclass is indented under subclass 147. Compounds wherein the acid radical contains nitrogen directly
attached to the carbon of an esterified carboxyl group.
| |||
![[List of Patents for class 560 subclass 149]](../ps.gif) 149 149 | Sulfoxy in acid moiety: | ||||
This subclass is indented under subclass 147. Compounds wherein the acid radical contains sulfur bonded
to oxygen.
| |||||
![[List of Patents for class 560 subclass 150]](../ps.gif) 150 150 | Sulfonyl or sulfinyl in acid moiety: | ||
This subclass is indented under subclass 149. Compounds wherein the acid radical contains the sulfinyl
group, R-S(=O)-R or the sulfonyl group, R-S(=O)2R.
| |||
![[List of Patents for class 560 subclass 151]](../ps.gif) 151 151 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 149. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
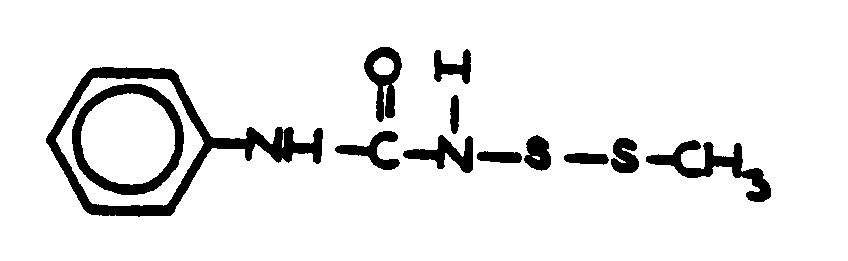
![[List of Patents for class 560 subclass 152]](../ps.gif) 152 152 | Thio ether in acid moiety: | ||
This subclass is indented under subclass 147. Compounds wherein the acid radical contains the group, R-S-R.
| |||
![[List of Patents for class 560 subclass 153]](../ps.gif) 153 153 | Nitrogen or halogen in acid moiety: | ||
This subclass is indented under subclass 152. Compounds wherein the acid radical contains nitrogen or
halogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 154]](../ps.gif) 154 154 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 152. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 155]](../ps.gif) 155 155 | Nitrogen in acid moiety other than as nitroso or isocyanate (e.g., amino acid esters, etc.): | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains covalently bonded
nitrogen other than in the form of isocyanate or nitroso groups.
| |||
![[List of Patents for class 560 subclass 156]](../ps.gif) 156 156 | Nitro bonded to carbon in acid moiety: | ||
This subclass is indented under subclass 155. Compounds wherein the acid radical contains the group -N(=O)2 bonded
to carbon.
| |||
![[List of Patents for class 560 subclass 157]](../ps.gif) 157 157 | Carbamic acid: | ||
This subclass is indented under subclass 155. Compounds wherein the acid radical contains a nitrogen directly
attached to the carbon of an esterified carboxyl group.
| |||
![[List of Patents for class 560 subclass 158]](../ps.gif) 158 158 | Polycarbamic: | ||
This subclass is indented under subclass 157. Compounds wherein the acid radical contains more than one
carbamic acid group.
| |||
![[List of Patents for class 560 subclass 159]](../ps.gif) 159 159 | Addition nitrogen in acid moiety: | ||||||
This subclass is indented under subclass 157. Compounds wherein the acid radical contains covalently bonded
nitrogen in addition to that present in the carbamic acid group.
| |||||||
![[List of Patents for class 560 subclass 160]](../ps.gif) 160 160 | Oxy in acid moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the acid radical contains an -OX group
attached to a noncarbonylic carbon, where X=C, H, an alcoholate
forming group not provided for above.
| |||
![[List of Patents for class 560 subclass 161]](../ps.gif) 161 161 | Halogen in acid moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the acid radical contains covalently bonded
halogen.
| |||
![[List of Patents for class 560 subclass 162]](../ps.gif) 162 162 | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the alcohol moiety contains a carbocyclic
group.
| |||
![[List of Patents for class 560 subclass 163]](../ps.gif) 163 163 | Aromatic alcohol moiety: | ||
This subclass is indented under subclass 162. Compounds wherein the alcohol moiety of the ester contains
an aromatic group.
| |||
![[List of Patents for class 560 subclass 164]](../ps.gif) 164 164 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 163. Compounds wherein the alcohol moiety of the ester contains
in addition to the esterified OH group, at least one other -OX group
attached to a noncarbonylic carbon, where X may be C, H, an alcoholate
forming group not provided for above, or an acyl group not provided
for above.
| |||
![[List of Patents for class 560 subclass 165]](../ps.gif) 165 165 | Sulfur or nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the alcohol moiety of the ester contains
covalently bonded nitrogen or sulfur.
| |||
![[List of Patents for class 560 subclass 166]](../ps.gif) 166 166 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the alcohol moiety is acyclic and in addition
to the esterified OH group contains another -OX attached to a noncarbonylic
carbon and X may be C, H, an alcoholate forming group not provided
for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 167]](../ps.gif) 167 167 | Halogen in alcohol moiety: | ||
This subclass is indented under subclass 157. Compounds wherein the alcohol moiety of the ester contains
covalently bonded halogen.
| |||
![[List of Patents for class 560 subclass 168]](../ps.gif) 168 168 | Amidine, azomethine, ketimine or oxime in acid moiety: | ||
This subclass is indented under subclass 155. Compounds in which the acid radical contains the group =N-C=N-
or the C=N-, identical with the structure obtained by reacting
an aldehyde or ketone with ammonia or an amine.
| |||
![[List of Patents for class 560 subclass 169]](../ps.gif) 169 169 | Additional nitrogen in acid moiety: | ||||||
This subclass is indented under subclass 155. Compounds in which the acid radical contains more than one
covalently bonded nitrogen.
| |||||||
![[List of Patents for class 560 subclass 170]](../ps.gif) 170 170 | Oxy, aldehyde or ketone group in acid moiety: | ||
This subclass is indented under subclass 155. Compounds wherein the acid radical contains the group -C(=O)X
bonded to carbon, where X is C or H; or the group -OX attached to
a noncarbonylic carbon, where X is C, H, and alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 171]](../ps.gif) 171 171 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 155. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 172]](../ps.gif) 172 172 | Halogen or unsaturation in acid moiety: | ||
This subclass is indented under subclass 155. Compounds wherein the acid radical contains covalently bonded
halogen or contains an ethylenic double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 173]](../ps.gif) 173 173 | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 155. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic group.
| |||
![[List of Patents for class 560 subclass 174]](../ps.gif) 174 174 | Aldehyde or ketone group in acid moiety: | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains the group -C(=O)X
attached to carbon, where X is C or H.
| |||
![[List of Patents for class 560 subclass 175]](../ps.gif) 175 175 | Preparing esters by carbonylation: |
| This subclass is indented under subclass 174. Processes wherein the ester is prepared through formation of a carboxyl group of a starting material by reaction with a carbonylating agent such as carbon monoxide either in the presence of an alcohol or by subsequent esterification of the formed acid. | |
![[List of Patents for class 560 subclass 176]](../ps.gif) 176 176 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 174. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 177]](../ps.gif) 177 177 | Aldehyde group in acid moiety: | ||
This subclass is indented under subclass 174. Compounds wherein the acid radical contains the aldehyde
group-C(=O)H.
| |||
![[List of Patents for class 560 subclass 178]](../ps.gif) 178 178 | Acetoacetic acid: | ||||
This subclass is indented under subclass 174. Compounds which are esters of acetoacetic acid, per se.
| |||||
![[List of Patents for class 560 subclass 179]](../ps.gif) 179 179 | Oxy in acid moiety: | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains the -OX group
attached to a noncarbonylic carbon, where X may be C, H, an alcoholate
forming group not provided for above, or an acyl group not provided
for above.
| |||
![[List of Patents for class 560 subclass 180]](../ps.gif) 180 180 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 179. Compounds wherein the acid radical contains more than one
carboxyl group, at least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 181]](../ps.gif) 181 181 | Unsaturation in acid moiety: | ||
This subclass is indented under subclass 180. Compounds wherein the acid radical contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 182]](../ps.gif) 182 182 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 180. Compounds wherein the alcohol moiety of the ester contains
in addition to the esterified OH group, an -OX group attached to
a noncarbonylic carbon, wherein X may be C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 183]](../ps.gif) 183 183 | Unsaturation in acid moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the acid radical contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 184]](../ps.gif) 184 184 | Halogen in acid moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the acid radical contains covalently bonded
halogen.
| |||
![[List of Patents for class 560 subclass 185]](../ps.gif) 185 185 | Acylated oxy in acid moiety: | ||||
This subclass is indented under subclass 179. Compounds wherein a hydroxy group of the esterified acid
radical has been esterified by an acyl group not provided for above.
| |||||
![[List of Patents for class 560 subclass 186]](../ps.gif) 186 186 | Polyoxy acid moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the acid radical contains more than one
oxy group.
| |||
![[List of Patents for class 560 subclass 187]](../ps.gif) 187 187 | Alkoxy in acid moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the X of the -OX group in the acid radical
is C which is part of an alkyl group.
| |||
![[List of Patents for class 560 subclass 188]](../ps.gif) 188 188 | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic group.
| |||
![[List of Patents for class 560 subclass 189]](../ps.gif) 189 189 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 179. Compounds wherein the alcohol moiety of the ester contains,
in addition to the esterified OH, another -OX group attached to
a noncarbonylic C, where X may be C, H, an alcoholate forming group
not provided for above, or an acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 190]](../ps.gif) 190 190 | Polycarboxylic acid: | ||||
This subclass is indented under subclass 129. Compounds which contain more than one carboxyl group, at
least one of which is esterified.
| |||||
![[List of Patents for class 560 subclass 191]](../ps.gif) 191 191 | Purification or recovery: |
| This subclass is indented under subclass 190. Processes which are directed to the purification, separation or recovery of acyclic polycarboxylic acid esters. | |
![[List of Patents for class 560 subclass 192]](../ps.gif) 192 192 | Halogen in acid moiety: | ||||
This subclass is indented under subclass 190. Compounds wherein the acid radical contains halogen covalently
bonded.
| |||||
![[List of Patents for class 560 subclass 193]](../ps.gif) 193 193 | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 190. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic group not provided for above.
| |||
![[List of Patents for class 560 subclass 194]](../ps.gif) 194 194 | Plural rings in alcohol moiety: | ||
This subclass is indented under subclass 193. Compounds wherein the alcohol moiety of the ester contains
more than one carbocyclic group.
| |||
![[List of Patents for class 560 subclass 195]](../ps.gif) 195 195 | Phosphorus or sulfur in alcohol moiety: | ||
This subclass is indented under subclass 190. Compounds wherein the alcohol moiety of the ester contains
sulfur or phosphorus covalently bonded.
| |||
![[List of Patents for class 560 subclass 196]](../ps.gif) 196 196 | Nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 190. Compounds wherein the alcohol moiety of the ester contains
nitrogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 197]](../ps.gif) 197 197 | Halogen in alcohol moiety: | ||
This subclass is indented under subclass 190. Compounds wherein the alcohol moiety of the ester contains
halogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 198]](../ps.gif) 198 198 | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 190. Compounds wherein the alcohol moiety of the ester contains,
in addition to the esterified OH, an -OX attached to a noncarbonylic
C, where X may be C, H, and alcoholate forming group not provided
for above, or an acyl group not provided for above.
| |||
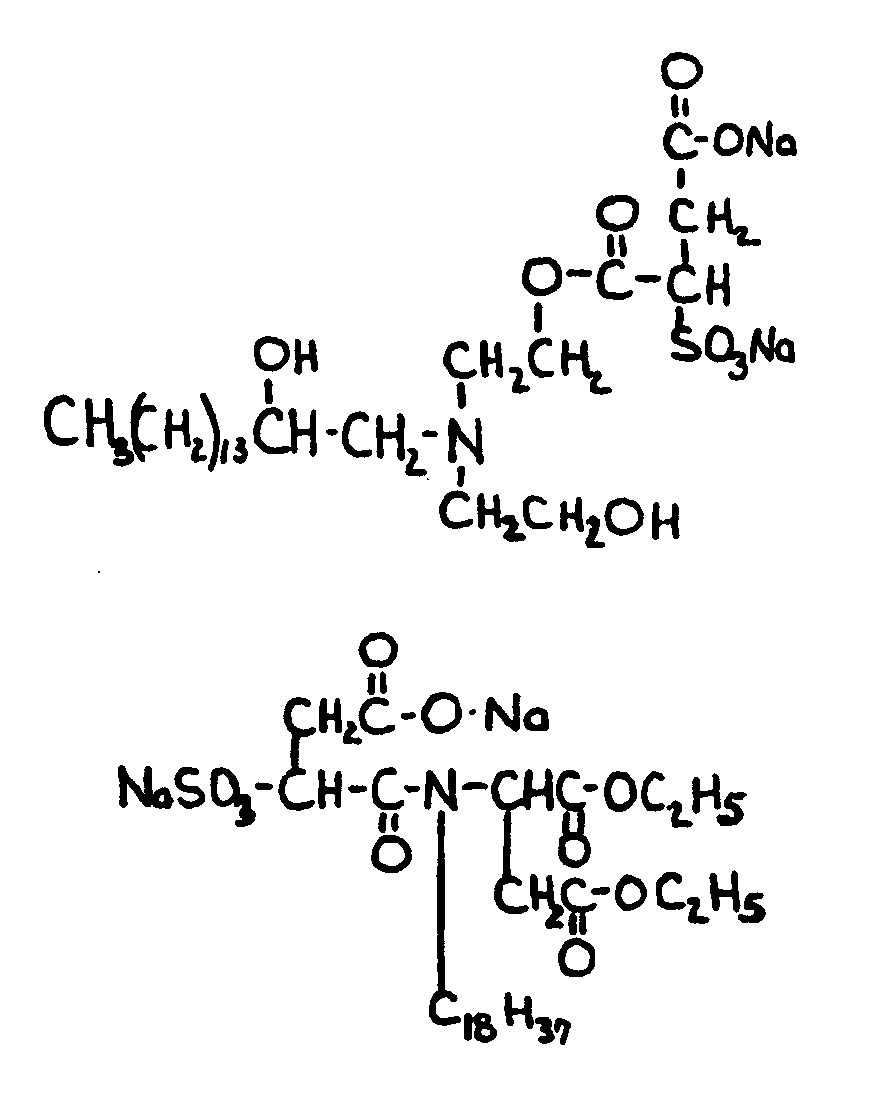
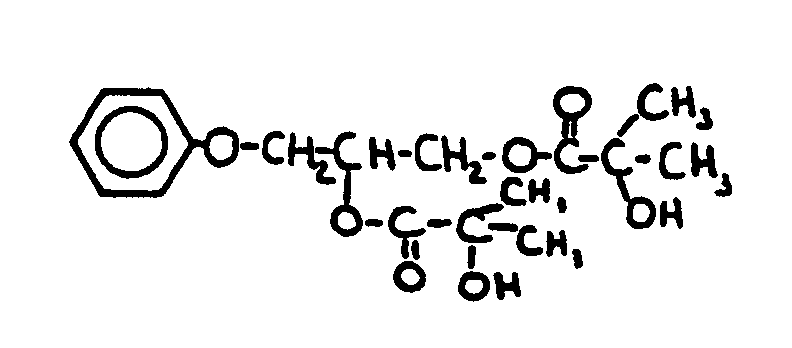
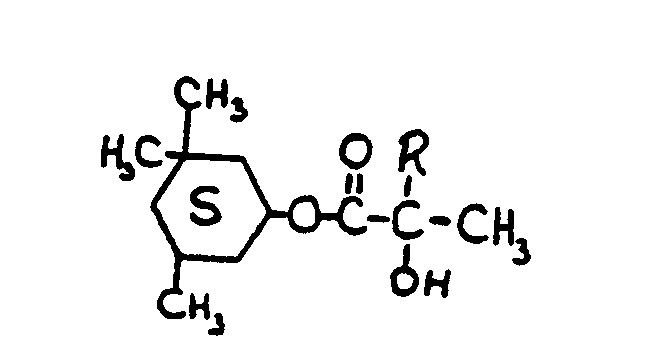
![[List of Patents for class 560 subclass 199]](../ps.gif) 199 199 | Additional monooxy alcohol or monocarboxylic acid (e.g., complex esters, etc.): | ||||||||||
This subclass is indented under subclass 198. Compounds in which an additional monohydric alcohol or monocarboxylic
acid or both have been reacted.
| |||||||||||
![[List of Patents for class 560 subclass 200]](../ps.gif) 200 200 | Preparing esters from alkylene oxides: |
| This subclass is indented under subclass 198. Processes in which an acyclic polycarboxylic acid ester is produced by reaction with an alkylene oxide. | |
![[List of Patents for class 560 subclass 201.]](../ps.gif) 201. 201. | Unsaturation in alcohol moiety: | ||||
This subclass is indented under subclass 190. Compounds wherein the alcoholic moiety of the ester contains
an ethylenic double bond or a triple bond.
SEE OR SEARCH THIS CLASS, SUBCLASS:
| |||||
![[List of Patents for class 560 subclass 202.]](../ps.gif) 202. 202. | Preparing esters for oligomerization: | ||
This subclass is indented under subclass 190. Processes in which a polycarboxylic acid ester is prepared
by interacting two to four monomeric units.
| |||
![[List of Patents for class 560 subclass 203.]](../ps.gif) 203. 203. | Preparing esters by alkylation or isomerization: |
| This subclass is indented under subclass 190. Processes in which a polycarboxylic acid ester is produced (a) by introducing an alkyl or alkylidene radical on the acid moiety, or (b) by forming an isomer of the starting material. | |
![[List of Patents for class 560 subclass 204.]](../ps.gif) 204. 204. | Preparing esters by esterification or cabonylation: |
| This subclass is indented under subclass 190. Processes in which a polycarboxlyic acid ester is produced (a) by a reaction which forms an ester linkage, or (b) by formation of a carboxyl group on a starting material by reaction with a carbonylating agent such as carbon monoxide with simultaneous or subsequent esterification of the acid with an alcohol. | |
![[List of Patents for class 560 subclass 205.]](../ps.gif) 205. 205. | Unsaturation in acid moiety: | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 206.]](../ps.gif) 206. 206. | Preparing esters for carbonylation: |
| This subclass is indented under subclass 205. Processes wherein an unsaturated acid ester is prepared through formation of a carboxyl group on a starting material by reaction with carbonylating agent such as carbon monoxide in the presence of an alcohol or by subsequent esterification of the formed acid. | |
![[List of Patents for class 560 subclass 207.]](../ps.gif) 207. 207. | Group VIII noble metal catalyst utilized: |
| This subclass is indented under subclass 206. Processes wherein the carbonylating is carried out in the presence of a catalyst which contains a noble metal from Group VIII, i.e., iridium, osmium, palladium, platinum, rhodium, or rubidium. | |
![[List of Patents for class 560 subclass 208.]](../ps.gif) 208. 208. | Formation of carboxyl group by oxidation: |
| This subclass is indented under subclass 205. Processes wherein the ester is produced by treatment of a starting material, such as an olefin or an aldehyde with oxygen or an oxygen producing material and esterifying the resulting acid. | |
![[List of Patents for class 560 subclass 209.]](../ps.gif) 209. 209. | Preparing esters from alkylene oxides: |
| This subclass is indented under subclass 205. Processes wherein the ester is produced by a reaction, where one of the reactants is an alkylene oxide. | |
![[List of Patents for class 560 subclass 210.]](../ps.gif) 210. 210. | Preparing esters from aldehydes: | ||||
This subclass is indented under subclass 205. Processes wherein the ester is prepared from an aldehyde.
SEE OR SEARCH THIS CLASS, SUBCLASS:
| |||||
![[List of Patents for class 560 subclass 211.]](../ps.gif) 211. 211. | Formation of ethylenic unsaturation: |
| This subclass is indented under subclass 205. Processes wherein the ester is produced by treating a monocarboxylic acid or ester to introduce unsaturation and may include subsequent esterification. | |
![[List of Patents for class 560 subclass 212.]](../ps.gif) 212. 212. | By dehydration or dealcoholization: |
| This subclass is indented under subclass 211. Processes wherein unsaturation is introduced by removal of an –OX group, where X is H or C. | |
![[List of Patents for class 560 subclass 213.]](../ps.gif) 213. 213. | By dehalogenation or dehydrohalogenation: |
| This subclass is indented under subclass 211. Processes wherein unsaturation is introduced by removal of a halogen or a hydrohalide. | |
![[List of Patents for class 560 subclass 214.]](../ps.gif) 214. 214. | By dehydrogenation: |
| This subclass is indented under subclass 211. Processes wherein unsaturation is introduced by removal of hydrogen. | |
![[List of Patents for class 560 subclass 215.]](../ps.gif) 215. 215. | Preparing esters from nitriles or amides: |
| This subclass is indented under subclass 205. Processes wherein the ester is produced by reaction of a nitrile or an amide. | |
![[List of Patents for class 560 subclass 216.]](../ps.gif) 216. 216. | Preparing esters by depolymerization: |
| This subclass is indented under subclass 205. Processes wherein the ester is produced by changing the state of a polymeric material to that of a lower polymeric form or to a monomer. | |
![[List of Patents for class 560 subclass 217.]](../ps.gif) 217. 217. | Preparing esters by ester interchange: | ||
This subclass is indented under subclass 205. Processes wherein the ester is produced by reaction of an
ester with an alcohol, an acid or an ester to produce a
different ester.
| |||
![[List of Patents for class 560 subclass 218.]](../ps.gif) 218. 218. | Purification or recovery: |
| This subclass is indented under subclass 205. Processes which are directed to the purification, recovery or separation of acyclic, unsaturated monocarboxylic acid esters. | |
![[List of Patents for class 560 subclass 219.]](../ps.gif) 219. 219. | Halogen in acid moiety: | ||
This subclass is indented under subclass 205. Compounds wherein the acid radical contains covalently bonded
halogen.
| |||
![[List of Patents for class 560 subclass 220.]](../ps.gif) 220. 220. | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 205. Compounds wherein the alcohol moiety of the ester contains
a carbocyclic group.
| |||
![[List of Patents for class 560 subclass 221.]](../ps.gif) 221. 221. | Aromatic alcohol moiety: | ||
This subclass is indented under subclass 220. Compounds wherein the carbocyclic group of the alcohol moiety
is aromatic.
| |||
![[List of Patents for class 560 subclass 222.]](../ps.gif) 222. 222. | Phosphorus, sulfur or nitrogen in alcohol moiety: | ||
This subclass is indented under subclass 205. Compound wherein the alcohol moiety contains phosphorus
or sulfur or nitrogen covalently bonded.
| |||
![[List of Patents for class 560 subclass 223.]](../ps.gif) 223. 223. | Halogen in alcohol moiety: | ||
This subclass is indented under subclass 205. Compounds wherein the alcohol moiety contains covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 224.]](../ps.gif) 224. 224. | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 205. Compounds wherein the alcohol moiety contains in addition
to the esterified hydroxyl group, an –OX group
attached to a noncarbonylic carbon, where X may be C, H, an
alcoholate forming group not provided for above, or an
acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 225.]](../ps.gif) 225. 225. | Unsaturation in alcohol moiety: | ||
This subclass is indented under subclass 205. Compounds wherein the alcohol moiety contains an ethylenic
double bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 226.]](../ps.gif) 226. 226. | Halogen in acid moiety: | ||
This subclass is indented under subclass 129. Compounds wherein the acid radical contains covalently bonded
halogen.
| |||
![[List of Patents for class 560 subclass 227.]](../ps.gif) 227. 227. | Flourine in acid moiety: | ||
This subclass is indented under subclass 226. Compounds wherein the halogen is flourine.
| |||
![[List of Patents for class 560 subclass 228.]](../ps.gif) 228. 228. | Cyclic alcohol moiety: | ||
This subclass is indented under subclass 226. Compounds wherein the alcohol moiety contains a carbocyclic
group.
| |||
![[List of Patents for class 560 subclass 229.]](../ps.gif) 229. 229. | Halogen in alcohol moiety: | ||
This subclass is indented under subclass 226. Compounds wherein the alcohol moiety contains covalently
bonded halogen.
| |||
![[List of Patents for class 560 subclass 230.]](../ps.gif) 230. 230. | Polyoxyl alcohol moiety: | ||
This subclass is indented under subclass 226. Compounds wherein the alcohol moiety contains. in
addition to the esterified hydroxyl, an –OX group
attached to noncarbonylic carbon, where X is C, H, an
alcoholate forming group not provided for above, or an
acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 231.]](../ps.gif) 231. 231. | Unsubstituted acids of the acetic series: | ||||
This subclass is indented under subclass 129. Compounds wherein the acid radical is saturated, unsubstituted, contains
less than seven carbon atoms in an unbroken chain attached to a
carboxyl group, and has the formula CnH2n+1COOR.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 232.]](../ps.gif) 232. 232. | Preparing esters by carbonylation: |
| This subclass is indented under subclass 231. Processes wherein the ester is prepared through formation of a carboxyl group on a starting material by reaction with a carbonylating agent such as carbon monoxide in the presence of an alcohol or by subsequent esterfication. | |
![[List of Patents for class 560 subclass 233.]](../ps.gif) 233. 233. | Of olefins: |
| This subclass is indented under subclass 232. Processes wherein the starting material is an olefin. | |
![[List of Patents for class 560 subclass 234.]](../ps.gif) 234. 234. | Preparing esters by ester interchange: | ||
This subclass is indented under subclass 231. Processes wherein the ester is produced by reacting an ester
with an alcohol, an acid or another ester to produce a
different ester.
| |||
![[List of Patents for class 560 subclass 235.]](../ps.gif) 235. 235. | From alkyl sulfates: | ||
This subclass is indented under subclass 234. Processes wherein an alkyl sulfate is used as the source
of another alcohol.
| |||
![[List of Patents for class 560 subclass 236.]](../ps.gif) 236. 236. | Preparing esters from halogenated hydrocarbons: | ||
This subclass is indented under subclass 231. Processes wherein the ester is prepared from a halogenated
hydrocarbon.
| |||
![[List of Patents for class 560 subclass 237.]](../ps.gif) 237. 237. | From alkenyl halides: | ||
This subclass is indented under subclass 236. Processes wherein the halogenated hydrocarbon is an alkenyl
halide, e.g., vinyl chloride, etc.
| |||
![[List of Patents for class 560 subclass 238.]](../ps.gif) 238. 238. | Preparing esters from aldehydes: | ||
This subclass is indented under subclass 231. Processes wherein the ester is prepared from an aldehyde.
| |||
![[List of Patents for class 560 subclass 239.]](../ps.gif) 239. 239. | Preparing esters by dehydrogenation of alcohols: | ||
This subclass is indented under subclass 231. Processes wherein the ester is prepared by dehydrogenation
of an alcohol.
| |||
![[List of Patents for class 560 subclass 240.]](../ps.gif) 240. 240. | Preparing esters from ethers: | ||
This subclass is indented under subclass 231. Processes wherein the ester is prepared from an ether,
including cyclic ethers.
| |||
![[List of Patents for class 560 subclass 241.]](../ps.gif) 241. 241. | Preparing esters from hydrocarbons: |
| This subclass is indented under subclass 231. Processes wherein the ester is prepared from a hydrocarbon. | |
![[List of Patents for class 560 subclass 241.1]](../ps.gif) 241.1 241.1 | By oxidation of hydrocarbon mixtures: |
| This subclass is indented under subclass 241. Processes wherein the ester is prepared by oxidizing a mixture of hydrocarbons. | |
![[List of Patents for class 560 subclass 242.]](../ps.gif) 242. 242. | From acetylenic hydrocarbons: | ||
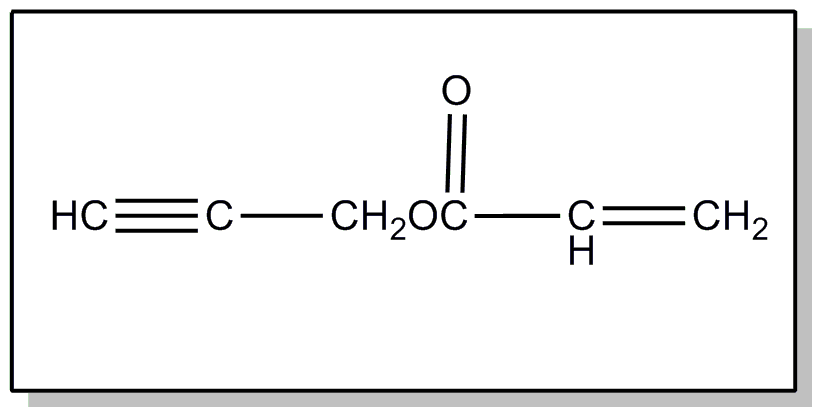
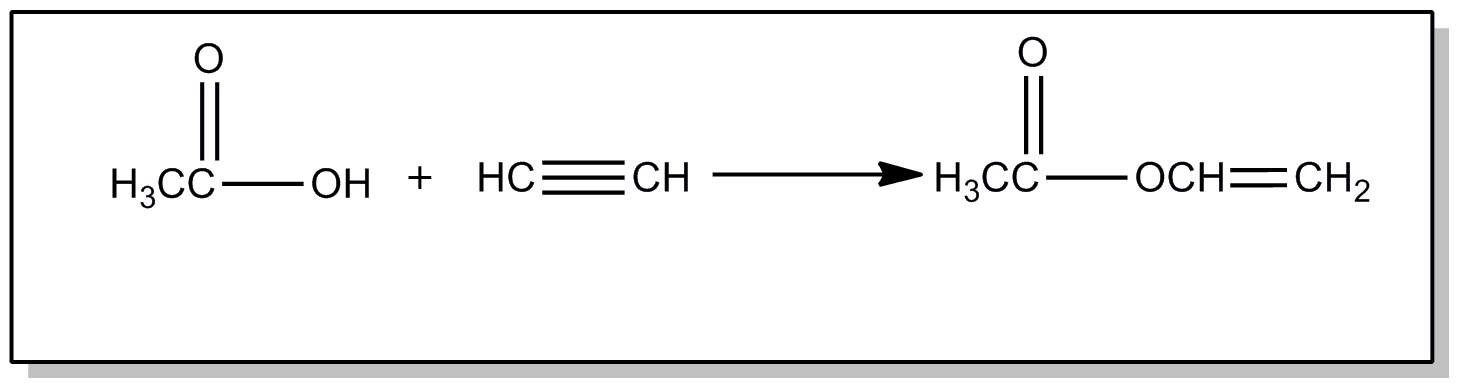
This subclass is indented under subclass 241. Processes wherein the hydrocarbon contains a triple bond.
| |||
![[List of Patents for class 560 subclass 243.]](../ps.gif) 243. 243. | From olefins utilizing Group VIII noble metal catalyst: | ||
This subclass is indented under subclass 241. Processes wherein the hydrocarbon is an olefin and the reaction
is carried out in the presence of a catalyst containing a Group
VIII noble metal.
| |||
![[List of Patents for class 560 subclass 244.]](../ps.gif) 244. 244. | From polyolefins: |
| This subclass is indented under subclass 243. Processes wherein the olefin contains more than one double bond, e.g., butadiene, etc. | |
![[List of Patents for class 560 subclass 245.]](../ps.gif) 245. 245. | Gas phase: |
| This subclass is indented under subclass 243. Processes wherein the reaction is carried out in a gas or vapor phase. | |
![[List of Patents for class 560 subclass 246.]](../ps.gif) 246. 246. | Preparing polyoxy alcohol esters from olefins: |
| This subclass is indented under subclass 241. Processes wherein the ester is prepared from an olefin and is equivalent in structure to an ester prepared from a polyoxy alcohol. | |
![[List of Patents for class 560 subclass 247.]](../ps.gif) 247. 247. | Preparing alkyl esters from olefins: |
| This subclass is indented under subclass 241. Processes wherein the ester is prepared from an olefin and is equivalent in structure to an ester prepared from an acyclic saturated monooxy alcohol. | |
![[List of Patents for class 560 subclass 248.]](../ps.gif) 248. 248. | Purification or recovery: |
| This subclass is indented under subclass 231. Processes which are directed to the purification, separation, or recovery of esters of unsubstituted acids of the acetic acid series. | |
![[List of Patents for class 560 subclass 249.]](../ps.gif) 249. 249. | Terpene alcohol moiety: | ||
This subclass is indented under subclass 231. Processes in which alcohol moiety of the ester is a terpene.
| |||
![[List of Patents for class 560 subclass 250.]](../ps.gif) 250. 250. | Nitrogen is alcohol moiety other than as nitro, nitroso or isocyanate: | ||
This subclass is indented under subclass 231. Compounds in which alcohol moiety contains nitrogen other
than in the form of an isocyanate, nitroso, or
nitro group.
| |||
![[List of Patents for class 560 subclass 251.]](../ps.gif) 251. 251. | Plural nitrogens in alcohol moiety: | ||||
This subclass is indented under subclass 250. Compounds wherein the alcohol moiety contains more than
one nitrogen.
| |||||
![[List of Patents for class 560 subclass 252.]](../ps.gif) 252. 252. | Polyoxy alcohol moiety: | ||
This subclass is indented under subclass 250. Processes in which alcohol moiety contains, in addition
to the esterified OH group, another –OX group
attached to a noncarbonylic C, where X may be C, H, an
alcoholate forming group not provided for above, or an
acyl group not provided for above.
| |||
![[List of Patents for class 560 subclass 253.]](../ps.gif) 253. 253. | Acyclic alcohol moiety: | ||
This subclass is indented under subclass 250. Processes in which alcohol moiety is acyclic.
| |||
![[List of Patents for class 560 subclass 254.]](../ps.gif) 254. 254. | Aromatic alcohol moiety: |
| This subclass is indented under subclass 231. Processes in which alcohol moiety contains a benzene ring
and is not provided for above.
| |
![[List of Patents for class 560 subclass 255]](../ps.gif) 255 255 | Plural rings in alcohol moiety: | ||
This subclass is indented under subclass 254. Compounds wherein the alcohol moiety contains at least one
additional carbocyclic group.
| |||
![[List of Patents for class 560 subclass 256]](../ps.gif) 256 256 | Polycyclo - alicyclic ring system in alcohol moiety: | ||||
This subclass is indented under subclass 231. Compounds wherein the alcohol moiety contains a polyalicyclic
nucleus in which the rings are joined either through two ortho positioned carbons
or through a carbon bridge or both.
SEE OR SEARCH CLASS:
| |||||
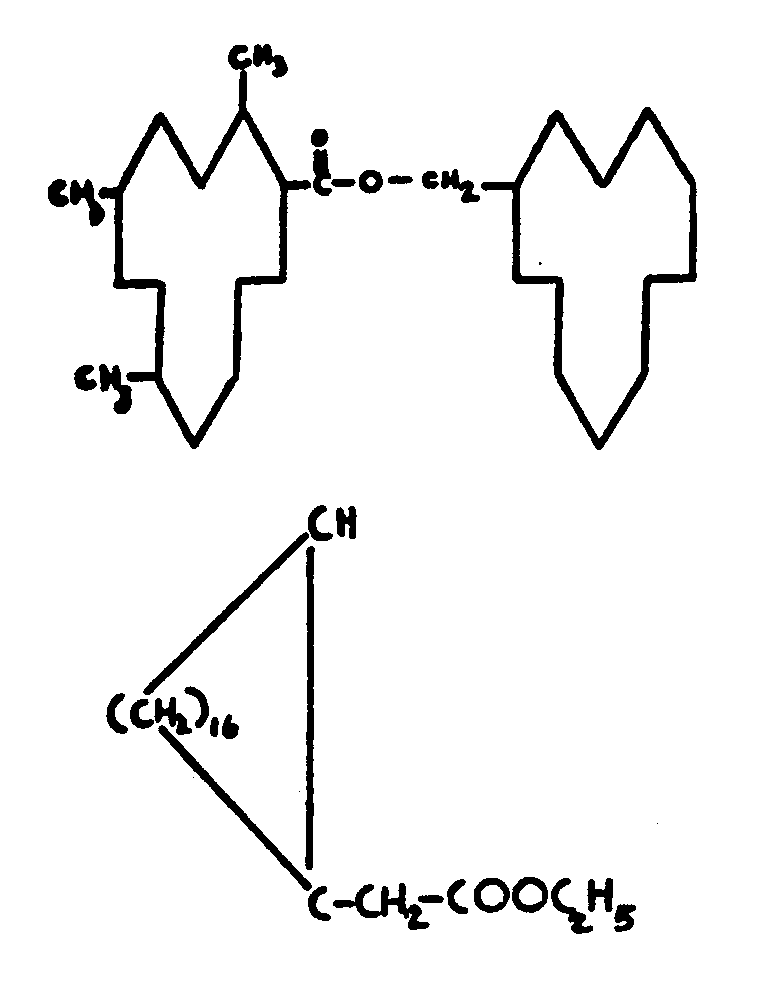
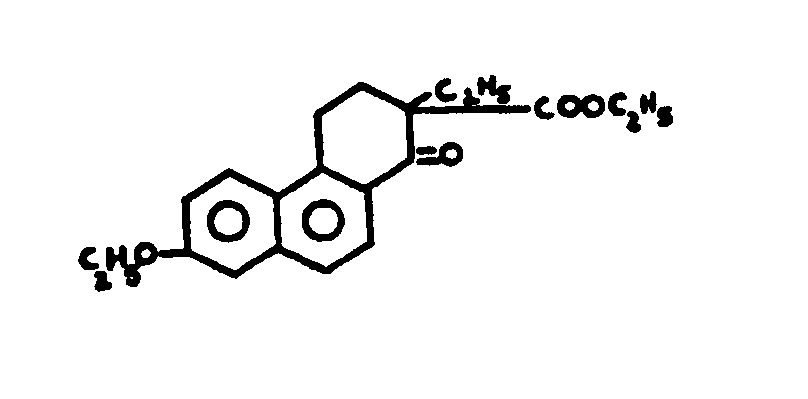
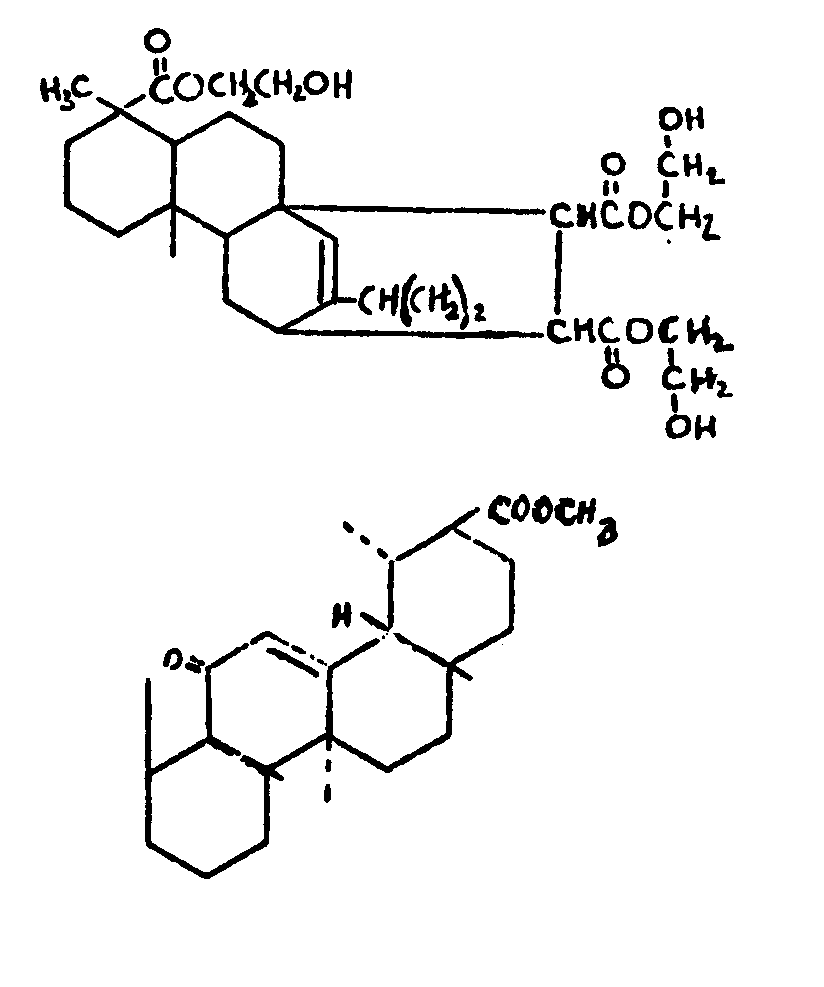
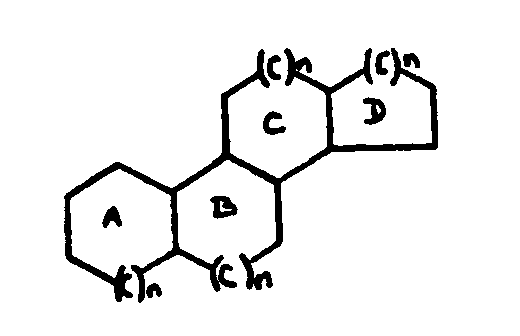
![[List of Patents for class 560 subclass 257]](../ps.gif) 257 257 | Nor- or homo-cyclopentanohydrophenan-threnes: | ||||
| This subclass is indented under subclass 256. Compounds wherein the alcohol moiety contains the structure,
illustrated below, wherein is 0-2, but all n"s may not
be equal to 1 at the same time.
| |||||
![[List of Patents for class 560 subclass 258]](../ps.gif) 258 258 | Nor-a ring: | ||
This subclass is indented under subclass 257. Compounds wherein n=0 in the A ring.
| |||
![[List of Patents for class 560 subclass 259]](../ps.gif) 259 259 | 2, 6, 6-trialkyl cyclohexenyl in alcohol moiety: | ||
This subclass is indented under subclass 231. Compounds wherein the alcohol moiety is charcterized by
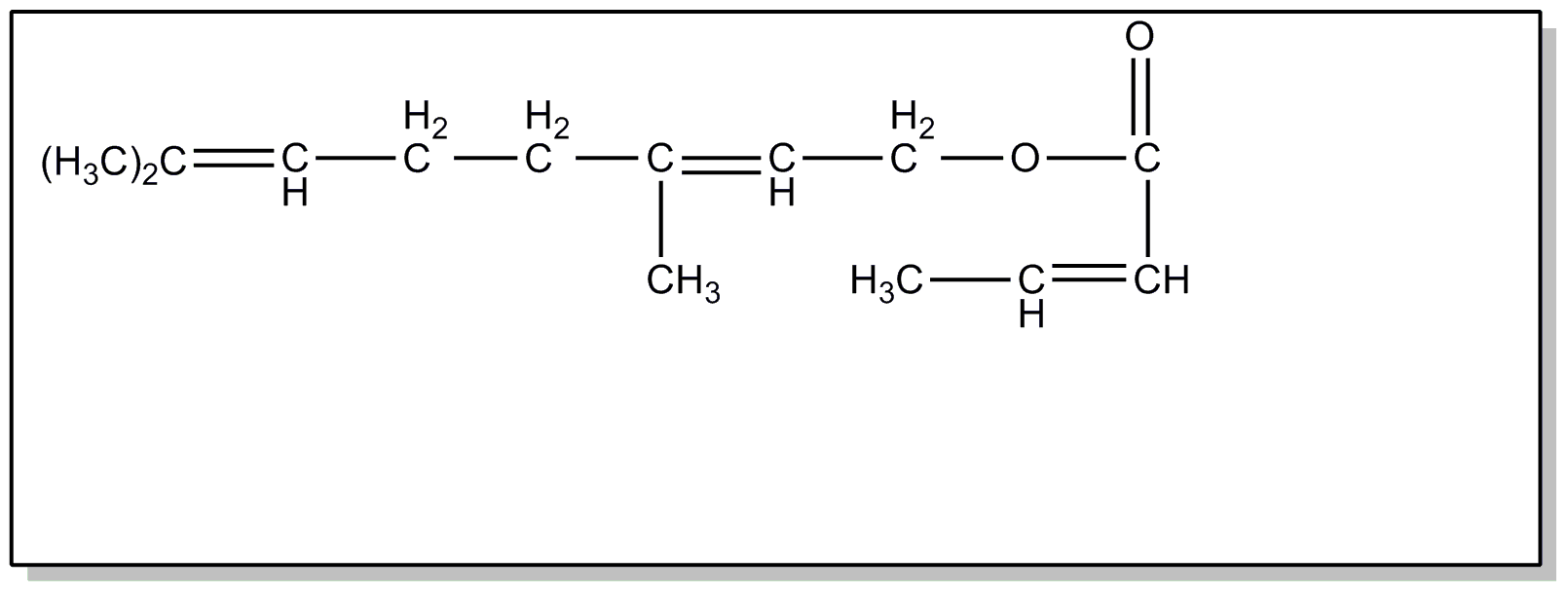
the presence of a 2, 6, 6-trialkyl cyclohexenyl group, e.g., carotenes.
| |||
![[List of Patents for class 560 subclass 260]](../ps.gif) 260 260 | Vitamin a alcohol moiety: | ||
This subclass is indented under subclass 259. Compounds wherein the alcohol moiety is Vitamin A.
| |||
![[List of Patents for class 560 subclass 261]](../ps.gif) 261 261 | Acyclic alcohol moiety having unsaturation: | ||
This subclass is indented under subclass 231. Compounds wherein the alcohol moiety is acyclic and contains
an ethylenic doule bond or a triple bond.
| |||
![[List of Patents for class 560 subclass 262]](../ps.gif) 262 262 | Substituted: | ||
This subclass is indented under subclass 261. Compounds wherein the alcohol moiety contains a substituent
other than a hydrocarbon and is not provided for above.
| |||
![[List of Patents for class 560 subclass 263]](../ps.gif) 263 263 | Acyclic polyoxy alcohol moiety: | ||
This subclass is indented under subclass 231. Compounds wherein the alcohol moiety is acyclic and contains
in addition to the esterified OH group, another -OX group attached
to a noncarbonylic C, where X may be C, H, an alcoholate forming
group not provided for above, or an acyl group not provided for
above.
| |||
![[List of Patents for class 560 subclass 264]](../ps.gif) 264 264 | Substituted: | ||
This subclass is indented under subclass 263. Compounds wherein the alcohol moiety contains a substituent
other than a hydrocarbon and is not provided for above.
| |||
![[List of Patents for class 560 subclass 265]](../ps.gif) 265 265 | Acyclic monohydric alcohol moiety: | ||
This subclass is indented under subclass 231. Compounds wherein the alcohol moiety is acyclic, saturated
and has no oxy group other than the one which is esterified.
| |||
![[List of Patents for class 560 subclass 266]](../ps.gif) 266 266 | Substituted: | ||
This subclass is indented under subclass 265. Compounds wherein the alcohol moiety contains a substituent
other than a hydrocarbon and is not provided for above.
| |||
![[List of Patents for class 560 subclass 300]](../ps.gif) 300 300
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the hypoholite group,
-O-halo, or the perhypohalite group, -O-O-halo, is bonded directly
to carbon, which carbon may be single bonded to any atom but may
be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 301]](../ps.gif) 301 301
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the cyanate group,
-O-C N is bonded directly to carbon, which carbon may be single
bonded to any atom but may be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 302]](../ps.gif) 302 302
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the group -C(=X)-X-nX-,
wherein the X"s may be the same or diverse chalcogens (i.e.,
oxygen, sulfur, selenium or tellurium), nX is a divalent chalcogen
or a chain of divalent chalcogens, and a single bonded X is bonded
directly to carbon, which carbon may be single bonded to any atom
but may be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 303]](../ps.gif) 303 303
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the sulfohydroxamate
group or a chalcogen analogue thereof, -S(=O)(=O-NH-X-wherein
X is chalcogen (i.e., oxygen, sulfur, selenium or tellurium) and
substitution may be made for hydrogen only, and wherein the X is
bonded directly to carbon, which carbon may be single bonded to
any atom but may be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 304]](../ps.gif) 304 304
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the peroxynitrate
group, -O-O-N(=O)(=O), is bonded directly to carbon,
which carbon may be single bonded to any atom but may be multiple
bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 305]](../ps.gif) 305 305
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the -X-X- group,
wherein the X"s are the same or diverse chalcogens (i.e.,
oxygen, sulfur, selenium or tellurium), bonded directly to boron and
to carbon, which carbon may be single bonded to any atom but may
be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 306]](../ps.gif) 306 306
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the perhalate group,
-O-halo(=O)(=O)(=O), is bonded directly
to carbon which carbon may be single bonded to any atom but may
be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 307]](../ps.gif) 307 307
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the -S(=O)(=O)-S-
group wherein the divalent sulfur is bonded directly to carbon,
which carbon may be single bonded to any atom but may be multiple
bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 308]](../ps.gif) 308 308
| |||
This subclass is indented under subclass 307. Compounds wherein the hexavalent sulfur of the -S(=O)(=O)-S-
group is bonded directly to oxygen.
| |||
![[List of Patents for class 560 subclass 309]](../ps.gif) 309 309
| |||
This subclass is indented under subclass 308. Compound wherein the -S(=O)(=O)-S- group is
attached indirectly to nitrogen by nonionic bonding.
| |||
![[List of Patents for class 560 subclass 310]](../ps.gif) 310 310
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the -S-(=O)-S-
group wherein the divalent sulfur is bonnded directly to carbon,
which carbon may be single bonded to any atom but may be multiple
bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 311]](../ps.gif) 311 311
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the perhydroxamate
group or a chalcogen analogue thereof, -C(=X)-NH-X-X-,
wherein the X"s may be the same or diverse chalcogens (i.e., oxygen,
sulfur, selenium or tellurium) and substitution may be made for
hydrogen only, and wherein the single bonded X is bonded directly to
carbon, which carbon may be single bonded to any atom but may be
multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 312]](../ps.gif) 312 312
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... having the hydroxmate group
or a chalcogen analogue thereof, -C(=X)-NH-X-, wherein
the X"s may be the same or diverse chalcogens (i.e., oxygen,
sulfur, selenium or tellurium) and substitution may be made for
hydrogen only, and wherein the single bonded X is bonded directly to
carbon, which carbon may be single bonded to any atom but may be
multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 313]](../ps.gif) 313 313
| |||
This subclass is indented under subclass 312. Compounds wherein the carbon of the -C(=X)-NH-X-
group is bonded directly to nitrogen.
| |||
![[List of Patents for class 560 subclass 314]](../ps.gif) 314 314
| |||
This subclass is indented under subclass 313. Compounds wherein the substituent nitrogen is bonded directly
to acyclic or alicyclic carbon, or wherein the single bonded X is
sulfur.
| |||
![[List of Patents for class 560 subclass 315]](../ps.gif) 315 315
| |||
This subclass is indented under subclass 312. Compounds wherein the carbon of the -C(=X)-NH-X-
group is bonded directly to a carbocyclic ring.
| |||
![[List of Patents for class 560 subclass 316]](../ps.gif) 316 316
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the hyponitrite
group, -O-N=N-O-, is bonded directly to carbon, which carbon
may be single bonded to any atom but may be multiple bonded only
to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 317]](../ps.gif) 317 317
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the -N=S=O
group is bonded directly to carbon, which carbon may be single bonded
to any atom but may be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 318]](../ps.gif) 318 318
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the terminal oxygen
of a -S(=O)(=O)-O-O- group is bonded directly
to carbon, which carbon may be single bonded to any atom but may
be multiple bonded only to carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 319]](../ps.gif) 319 319
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the perthioimidate
group, HN=CH-S-S-, may be single bonded directly to any
atom but may be multiple bonded only to carbon, and substitution
may be made for hydrogen only.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 330]](../ps.gif) 330 330
| |||||
This subclass is indented under subclass 1. Compounds under Class 532, ... wherein the isocyanate group,
-N=C=O, is bonded directly to carbon, which carbon
may be single bonded to any atom but may be multiple bonded only to
carbon.
SEE OR SEARCH CLASS:
| |||||
![[List of Patents for class 560 subclass 331]](../ps.gif) 331 331
| |
| This subclass is indented under subclass 330. Products which contain an isocyanate ester in admixture with a preserving or stabilizing agent whose sole function is to prevent physical or chemical change. | |
![[List of Patents for class 560 subclass 332]](../ps.gif) 332 332
| |
| This subclass is indented under subclass 331. Products wherein the preserving or stabilizing agent contains nitrogen. | |
![[List of Patents for class 560 subclass 333]](../ps.gif) 333 333
| |
| This subclass is indented under subclass 331. Products wherein the preserving or stabilizing agent contains phosphorus, silicon or a phenolic hydroxy group. | |
![[List of Patents for class 560 subclass 334]](../ps.gif) 334 334
| |||
This subclass is indented under subclass 330. Compounds which contain the carbodiimide group, -N=C=N-.
| |||
![[List of Patents for class 560 subclass 335]](../ps.gif) 335 335
| |||
This subclass is indented under subclass 330. Compounds which contain the biuret group, -NH-C(=0)-NH-C(=O)
-NH-, wherein substitution may be made for hydrogen only.
| |||
![[List of Patents for class 560 subclass 336]](../ps.gif) 336 336
| |
| This subclass is indented under subclass 330. Processes which are directed to the preparation, purification, recovery, or treatment in any way of an isocyanate ester. | |
![[List of Patents for class 560 subclass 337]](../ps.gif) 337 337
| |
| This subclass is indented under subclass 336. Processes wherein isocyanic acid, H-N=C=O, or a salt thereof, is employed as a reactant. | |
![[List of Patents for class 560 subclass 338]](../ps.gif) 338 338
| |
| This subclass is indented under subclass 336. Processes which involve the formation of the isocyanate group, -N=C=O. | |
![[List of Patents for class 560 subclass 339]](../ps.gif) 339 339
| |
| This subclass is indented under subclass 338. Process wherein there is utilized a reactant which contains the cyanate group, -O-C=N. | |
![[List of Patents for class 560 subclass 340]](../ps.gif) 340 340
| |
| This subclass is indented under subclass 338. Process wherein there is utilized a reactant which contains a hetero ring. | |
![[List of Patents for class 560 subclass 341]](../ps.gif) 341 341
| |
| This subclass is indented under subclass 338. Processes wherein carbon monoxide is utilized in any way. | |
![[List of Patents for class 560 subclass 342]](../ps.gif) 342 342
| |||
This subclass is indented under subclass 341. Processes wherein there is utilized a reactant which contains
a nitro group bonded directly to carbon.
| |||
![[List of Patents for class 560 subclass 343]](../ps.gif) 343 343
| |
| This subclass is indented under subclass 338. Processes wherein there is utilized a reactant that contains the azide group, -N3. | |
![[List of Patents for class 560 subclass 344]](../ps.gif) 344 344
| |
| This subclass is indented under subclass 338. Processes wherein there is utilized a reactant that contains the -NH-C (=O)-NH- group, wherein substitution may be made for hydrogen only. | |
![[List of Patents for class 560 subclass 345]](../ps.gif) 345 345
| |
| This subclass is indented under subclass 338. Processes wherein there is utilized a reactant that contains the carbamate group, -O-C(=O)-NH, wherein substitution may be made for hydrogen only. | |
![[List of Patents for class 560 subclass 346]](../ps.gif) 346 346
| |
| This subclass is indented under subclass 338. Processes wherein there is utilized a reactant that contains carbon double or triple bonded to nitrogen. | |
![[List of Patents for class 560 subclass 347]](../ps.gif) 347 347
| |||
This subclass is indented under subclass 338. Processes wherein there is utilized a carbonyl dihalide
reactant, X-C(=O)-X, wherein X represents halogen.
| |||
![[List of Patents for class 560 subclass 348]](../ps.gif) 348 348
| |
| This subclass is indented under subclass 338. Processes wherein there is utilized a carbamyl halide reactant, halo-C(=O)-NH-, wherein substitution may be made for hydrogen only. | |
![[List of Patents for class 560 subclass 349]](../ps.gif) 349 349
| |
| This subclass is indented under subclass 336. Processes wherein an isocyanate ester is halogenated. | |
![[List of Patents for class 560 subclass 350]](../ps.gif) 350 350
| |||
This subclass is indented under subclass 336. Processes wherein an isocyanate group on one reactant and
a different functional group on a second reactant undergo an exchange
reaction.
| |||
![[List of Patents for class 560 subclass 351]](../ps.gif) 351 351
| |
| This subclass is indented under subclass 336. Processes wherein an iscoyanate ester of known structure is reacted to yield products of indeterminate structure. | |
![[List of Patents for class 560 subclass 352]](../ps.gif) 352 352
| |
| This subclass is indented under subclass 336. Processes wherein an isocyanate ester is separated from impurities, or from the reaction medium. | |
![[List of Patents for class 560 subclass 353]](../ps.gif) 353 353
| |
| This subclass is indented under subclass 352. Processes wherein a metal or an epoxy compound is utilized. | |
![[List of Patents for class 560 subclass 354]](../ps.gif) 354 354
| |||
This subclass is indented under subclass 330. Compounds which contain a polycyclo ring system having an
alicyclic ring as onne of the cyclos.
| |||
![[List of Patents for class 560 subclass 355]](../ps.gif) 355 355
| |||
This subclass is indented under subclass 330. Compounds wherein the isocyanate group is bonded directly
to an acyclic carbon.
| |||
![[List of Patents for class 560 subclass 356]](../ps.gif) 356 356
| |||
This subclass is indented under subclass 355. Compounds wherein the isocyanate group is attached indirectly
to halogen by acyclic nonionic bonding.
| |||
![[List of Patents for class 560 subclass 357]](../ps.gif) 357 357
| |||
This subclass is indented under subclass 355. Compounds wherein the isocyanate group is attached indirectly
by acyclic nonionic bonding to chalcogen, which is single bonded
directly to carbon.
| |||
![[List of Patents for class 560 subclass 358]](../ps.gif) 358 358
| |||
This subclass is indented under subclass 330. Compounds wherein the isocyanate group is bonded directly
to a benzene ring.
| |||
![[List of Patents for class 560 subclass 359]](../ps.gif) 359 359
| |||
This subclass is indented under subclass 358. Compounds wherein isocyanate groups are bonded directly
to more than one benzene ring.
| |||
![[List of Patents for class 560 subclass 360]](../ps.gif) 360 360
| |||
This subclass is indented under subclass 358. Compounds wherein plural isocyanate groups are bonded directly
to the same benzene ring.
| |||