CPC Definition - Subclass C09B
This place covers:
- Dyes (coloured compounds being at least partially soluble in a liquid medium; thereby the chemical structure of the chromophore might be relevant for the sub-group given for a certain dye class [e.g. anthracene dyes (C09B 1/00), monoazo dyes (C09B 29/00), quinacridones (C09B 48/00)] etc.)] or functional groups attached to the chromophor might be relevant [e.g. reactive dyes (C09B 62/00), Dyes containing a splittable water solubilizing group (C09B 69/08)]; further, polymeric dyes (C09B 69/10) are also enclosed in C09B, which are reaction products of dyes with monomers or with macromolecular compounds; thereby the dye might be a repeating unit in the polymer chain or it might be connected by a covalent bond to the polymer chain as a pending group
- Pigments (coloured compounds being insoluble in liquid systems)
- Compositions comprising dyes and/or pigments and further additives (e.g. comprising polymeric dispersing agents (C09B 67/009)
- Blends of at least two dyes and/or pigments (C09B 67/0033)
- Preparation processes to prepare dyes or pigments (e.g. special methods of performing the coupling reaction of azo dyes (C09B 41/00)
- Dye or pigment preparations of special physical nature, e.g. tablets, films, extrusion, microcapsules, sheets, pads, bags with dyes (C09B 67/0097)
- Treatment of dyes or pigments without chemical reactions in order to influence the physical properties (classified in the main group C09B 67/00), e.g. acid pasting (C09B 67/0017), grinding, milling C09B 67/0002), coating C09B 67/0004), flushing (C09B 67/0021), etc.
In main group (C09B 68/00), the surface modification of pigments with chemical reactions is covered. Thereby the establishing of covalent/complex bonds of chemical groups on the surface of the pigment is the point of interest.
Certain fluorescent dyes or pigments of specific structure like Fluorescein derivatives (C09B 11/08), Rhodamine derivatives (C09B 11/24), Stilbene dyes (C09B 23/148) etc.); thereby it is mentioned, that there is no main- or subgroup which explicitly mentions properties like luminescense, fluorescense or phosphorescense; luminescent compounds are merely classified by the chemical structure of their chromophore
- Dyes, especially pigments having a certain crystal modification; special X-ray patterns (C09B 67/0025)
- Purification, Precipitation, or Filtration of dyes or pigments (C09B 67/0096)
- Dyestuff salts, e.g. salts of acid dyes with basic dyes; thereby the counter ion of the dye might not be a standard ion like Na+, K+, Ca2+, NH4+ or Cl-, SO42-, NO3- etc., but rather a complex non-common ion like a charged dyestuff itself or similar ions (C09B 69/02)
- The term "closely related Compounds" in the title of this sub class covers compounds, which could be seen as precursors for dyes becoming dyes after a minor modification, e.g. a chemical reaction or pH-change; so there is a clear structural similarity between the closely related compound and the dye.
The subclass C09B may overlap with many other subclasses relating to the use of dyes or pigments. In general, a C09B class is given to documents which describe dyes/pigments appearing to be novel as such or compositions/ preparations of dyes/pigments which appear to be novel; further, documents disclosing novel preparation processes for dyes/pigments should be put into C09B.
E.g., a document describing inks (C09D 11/00), which comprise already known dyes or pigments, should only be put into C09D 11/00; in case the inks comprise dyes or pigments not yet known in the prior art, the corresponding C09B sub-group should be given.
This place does not cover:
Attention is drawn to the following places, which may be of interest for search:
Places in relation to which this subclass is residual or which may be of interest for search:
Dyes/pigments for colouring foodstuff | |
Cosmetic or similar toilet preparations containing organic compounds (depending on the chromophore structure of the used dye/pigment, the corresponding sub group below A61K 8/30 should be identified for classification/search) | |
Medicinal preparations containing organic active ingredients (depending on the chromophore structure of the used dye/pigment, the corresponding sub group below A61K 31/00 should be identified for classification/search) | |
Preparations for testing in vivo Preparation for luminescence or biological staining (depending on the kind of the used dye/pigment, the corresponding sub group below A61K 49/001 should be identified for classification/search) | |
Preparations for temporary colouring the hair, e.g. direct dyes | |
Preparations for permanently dyeing the hair | |
Dyes/pigments used in thermography, e.g. in contact thermal transfer or sublimation processes | |
Mass colouring of high-molecular organic compounds, organic ingredients like optical brightening agents, organic pigments | |
Coatings | |
Inks | |
Pigment pastes | |
Dyeing or printing textiles; dyeing leather, furs, or solid macromolecular substances in any form | |
Dyes/pigments for dyeing paper, cardboard | |
Investigating or analysing materials by specific methods not covered by the preceding groups; with fluorescent label | |
Investigating or analysing materials by specific methods not covered by the preceding groups; involving labelled substances | |
Lenses | |
Contact lenses | |
Optical elements other than lenses, containing organic substances, e.g. dyes, inks or pigments | |
Optical elements other than lenses; Absorbing filters; absorbing filters, containing organic substances, e.g. dyes, inks or pigments | |
Dyes/pigments used in photosensitive materials | |
Dyes/pigments for colouring filters used e.g. in LCD's | |
Dyes/pigments used in photoconductive layers | |
Recording or reproducing by optical means, containing dyes, e.g. layers in DVD's, CD's, Blue rays | |
Electrolytic light sensitive devices using dyes (Dye Sensitized Solar Cells, DSSc) | |
Light sensitive devices; comprising an organic dye as the active light absorbing material, e.g. adsorbed on an electrode or dissolved in solution | |
Solid state devices using organic materials (e.g. dyes) as the active part, or using a combination of organic materials with other materials as the active part (Organic solar cells, organic light emitting devices [OLEDs]) |
In this subclass, in the absence of an indication to the contrary, a compound
is classified in the last appropriate place ('Last Place Rule')
As an example the following explanation is given: In the sub-group C09B 1/02 hydroxy-anthraquinones are classified. We search a sub-group for the compound 1-hydroxy-2-chloro-anthraquinone. The correct sub-group is C09B 1/10.
Now we search a sub-group for the compound 1-hydroxy-2-chloro-3-nitro-anthraquinone; again, the correct sub-group is C09B 1/10. In case we search a sub-group for the compound 1-hydroxy-2-chloro-3-sulfo-anthraquinone, we have to choose, following the 'Last Place Rule', the sub-group C09B 1/12; to avoid a loss of information, also the group C09B 1/10 should be considered.
In this place, the following terms or expressions are used with the meaning indicated:
Onium group | ionic groups bearing a positive charge comprising nitrogen, phosphor etc. as the charged atom |
Aralkyl, arylalkyl | unless other specified (exception: C09B 1/526), both sequences alkyl-aryl and aryl-alkyl are meant (see e.g. C09B 1/514 and C09B 1/515) |
Carbocyclic ring | here aromatic as well as a non-aromatic rings are mentioned (no heterocyclic rings) |
Sulfonated | having a SO3H or SO3‾ group attached |
Sulfo | SO3H |
Sulfonat | SO3‾ |
Leuco form | the form of a reduced dye which is normally uncoloured or only slightly coloured compared to the dye itself (e.g. indigo and its leuco-form) |
Vat dyeLeuco dye | sometimes insoluble dyes (e.g. indigoid dyes) are transferred into their soluble derivatives (e.g. leuco form) by e.g. reduction and then contacted with the material to be coloured; the soluble form is then converted into its insoluble form e.g. by oxidation; such dyes are called 'vat dyes' (in German: Küpenfarbstoff) |
Cyanine dyes | specific polymethine dyes having N-heterocyclic rings at both end of the polymethin chain (push-pull-system) |
Acid dyes | water-soluble anionic dyes |
Basic dyes | water-soluble cationic dyes |
Substantive dyes (or Direct dyes) | are directly applied to the fibre from aqueous solution; especially suitable for cellulosic fibres |
Mordant dyes | see definition below in the Annex for C09B 65/00 |
Reactive dyes | see definition below in the Annex for C09B 62/00 |
Disperse dyes | are generally water insoluble; the dyes are finely ground in the presence of a dispersing agent; their main use is to dye polyester |
Sulfur dyes | see definition below for C09B 49/00 |
Lakes | A dye made insoluble in order to have pigmentary properties by precipitating the water soluble form of the dye (bearing SO3H resp. COOH groups) by salting out with cations of the rare earth metals (Ca2+, Mg2+, also Al3+) |
In patent documents, the following abbreviations are often used:
LCD | Liquid Crystal Display |
DSSc | Dye sensitised solar cells |
LED | Light Emitting Diode |
OLED | Organic Light Emitting Diode |
2D | two dimensional |
3D | three dimensional |
C.I. | means Colour Index (C.I.); refers to colorants indexed in the Colour Index International [http://www.colour-index.org/login.aspx] |
µm | micrometer (10-6 meter) |
Nm | nanometer (10-9 meter) |
CF | Color Filter |
TFT | Thin Film Transistor |
DPP | Diketopyrrolopyrrole (a type of pigment, see C09B 57/004) |
DVD | Digital Versatile Disc |
CD | Compact Disc |
This place covers:
For example:
 or
or 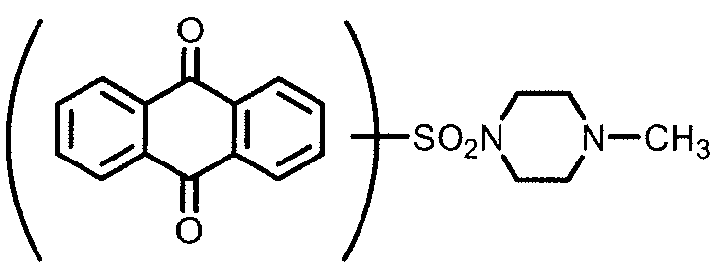
Further details of subgroups:
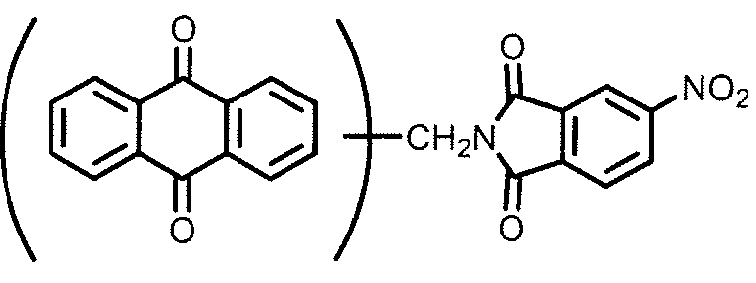
Dyes containing onium groups, e.g. ammonium groups:
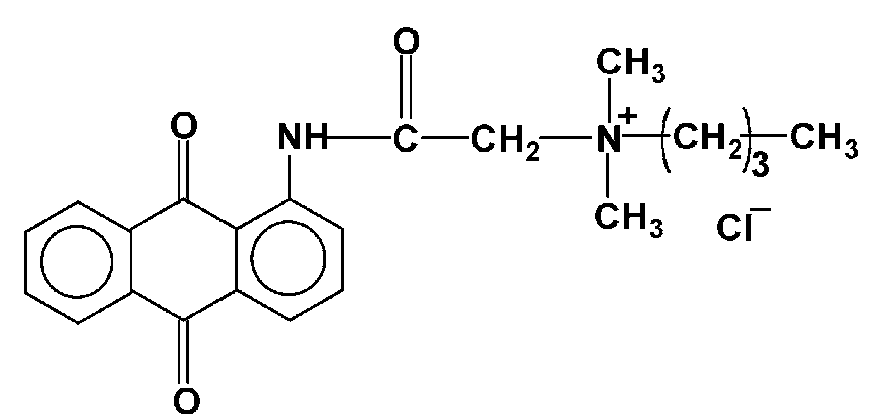
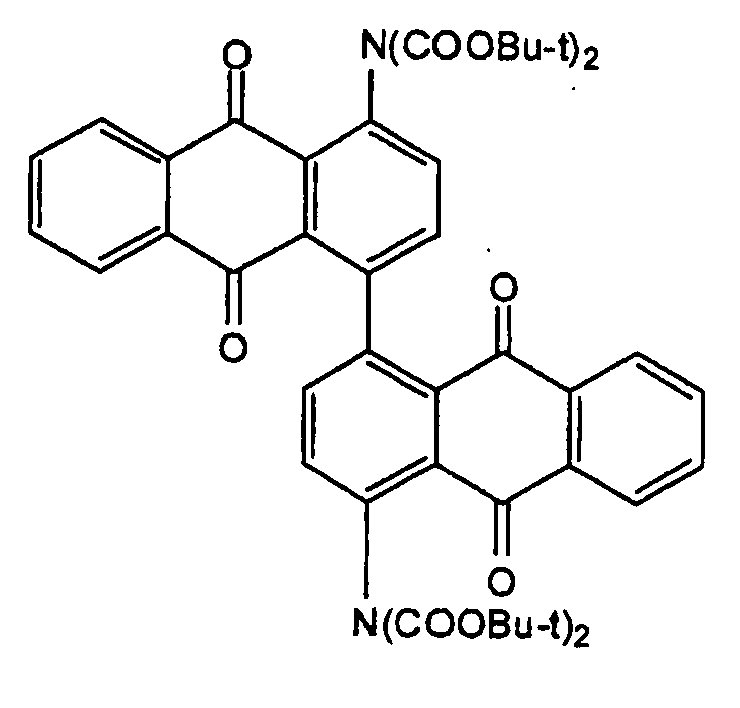
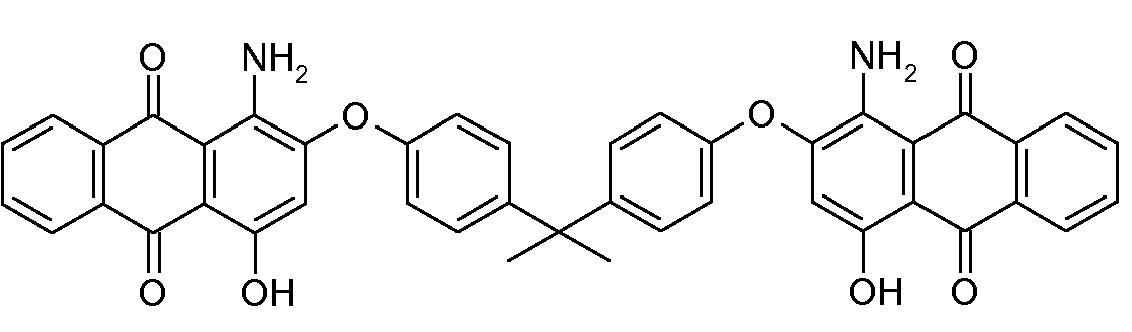
Di-anthraquinonyl and derivatives compounds:
at least two anthraquinones linked directly (see example left) or by a chemical linker (right example); in case the linker is represented by -NH- or the substituted derivative thereof, C09B 1/48 takes precedence
 ;
; 
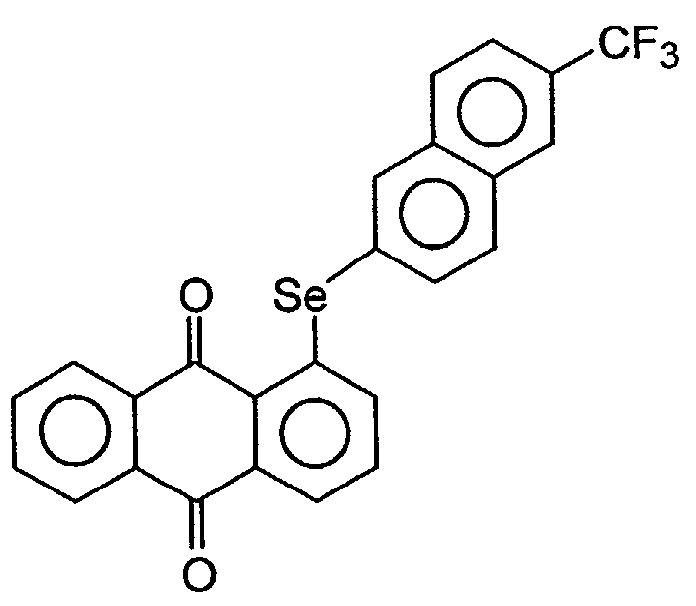
Seleno-anthraquinones
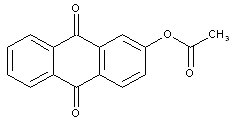
Hydroxy anthraquinones; Ethers or esters thereof
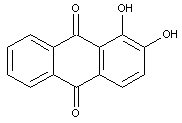
Dyes containing only OH groups
Dyes containing halogen
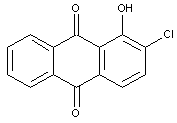
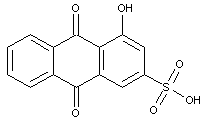
Dyes containing sulfonic acid groups
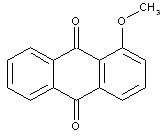
Dyes containing ether groups, e.g.:
Amino anthraquinones, e.g.:
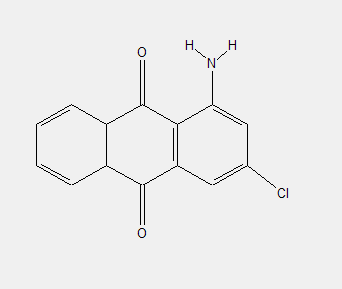
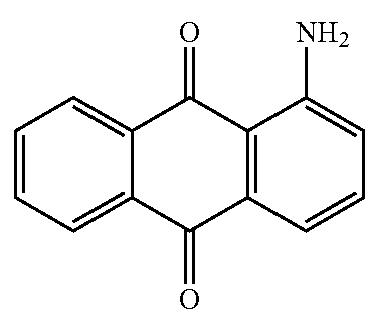
Dyes with no other substituents than the amino groups
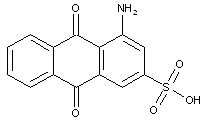
sulfonated amino anthraquinones, e.g.:
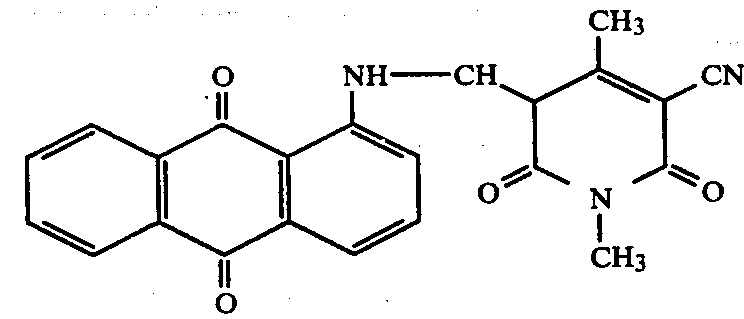
Dyes with an unsaturated C on the N atom attached to the nucleus; for C=O, C=S: C09B 1/36 takes precedence
also in case of acylated amino groups: C09B 1/36 takes precedence
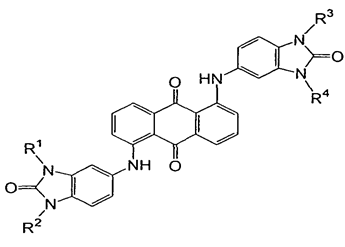
Dyes with amino groups substituted by heterocyclic radicals; for triazinic or
analogous heterocyclic radicals: C09B 1/46 takes precedence
Dyes with amino groups and onium groups
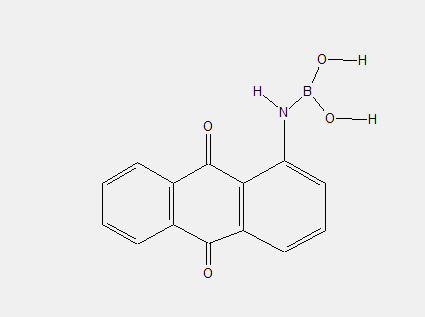
amino groups substituted by inorganic radicals, e.g.:
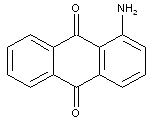
Dyes with unsubstituted amino groups
sulfonated
sulfonated dyes with unsubstituted amino groups

Dyes with amino groups substituted by hydrocarbon radicals
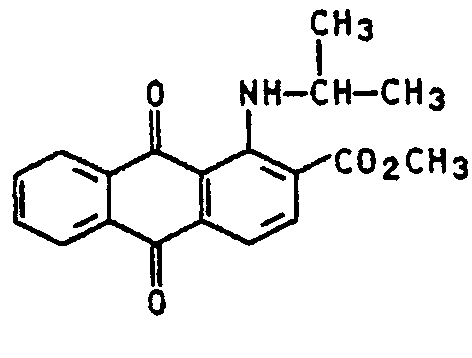
Dyes with no other substituents than the substituted amino groups
Dyes with amino groups substituted by hydrocarbon radicals and no other substituents than the substituted amino groups
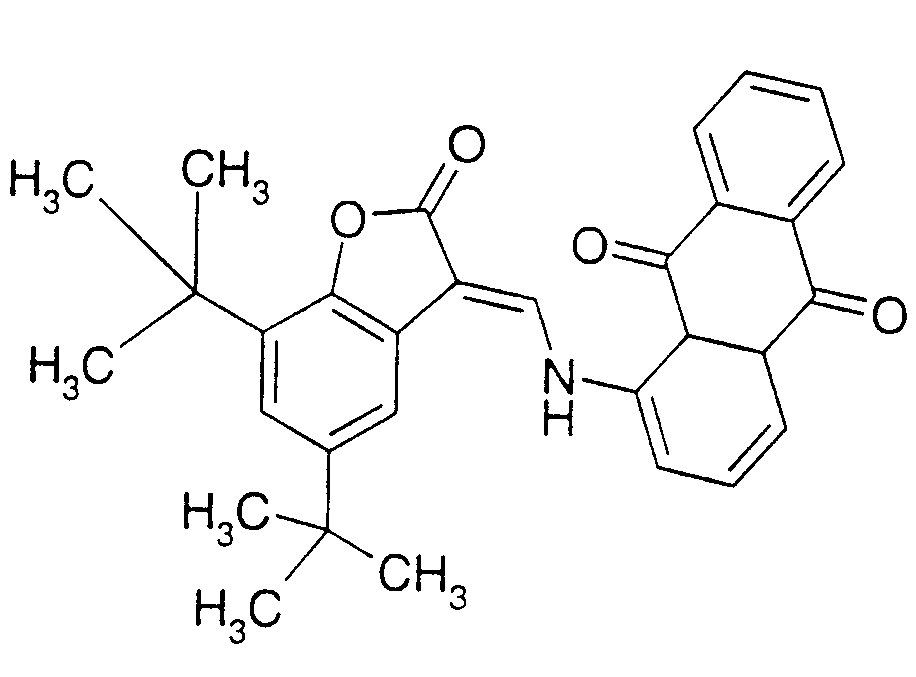
sufonated dyes with amino groups substituted by hydrocarbon radicals:
in case that both the anthracene nucleus and its substituent are sulfonated
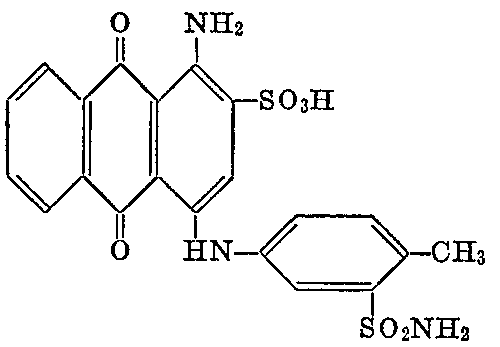
Dyes with amino groups substituted by alkyl, aralkyl, or cyclo-alkyl groups
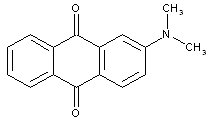
Sulfonated dyes with amino groups substituted by alkyl, aralkyl, or cyclo-alkyl groups:
in case that both the anthracene nucleus and its substituent are sulfonated
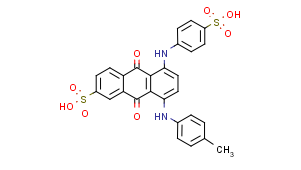
Sulfonated dyes with amino groups substituted by alkyl, aralkyl, or cyclo-alkyl groups:
only sulfonated in the anthracene nucleus
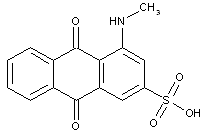
Dyes with amino groups substituted by aryl groups (anthrimides C09B 1/48 take precedence)
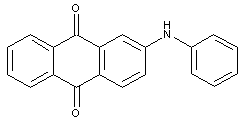
sulfonated dyes with amino groups substituted by aryl groups:
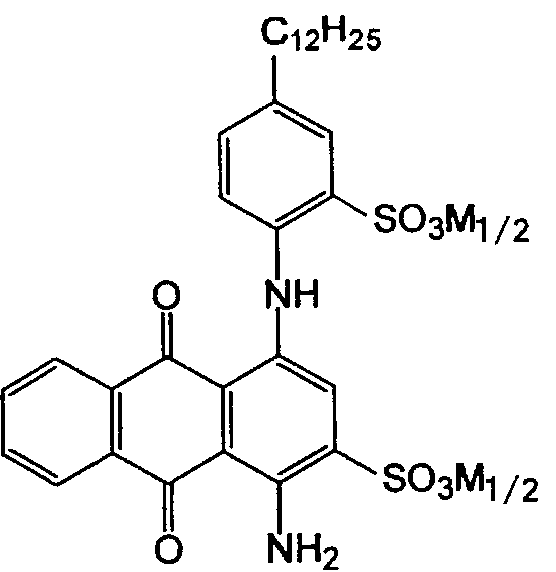
in case that both the anthracene nucleus and the aryl substituent are sulfonated
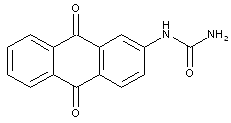
Urea or thiourea derivatives
the acyl groups being residues of an aliphatic or araliphatic carboxylic acid
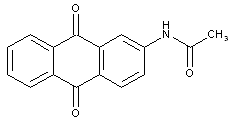
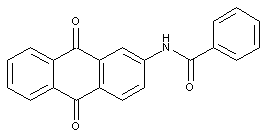
the acyl groups being residues of an aromatic carboxylic acid
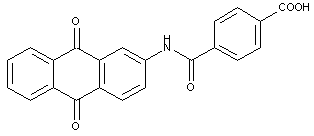
the acyl groups being residues of a dicarboxylic acid; this sub group does not contain any documents (see comment to sub group C09B 1/43 below)
Dicarboxylic acids: this sub group covers in principle the same compounds as the sub group C09B 1/425 above; however, this sub group contains documents. It is foreseen to bring this matter in accordance with the classification Rules in a later request.
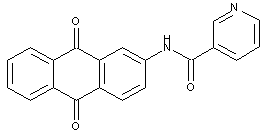
the acyl groups being residues of a heterocyclic carboxylic acid
anthraquinone dyes with acylated amino groups, the acyl groups being residues of cyanuric acid or an analogous heterocyclic compound
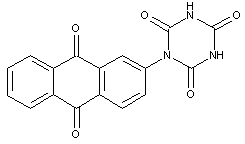
attached to two or more anthraquinone rings
anthraquinone dyes with acylated amino groups, the acyl groups being residues of cyanuric acid or an analogous heterocyclic compound and being further attached to two or more anthraquinone rings
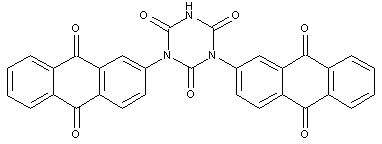
the acyl groups being residues of a sulfonic acid
anthraquinone dyes with acylated amino groups, the acyl groups being residues of a sulfonic acid
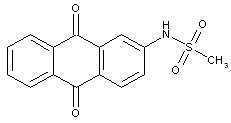
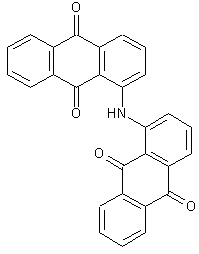
Anthrimides
two anthraquinone chromophores directly linked by -NH- or a substituted derivative thereof
Amino-hydroxy anthraquinones; Ethers or esters thereof [Seleno-anthraquinones C09B 1/007 take precedence]
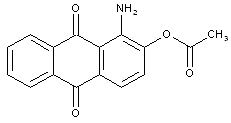
unsubstituted amino-hydroxy anthraquinone
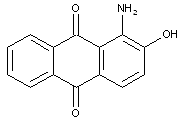
N-substituted amino-hydroxy anthraquinone
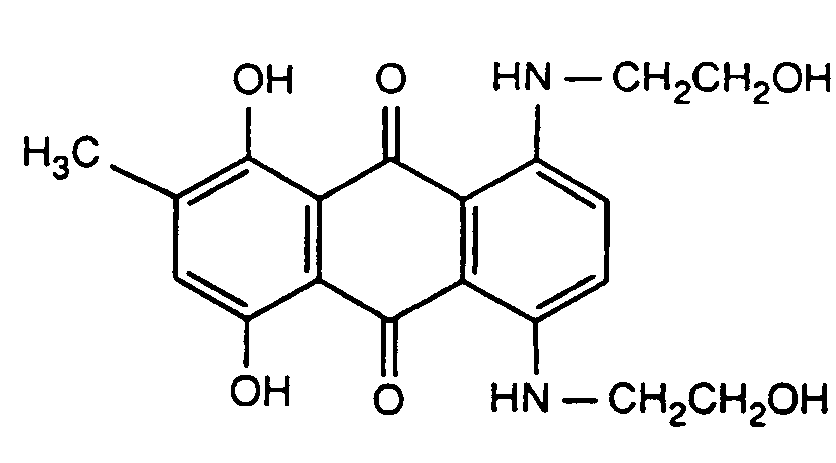
N-substituted amino-hydroxy anthraquinone, with only amino and hydroxy groups
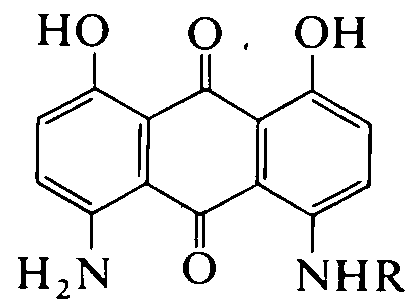
N-aryl derivatives of amino-hydroxy anthraquinones (N-aralkyl derivatives C09B 1/515 take precedence)
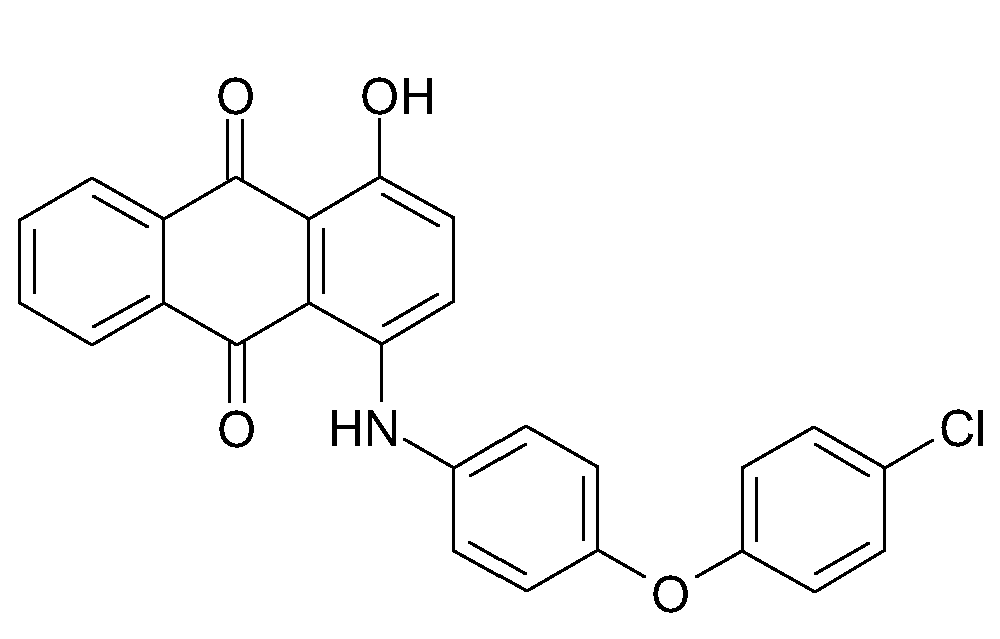
N-substituted amino-hydroxy anthraquinones, with only amino and hydroxy groups
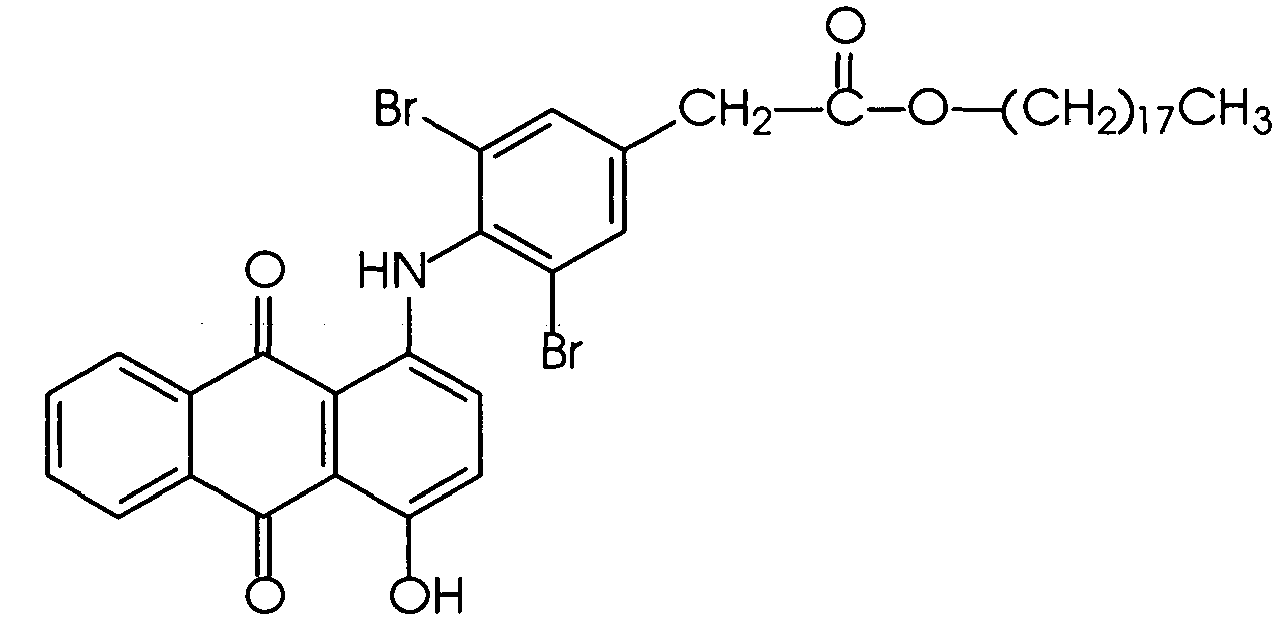
N-alkyl, N-aralkyl or N-cycloalkyl derivatives of substituted amino-hydroxy anthraquinones
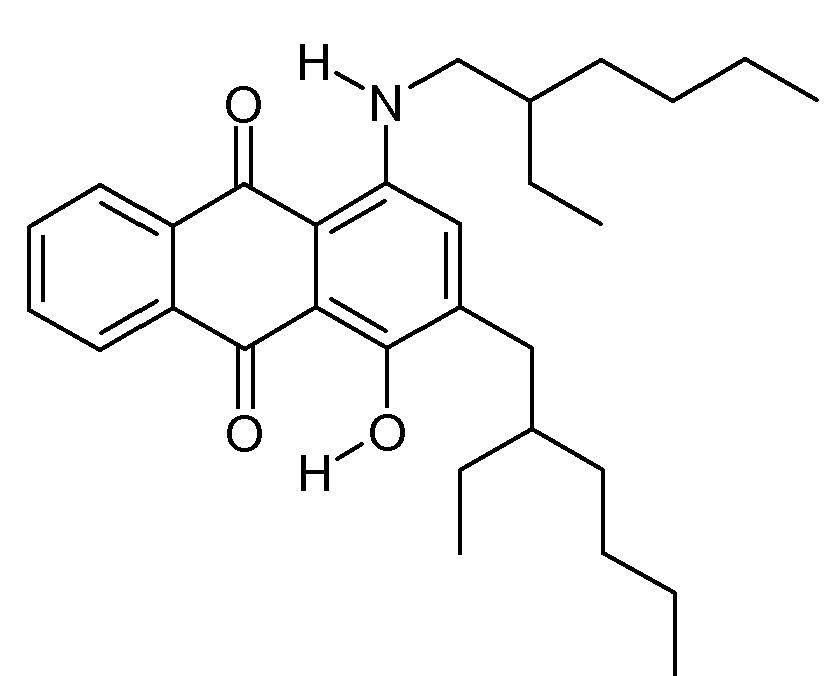
N-alkyl, N-aralkyl or N-cycloalkyl derivatives of amino-hydroxy anthraquinone with only amino and hydroxy groups
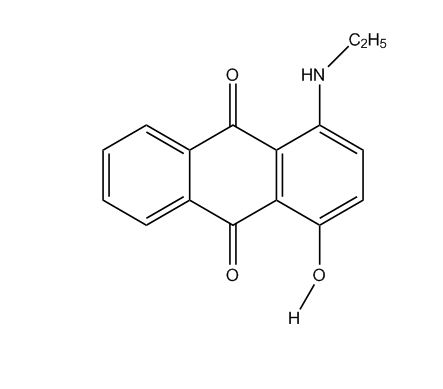
N-acylated derivatives
of amino-hydroxy anthraquinones
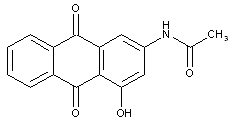
sulfonated amino-hydroxy anthraquinones:
in case that both the anthracene nucleus and the substituent are sulfonated
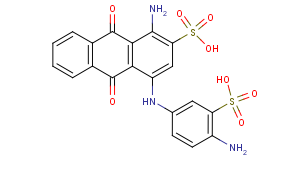
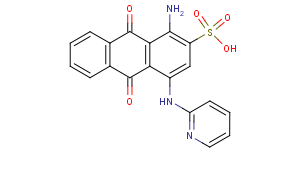
sulfonated N-substituted amino-hydroxy anthraquinones:
with substituents not covered by C09B 1/525 - C09B 1/528 (like heterocyclic, see example)
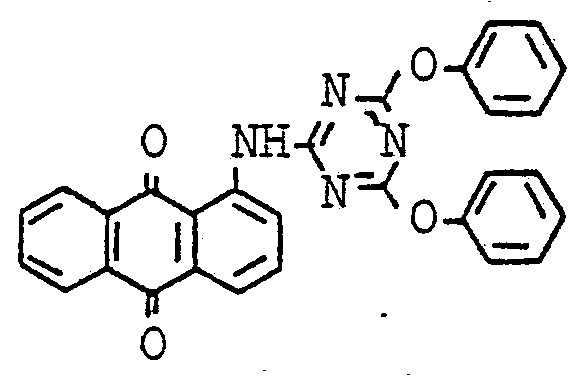
etherified derivatives of amino-hydroxy anthraquinones
with substituents not covered by C09B 1/542 - C09B 1/547 (like heterocyclic, see examples)
 or
or 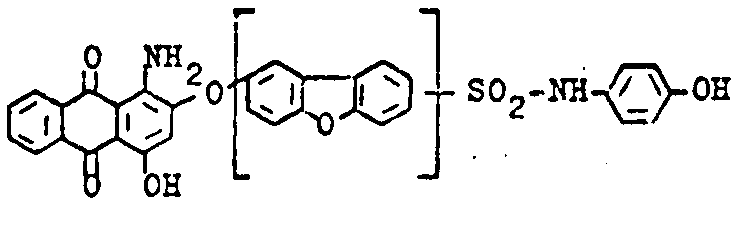
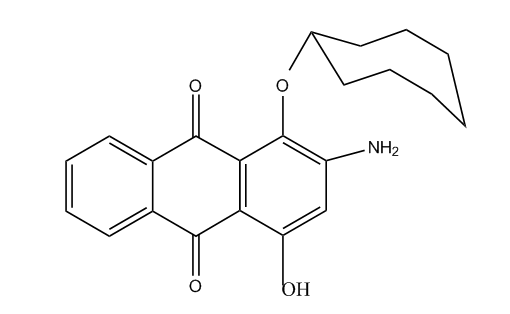
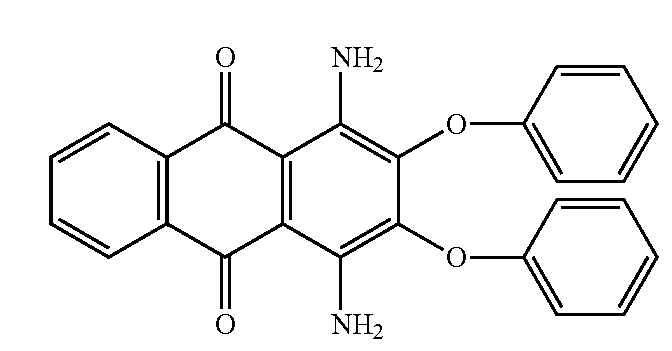
Amino-hydroxy anthraquinones with aliphatic, cycloaliphatic, araliphatic or aromatic ether groups, e.g.:
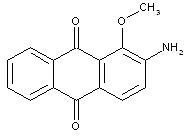
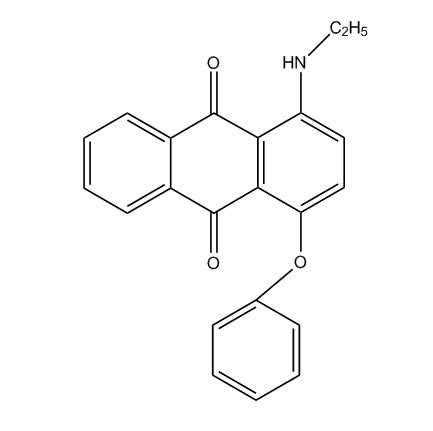
C09B 1/542 contains documents having both types of anthraquinones: compounds with aliphatic ether groups and compounds with aromatic ether groups (thereby cases are included, where the kind of ether group can vary). C09B 1/545 contains only documents with compounds with aliphatic ethers, thereby C09B 1/547 deals with compounds with aromatic ethers; in case documents contain compounds having both types of ether groups, the last place rule should be applied (C09B 1/547); in order to avoid a loss of information, also the sub group C09B 1/542 should be considered here
Amino-hydroxy anthraquinones with aliphatic, cycloaliphatic or araliphatic ether groups

Amino-hydroxy anthraquinones with aromatic ether groups
 or
or 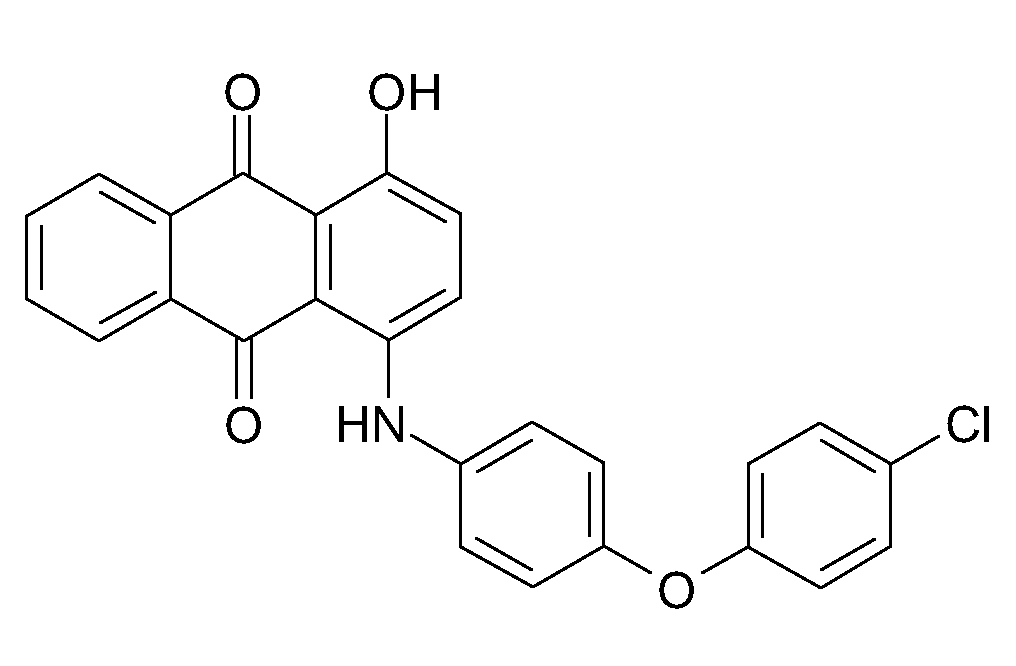
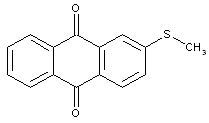
Mercapto-anthraquinones
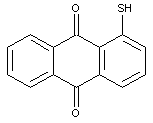
Mercapto-anthraquinones with mercapto groups substituted by aliphatic, cycloaliphatic, araliphatic or aryl radicals
for these sub groups C09B 1/58, C09B 1/585 and C09B 1/60 the same classification rule applies as in the above mentioned case concerning the sub groups C09B 1/542, C09B 1/545 and C09B 1/547
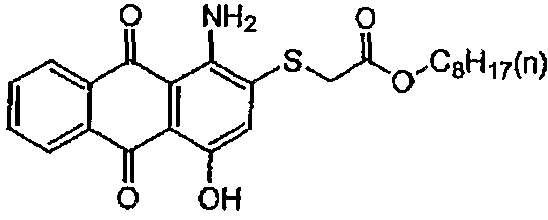
substituted by aryl radicals
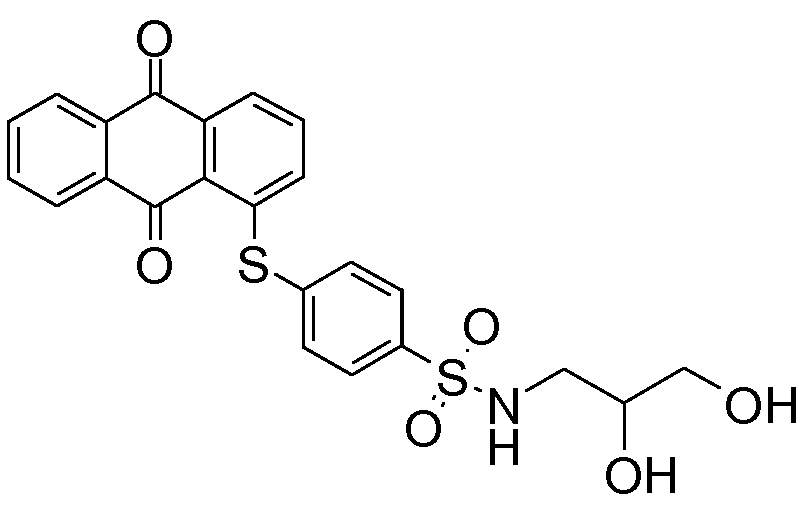
substituted by aliphatic, cycloaliphatic or araliphatic radicals;
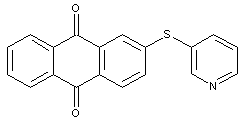
Mercapto-anthraquinones with mercapto groups substituted by a heterocyclic ring
This place covers:
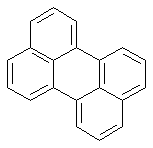
First, the anthracene nucleus is simply the aromatic ring system consisting of three 'fused' six-membered rings:
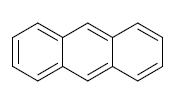 ---> anthracene 'ground structure'
---> anthracene 'ground structure'
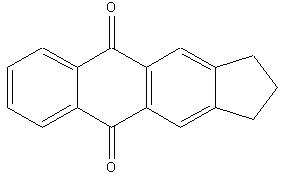
Condensed with one or more carbocyclic rings means, that, additionally to the ground structure, further rings are added by condensing them 'along to a bond'. In the three examples below an anthrone, which is based on an anthracene ground structure which additionally comprises a keto-group in the middle, is further condensed with saturated 'six-membered' rings (two compounds at the left) or with a saturated 'five-membered' ring (compound at the right). The term carbocyclic means, that hetero-atoms are excluded as members of the ring atoms (see here also the main group C09B 5/00, which deals with anthracene nucleus condensed with heterocyclic rings).
The fourth compound at the bottom could be considered to comprise two basic anthracene ground structures:
For example, documents disclosing compounds like:
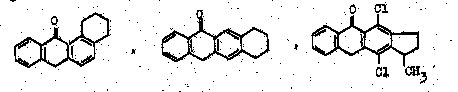
Care should be taken with view to the sub group C09B 3/78, which comprises documents disclosing one (or possibly more) specific structures not covered by the preceding subgroups C09B 3/02 up to C09B 3/76, while C09B 3/00 covers documents disclosing several structures falling under different subgroups from C09B 3/02 - C09B 3/76; all documents which are not covered by the groups C09B 3/02 up to C09B 3/76 should be put into C09B 3/78
Further details of subgroups:
Benzanthrones
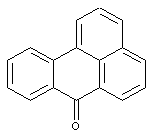
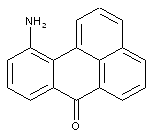
Amino derivatives
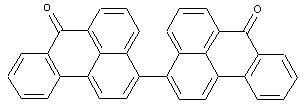
Dibenzanthronyls
at least two benzanthrones moieties linked directly or by a chemical linker
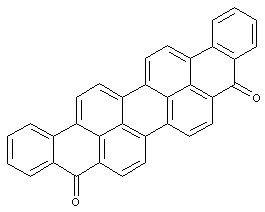
Perylene derivatives
care has to be taken with view to sub group C09B 5/62
Dibenzanthrones, Isodibenzanthrones
at least two benzanthrones moieties condensed together
Pyranthrones
Amino derivatives of pyranthrones
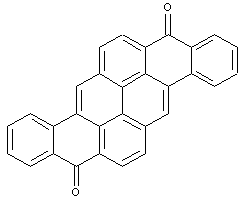
Dibenzopyrenequinones
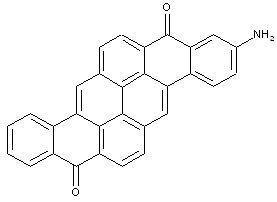
Amino derivatives of dibenzopyrenequinones
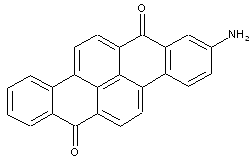
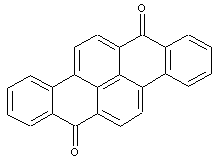
Benzanthraquinones
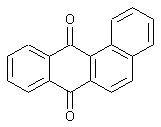
Anthanthrones
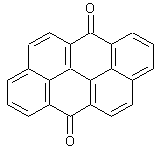
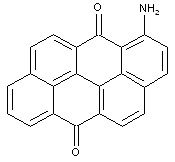
Amino derivatives of anthanthrones
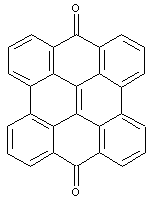
Benzo-, naphtho-, or anthra-dianthrones
Other dyes in which the anthracene nucleus is condensed with one or more carbocyclic rings (see the comment to main group C09B 3/00)
This place covers:
For example:
 or
or  or
or
Further details of subgroups:
the heterocyclic rings being condensed in peri-position and in 1-2 or 2-3 position
Dyes with anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings, the heterocyclic rings being condensed in peri-position and in 1-2 or 2-3 position and not covered/provided by any of the C09B 5/004 - C09B 5/008 sub groups (e.g. N and S-containing hetero rings, example)
only O-containing heterorings
the heterocyclic rings being condensed in peri-position (see example) and in 1-2 or 2-3 position
only S-containing heterorings
the heterocyclic rings being condensed in peri-position and in 1-2 or 2-3 position
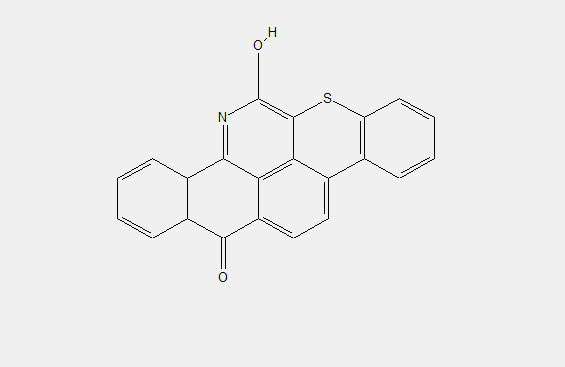
the heterocyclic rings being only condensed in peri-position
with only N-containing heterorings
the heterocyclic rings being only condensed in peri-position
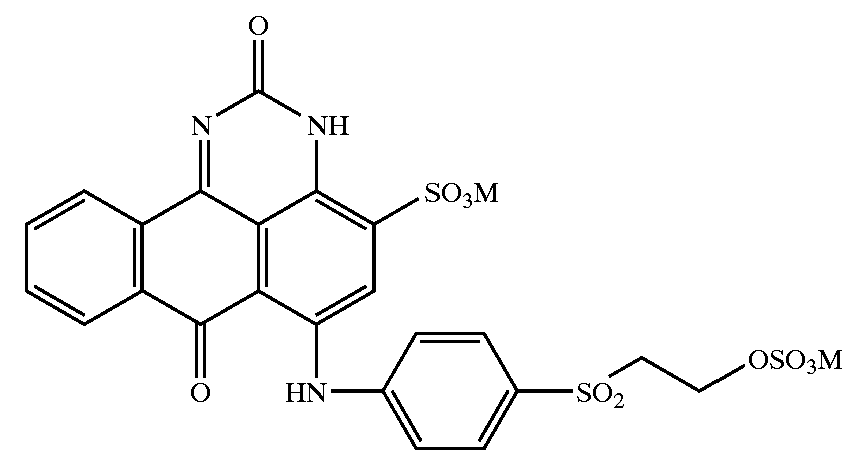
Pyrazolanthrones
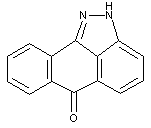
Benzanthronyl-pyrazolanthrone condensation products
'condensation' in this context means obviously, that also two chromophores could be linked by a single covalent bond (see formula below)
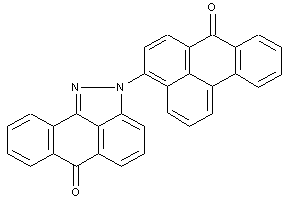
Dipyrazolanthrones
at least two pyrazolanthrones moieties linked directly or by a chemical linker
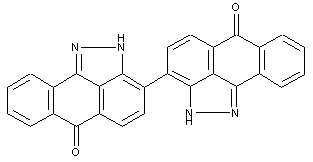
Condensation products of dipyrazolanthrones
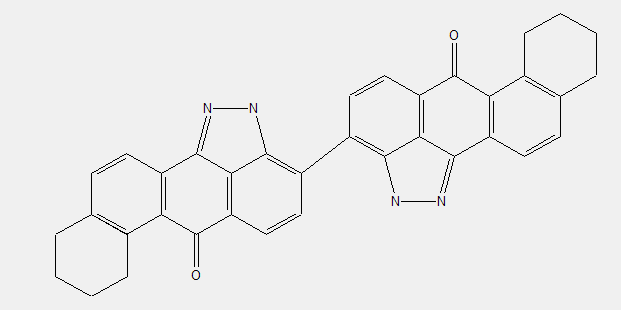
Isothiazolanthrones (see example); Isoxazolanthrones; Isoselenazolanthrones
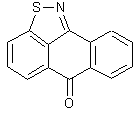
Thiophenanthrones
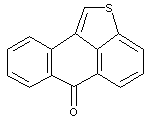
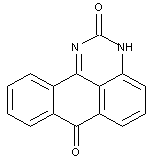
Benz-azabenzanthrones (anthrapyridones)
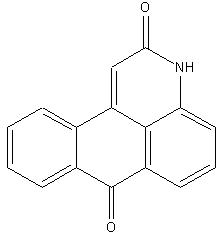
Benz-diazabenzanthrones, e.g. anthrapyrimidones
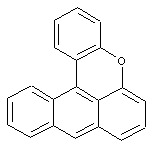
Coeroxene (see example); Coerthiene; Coeramidene; Derivatives thereof
Flavanthrones
the heterocyclic ring(s) being condensed with an anthraquinone nucleus in 1-2 (see example) or 2-3 position
Dyes with an anthracene nucleus condensed with one or more heterocyclic rings with or without carbocyclic rings, wherein the heterocyclic ring(s) being condensed with an anthraquinone nucleus in 1-2 (see example) or 2-3 position
the heterocyclic ring(s) being condensed with an anthraquinone nucleus in 1-2 (see example) or 2-3 position and not provided for in one of the sub groups C09B 5/26 - C09B 5/62
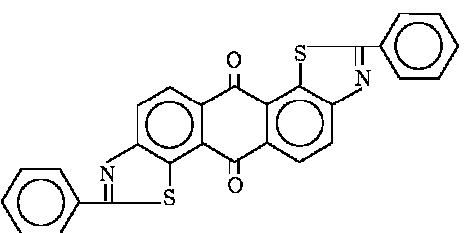
with only oxygen-containing heterorings
the heterocyclic ring(s) being condensed with an anthraquinone nucleus in 1-2 (see example) or 2-3 position
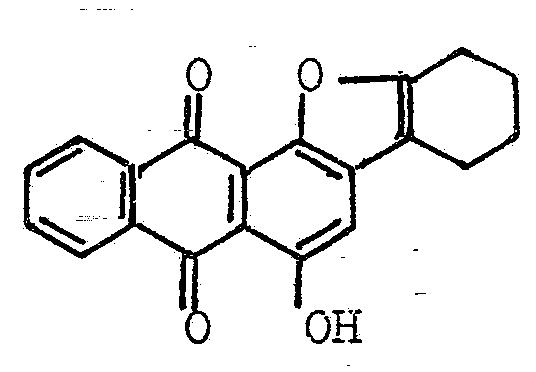
Phtaloyl isoindoles
the isoindole ring being condensed with an anthraquinone nucleus in 1-2 or 2-3 position (see example)
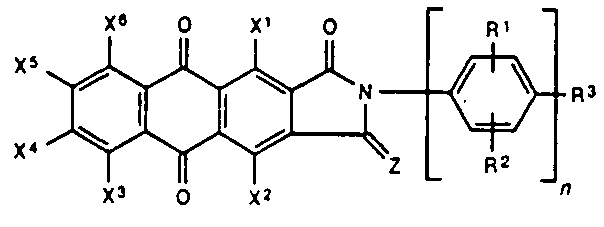
5,6-phtaloyl-dihydro-isoindoles
1,3 oxo or imino derivatives
means that the oxo or imino function is independently located in the 1,3 - position (below you see the 1,3-oxo derivative)
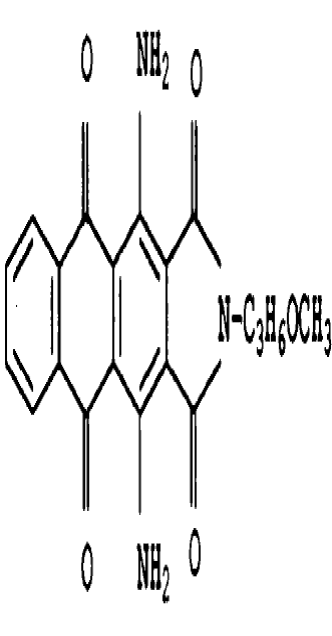
1-oxo-3-imino derivatives, e.g.:
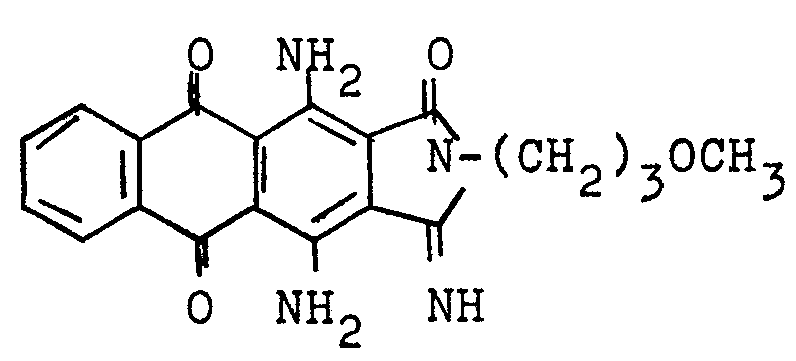
Carbazoles of the anthracene series
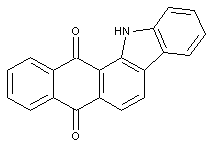
Anthrimide carbazoles
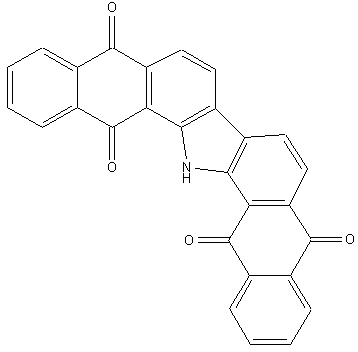
1,2-azoles of the anthracene series
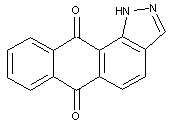
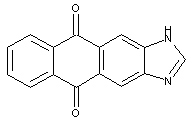
1,3-azoles of the anthracene series
Anthraquinone acridones or thioxanthones
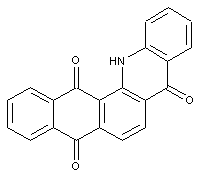
Anthraquinone acridones (see example below), anthraquinone thioxanthones (with S instead of NH)
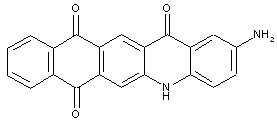
Amino acridones
Compounds containing acridone and carbazole rings
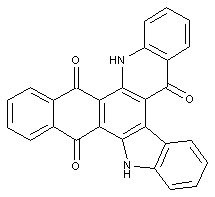
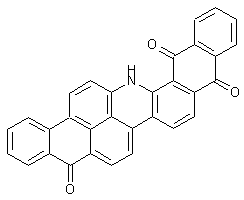
Condensation products of benzanthronyl-amino anthraquinones
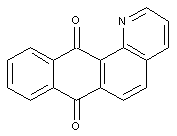
Pyridino anthraquinones
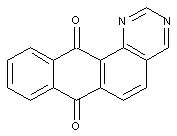
Azines of the anthracene series
(example below: 1,3-diazine)
Para-diazines
condensed with 1,4-diazine
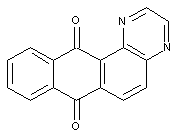
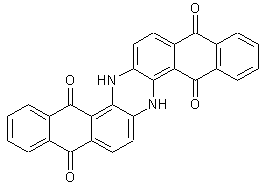
Bis-anthraquinonediazines (indanthrone)
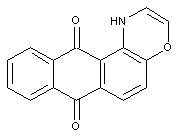
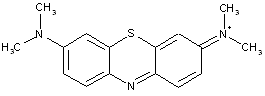
Thiazines; Oxazines (the example below is the oxazine)
the example below is the oxazine; thiazines have S instead of O
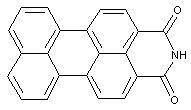
Cyclic imides or amidines of peri-dicarboxylic acids of the anthracene, benzanthrene, or perylene series
care has to be taken with view to sub group C09B 3/14
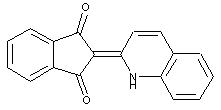
This place covers:
Indigoid dyes and derivatives not covered/provided by its sub-groups (example up: wherein X is O, S, etc., while Y means NH; O, S, etc. or example down)
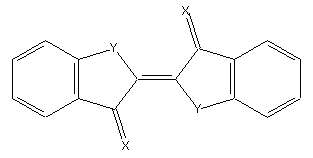 or
or 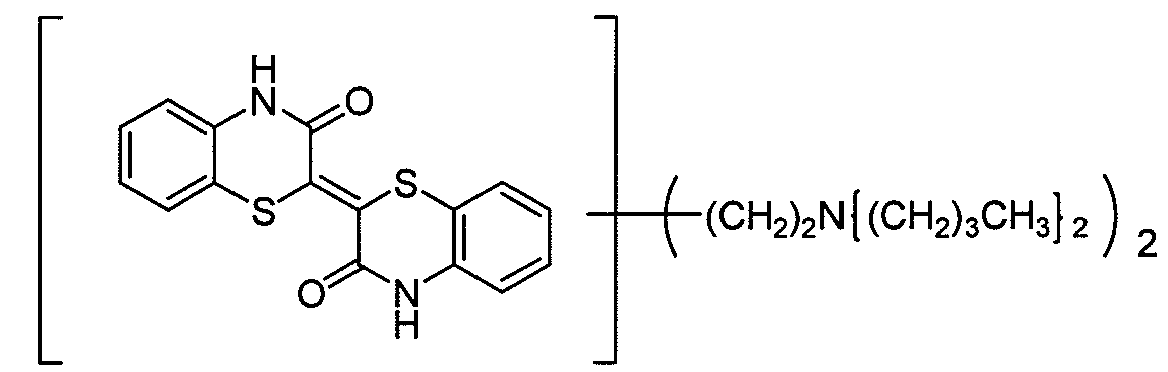
Further details of subgroups:
Bis-indole indigos
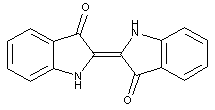
Indone-thionaphthene indigos
Other indole-indigos
not covered by the upper groups: e.g. with other heteroatom instead of NH (like O, see bis-oxodihydro-indolylene-benzodifuranone from example)
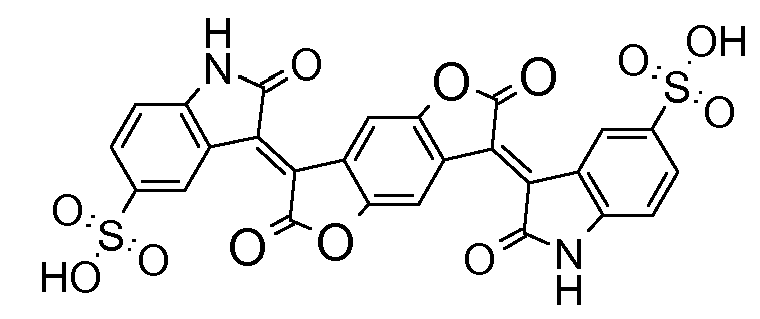
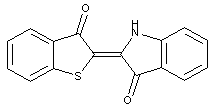
Bis-thionaphthene indigos
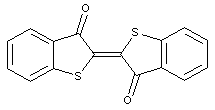
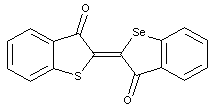
Other thionaphthene indigos
not covered by the upper groups (see example with Se)
This place covers:
Diaryl- or triarylmethane dyes; generally the coloured forms of these dyes have a sp2-hybridisation at the central C-atom
Further details of subgroups:
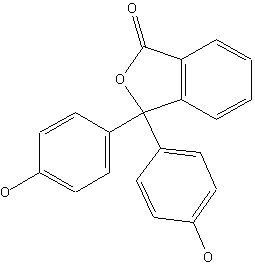
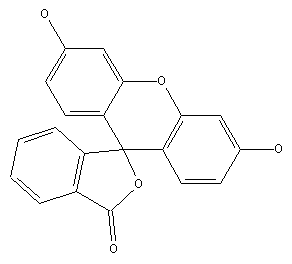
Phthaleins, e.g. Phenolphthaleine (left) and Fluoresceine (right)
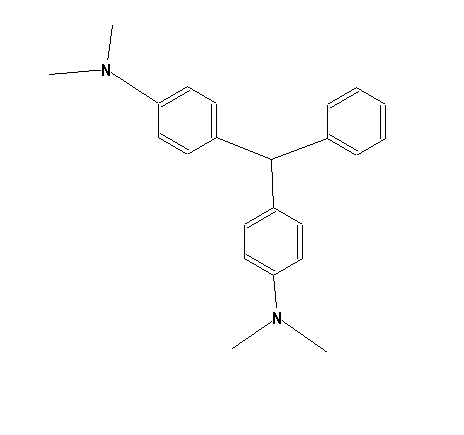
Amino derivatives of triarylmethanes without any OH group bound to an aryl nucleus
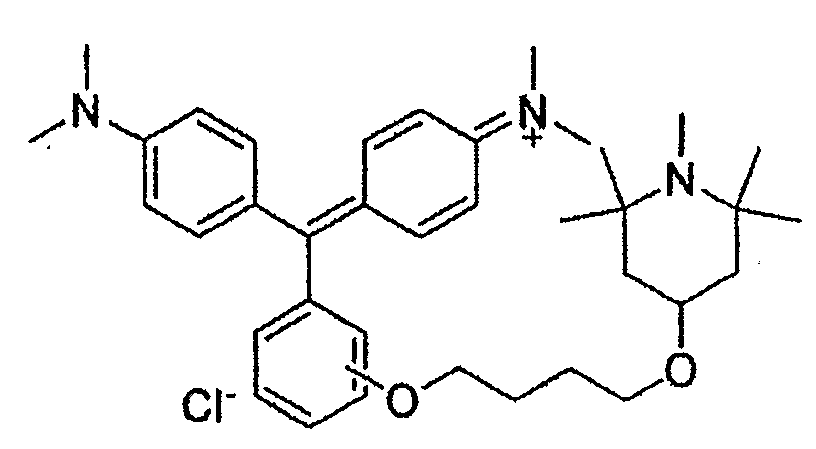
Amino derivatives of triarylmethanes containing OH groups bound to an aryl nucleus and derivatives thereof (like ethers (example) and esters)
Phthaleins containing amino groups (see left); example for a phthalane (see right):
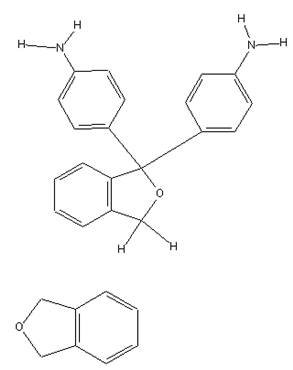
thereby the phthalane itself has the structure:
fluorans:
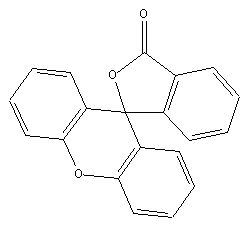
phthalides:
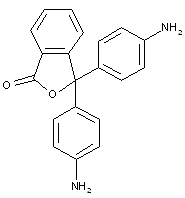
example for rhodamine:
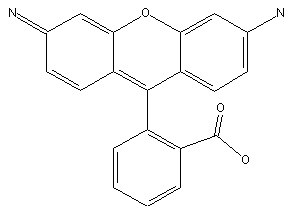
Phthaleins having both OH and amino substituent(s) on an aryl ring
Triarylmethane dyes in which at least one of the aromatic nuclei is heterocyclic
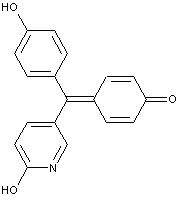
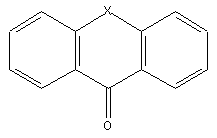
Pyronines (xanthon: X=O; thioxanthon: X=S; selenoxanthan X=Se; telluroxanthon X=Te)
This place covers:
Oxyketone dyes not covered by its sub groups (example)
Further details of subgroups:
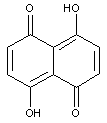
Oxyketone dyes of the naphthalene series, e.g. naphthazarin
Oxyketone dyes of the pyrene series
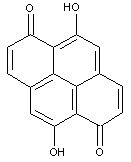
Oxyketone dyes of the acetophenone series, means here: derivatives of:
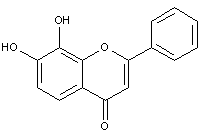
This place covers:
Illustrative example of subject matter classified in this group.
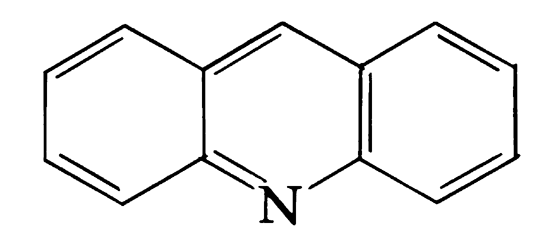
This place covers:
Illustrative example of subject matter classified in this group.
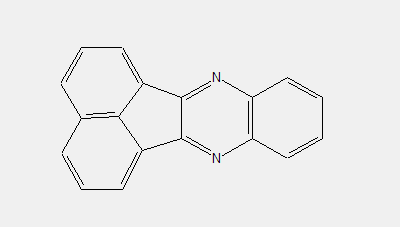
Further details of subgroups:
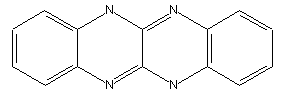
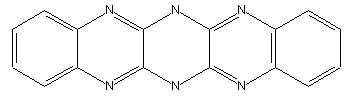
Azine dyes containing at least four ortho-condensed rings with at least two N-atoms in the system, e.g. fluoflavine (left), fluorubine (right)
 ,
,
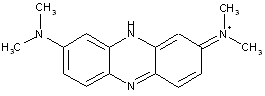
Azine dyes of the benzene series
Nigrosines are black azine dyes closely related to indulines, thereby their structures are not clearly defined; they should be classified here in C09B 17/02
Azine dyes of the naphthalene series
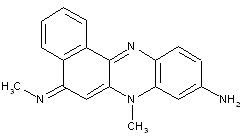
Fluorindine or its derivatives
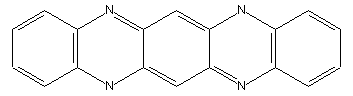
This place covers:
Illustrative example of subject matter classified in this group.
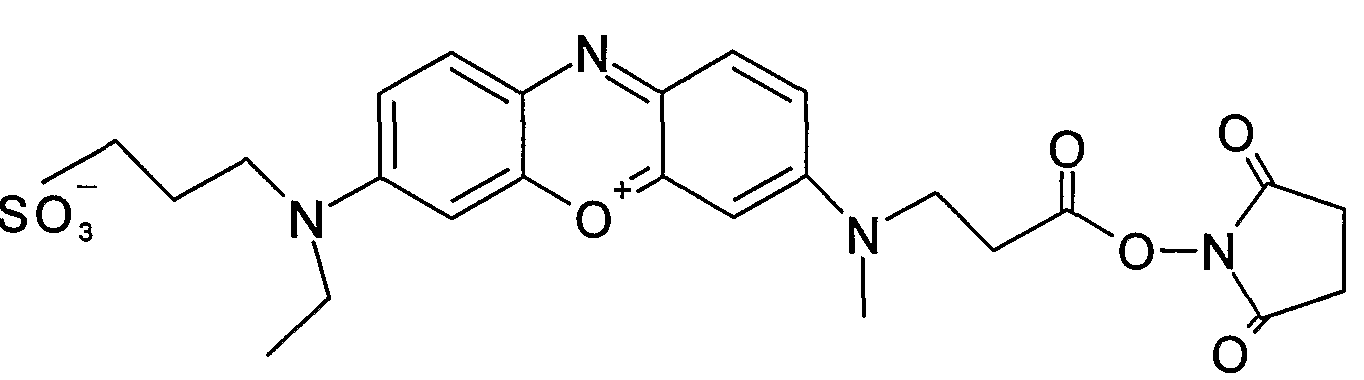
Further details of subgroups:
Gallocyanine dyes
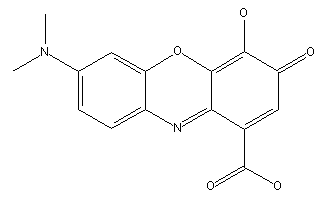
Bisoxazines prepared from aminoquinones
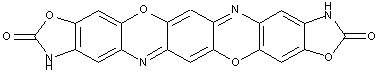
This place covers:
Illustrative example of subject matter classified in this group.
This place covers:
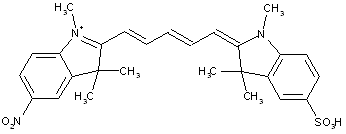
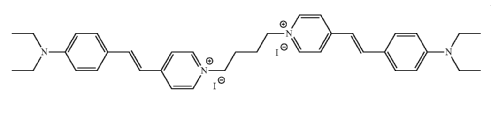
Methine or polymethine dyes, e.g. cyanine dyes; thereby methine or polymethine dyes comprise one or more CH-moieties linked with each other in order to establish a conjugated system: -CH[=CH-]x=, thereby x is zero or bigger.
Concerning the classification method with view to the sub groups C09B 23/02 up to C09B 23/086 and C09B 23/10 up to C09B 23/107: in case a document comprises at the same time dyes with one, three, five and seven methin groups in the chain, put it into the sub groups C09B 23/04, C09B 23/06, C09B 23/083 and C09B 23/086; in case a document comprises at the same time dyes with e.g. two and four methin groups in the chain, put it into the sub groups C09B 23/105 and C09B 23/107. Although this stays in contradiction to the classification philosophy of the last-place-rule, it has been done so in the past.
Further details of subgroups:
[N :substituted on the polymethine chain]
the polymethine chain being part of a carbocyclic ring, (e.g. benzene, naphtalene, cyclohexene, cyclobutenene-quadratic acid)
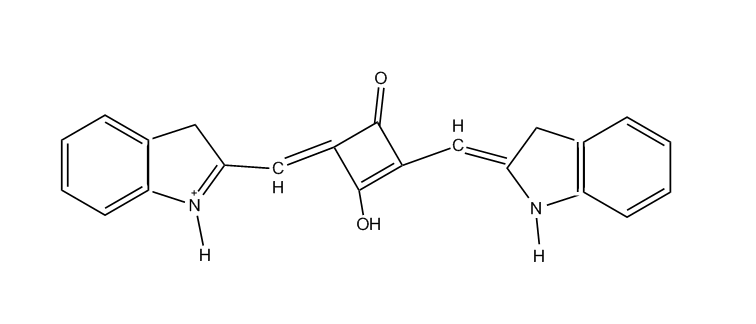
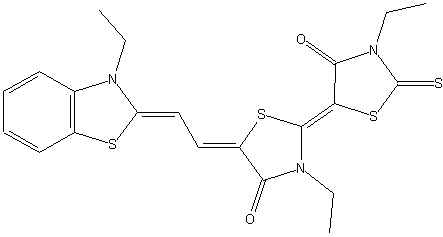
the polymethine chain being part of an heterocyclic ring, thereby the heteroring being rhodanine (left) in the chain (see example right)
 ;
; 
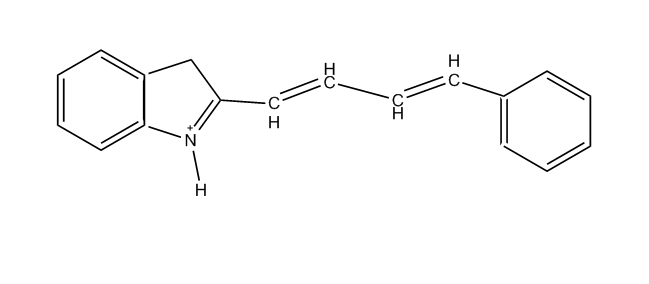
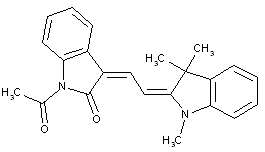
[N :having only one heterocyclic ring atom at one end of the methin chain, e.g. hemicyanines, hemioxonol (styryl dyes see C09B 23/14)]
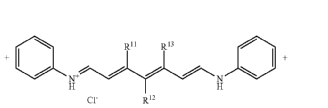
Methine dyes having only one heterocyclic ring at one end of the methine chain, e.g. hemicyanine dyes (see formula below)
Monomethine dyes, wherein the polymethine chain contains only one ![]() CH group
CH group
Cyanine dyes, wherein the polymethine chain contains three ![]() CH group
CH group
Cyanine dyes, wherein the polymethine chain contains more than three ![]() CH group
CH group

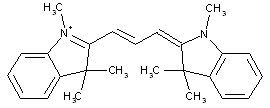
Cyanine dyes, wherein the polymethine chain contains five ![]() CH group
CH group
The polymethine chain containing an even number of CH-groups styryl dyes C09B 23/14 (C09B 23/14 takes precedence)
two heterocyclic rings linked carbon-to-carbon (C09B 7/00 takes precedence)
Cyanine dyes, wherein the polymethine chain contains an even number like two ![]() CH groups
CH groups
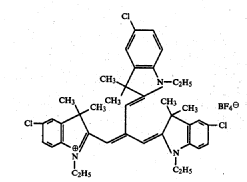
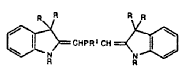
the polymethine chain being branched branched means that the substituent on the polymethine chain forms a new conjugated system ,e.g. most trinuclear cyanine dyes
Styryl dyes
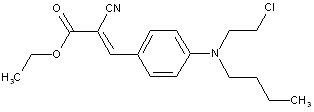
Bis styryl dyes containing two radicalsC6H5-CH=CH-
the ethylene chain carrying a heterocyclic residue, e.g. heterocycle-CH=CH-C6H5
(Benzo)thiazolstyrylamino dyes
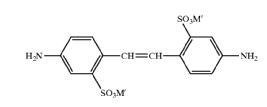
Stilbene dyes containing the moiety-C6H5-CH=CH-C6H5 (stilbene azo dyes C09B 29/00)
the polymethine chain containing hetero atoms
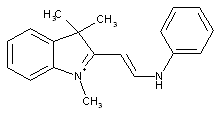
containing only phosphorus atoms, i.e. phosphacyanine
This place covers:
Illustrative example of subject matter classified in this group.
This place covers:
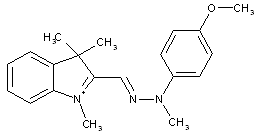
Hydrazones could be considered as chemical entities resulting from a reaction of a ketone with hydrazine:
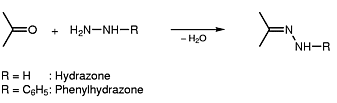
Hydrazone dyes represent therefore chromophores in which e.g. two aromatic systems are linked by such a hydrazone moiety (see formula below, left).
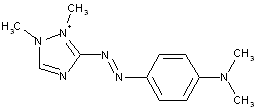
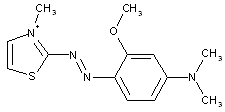
Triazene dyes could be considered as chromophores which comprise a chemical group (e.g. a linker) showing a sequence of three nitrogen atoms in a row; the conjugation (alternating double and single bonds) is still established over the whole chromophore (see formula below, right).
For example:
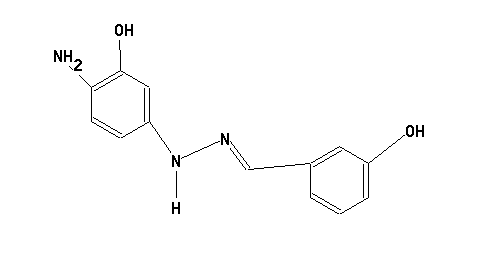 or
or 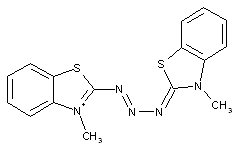
Further details of subgroups:
Hydrazone dyes (for hydrazone-azo dyes: C09B 56/18 takes precedence)

Cationic
Triazene dyes (triazene-azo dyes C09B 56/20)

This place covers:
Further examples for preparing azo dyes could also be e.g. the condensation of nitro compounds under basic conditions; a coupling reaction starting from two amines and an oxidoreductase enzyme; reaction between e.g. an N-heterocyclic hydrazine with a phenanthrene quinone etc.
This place covers:
Azo dyes could be considered as compounds which are built up by aromatic or heteroaromatic systems which are connected with each other via one or more entities of the so-called azo group; the azo group is the bivalent linker -N=N- comprising two nitrogen atoms. Thereby the conjugation (alternating double and single bonds) is established over the whole chromophore. Monoazo dyes simply comprise merely one azo-group which links two chemical groups (e.g. aromatic systems like the phenyl group) to result in a coloured compound. The scheme below shows the synthesis of a typical mono-azo dye.
Monoazo Dyes prepared in the conventional manner as shown in the scheme here below:
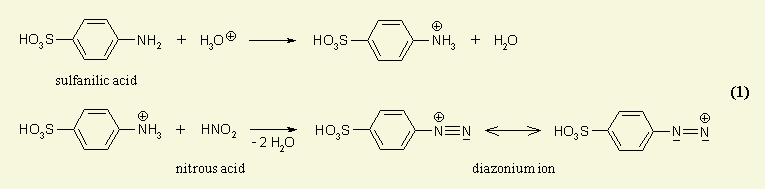
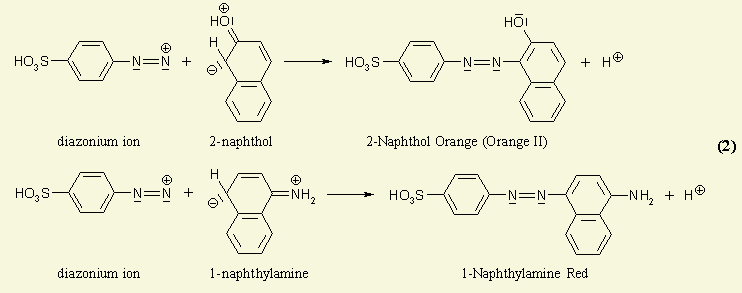
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
In case the "coupling direction" is not described in the document/application, both possible coupling directions should be classified
Further details of subgroups:
from diazotized anilines
from diazotized amino naphthalene
[N :from diazotized amInopolycyclic rings]
[N :from diazotized aminoanthracene]
Isothiazoles or condensed isothiazoles
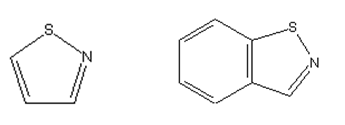
from other diazotized amino heterocyclic rings
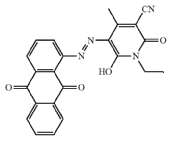
from diazotised o-amino-hydroxy compounds
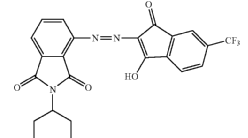
from coupling components containing amino as the only directing group
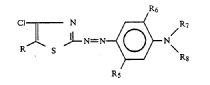
Amino benzenes
free of acid groups
substituted by (see formulas below), thereby X being O,S,NR; R being hydrocarbonyl
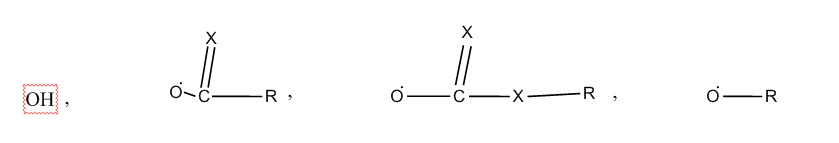
substituted by -CO...
having the substitution below:

having the substitution below:

having the substitution below:
having the substitution below:
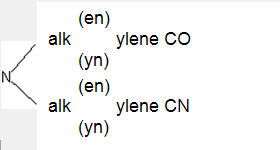
having the substitution below:
having the substitution below:

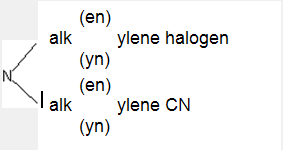
having the substitution below:
; represents e.g. the following structures: 
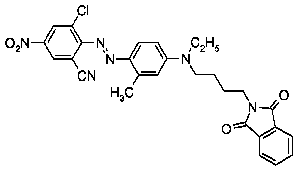
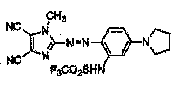
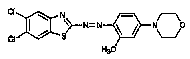
having the substitution below:

Amino naphthalenes
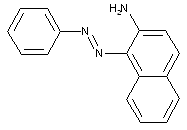
from coupling components containing hydroxyl as the only directing group
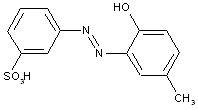
of the benzene series

Hydroxy carboxylic acids
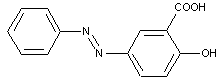
Naphthol-sulfonic acids
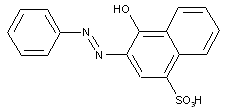
ortho-Hydroxy carbonamides
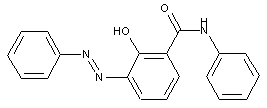
of the naphthalene series
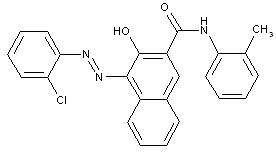
of heterocyclic compounds
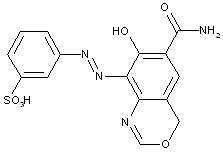
Amino phenols
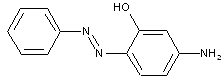
Amino naphthols
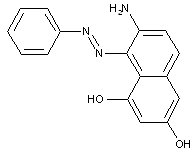
Amino naphtholsulfonic acid
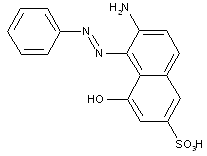
from coupling components containing a reactive methylene group
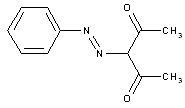
Aceto- or benzoylacetylarylides
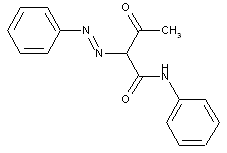
from other coupling components
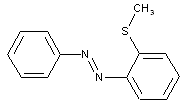
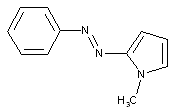
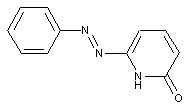
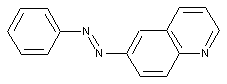
from heterocyclic compounds
containing only a nitrogen as heteroatom
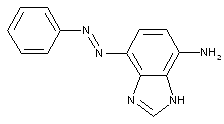
containing a five-membered heterocyclic ring with only one nitrogen as heteroatom
containing a six-membered heterocyclic with only one nitrogen as heteroatom
from quinolines or hydrogenated quinolines
containing a 1,2-diazoles or hydrogenated 1,2-diazoles
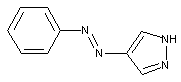
containing amino-1,2-diazoles
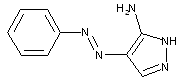
containing a six-membered heterocyclic ring with two nitrogen atoms
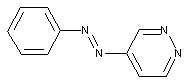
from a pyrimidine ring
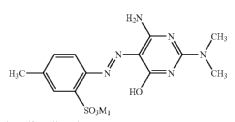
Barbituric acid and derivatives thereof
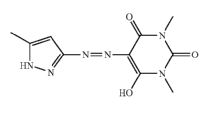
This place covers:
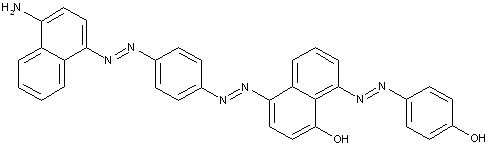
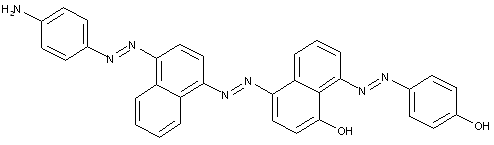
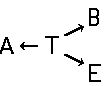
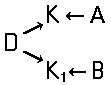
Disazo and polyazo dyes of the type A->B->C, A->B->C->D, or the like, prepared by diazotising and coupling and the following main groups up to C09B 35/00:
the arrows (->) indicate the coupling direction; in most cases the letter K represents the coupling component of the last step.
In case the "coupling direction" is not described in the document/application, both possible coupling directions should be classified
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
Further details of subgroups:
Amino-benzenes
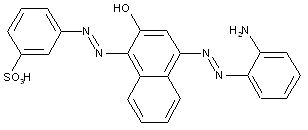
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
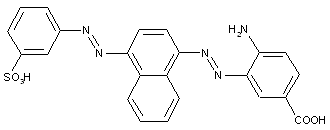
Amino naphthalenes
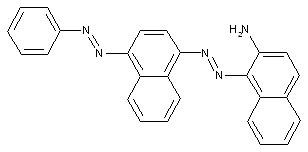
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
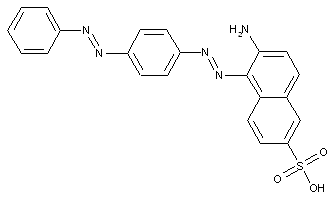
Phenols
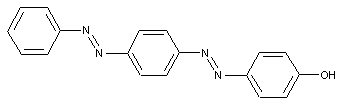
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
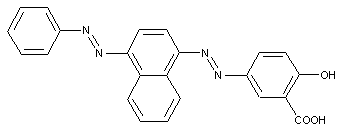
Naphthols
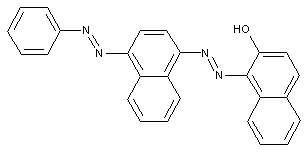
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
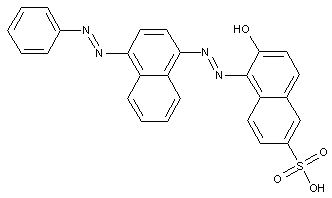
ortho-Hydroxy carboxylic acid amides
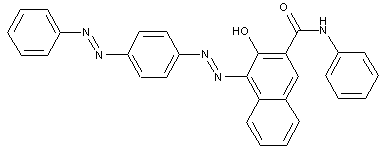
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
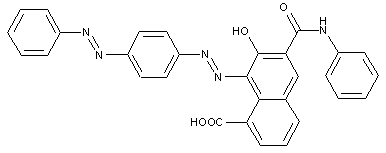
from a coupling component "C" containing directive hydroxyl and amino groups
containing acid groups, e.g. -CO2H, -SO3H, -PO3H2, -OSO3H, -OPO2H2; Salts thereof
Aceto- or benzoyl-acetylarylides
from other coupling components "C"
Heterocyclic components
1,2-Diazoles
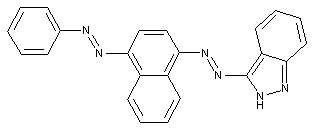
Pyrazoles
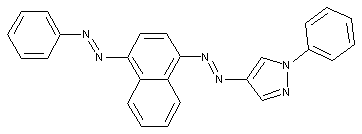
Indoles
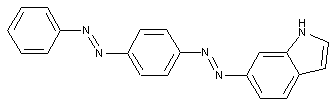
containing a six-membered ring with one nitrogen atom as the only ring hetero-atom
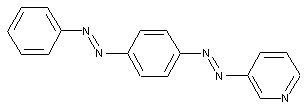
Quinolines or hydrogenated quinolines
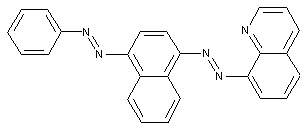
Trisazo dyes
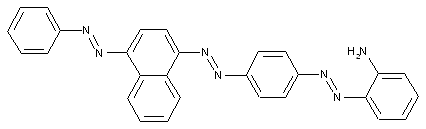
from a coupling component "D" containing a directive amine group

from a coupling component "D" containing a directive hydroxyl group
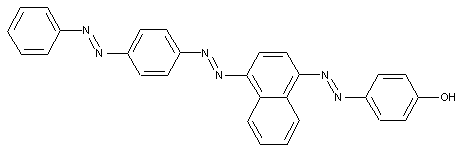
from a coupling component "D" containing directive hydroxyl and amino groups
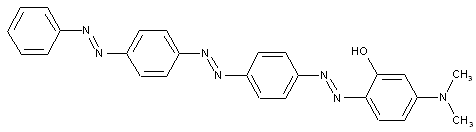
from a coupling component "D" containing reactive methylene groups
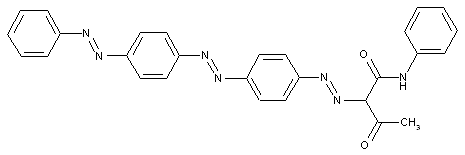
from other coupling components "D"
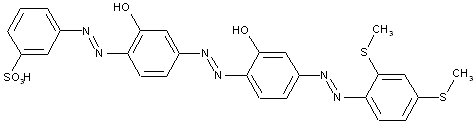
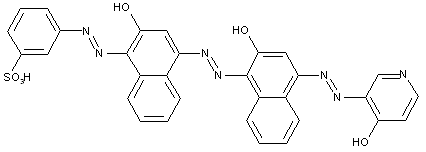
Heterocyclic compounds
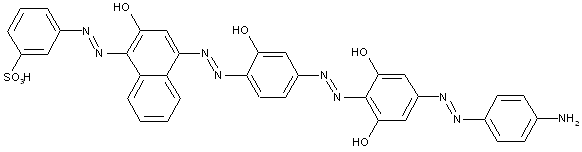
Other polyazo dyes
This place covers:
The arrows (->) indicate the coupling direction; in most cases the letter K represents the coupling component of the last step.
In case the "coupling direction" is not described in the document/application, both possible coupling directions should be classified.
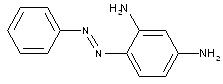
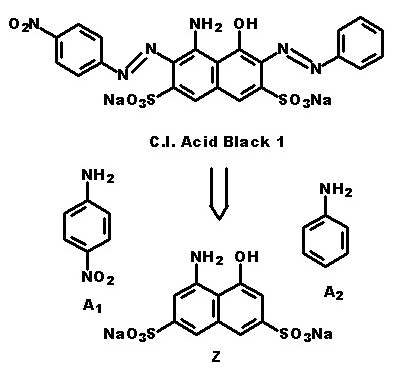
In the following scheme one can derive the diazotising and coupling direction carried out by the synthesis of C.I. Acid Black 1. The two aniline derivatives A1 (= 4-nitro-aniline) and A2 (= aniline) are transferred to their diazo components by diazotising with sodium nitrite under acid conditions (e.g. with HCl). The obtained diazo components are then coupled in the 2- and 7- position of the amino-naphthol coupling component to give C.I. Acid Black 1.
This is a typical synthesis for a disazo dye falling under the reaction scheme A--->K<---B.
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
Further details of subgroups:
Disazo dyes in which the coupling component is a dihydroxy or polyhydroxy compound
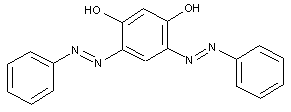
the coupling component being a bis-phenol
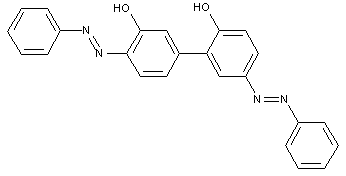
the coupling component being a bis-naphthol
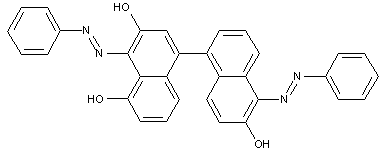
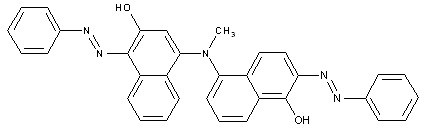
the coupling component being a bis-(naphthol-amine)
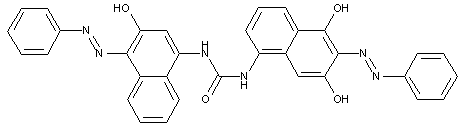
the coupling component being a bis-(naphthol-urea)
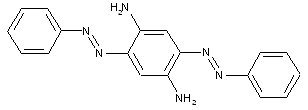
in which the coupling component is a diamine or polyamine
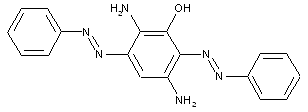
in which the coupling component is a hydroxy-amino compound
in which the coupling component is an amino naphthol
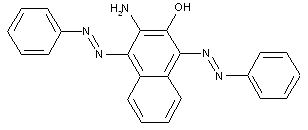
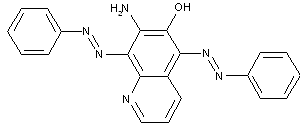
in which the coupling component is a hetero-cyclic compound
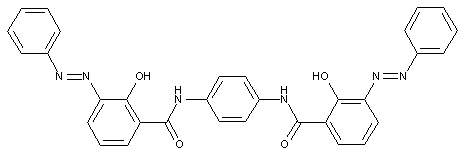
in which the coupling component is a bis -(-o-hydroxy-carboxylic- acid amide)
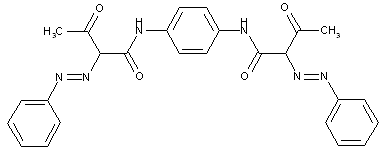
in which the coupling component is a bis-(aceto-acetyl amide) or a bis-(benzoyl-acetylamide)
from other coupling components
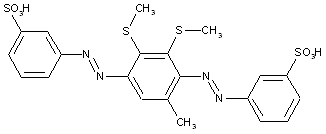
Trisazo dyes of the type A->B->K<-C
Trisazo dyes of the type;
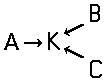
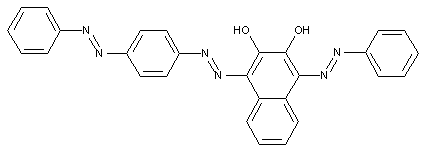
Tetrazo dyes of the type A->B->C->K-D
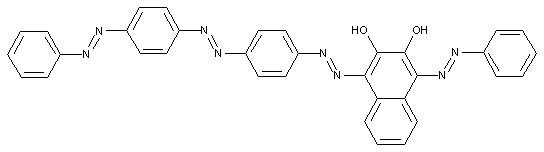
Tetrazo dyes of the type A ![]() B
B ![]() K
K ![]() C
C ![]() D
D
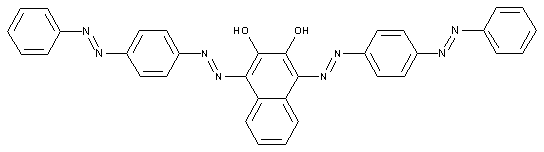
Tetrazo dyes of the type
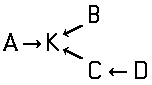
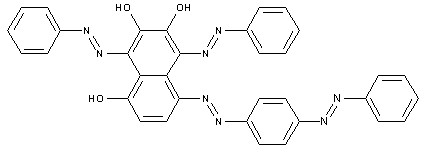
Tetrazo dyes of the type
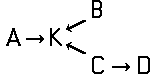
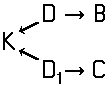
This place covers:
The arrows (->) indicate the coupling direction; in most cases the letter K represents the coupling component of the last step.
In case the "coupling direction" is not described in the document/application, both possible coupling directions should be classified.
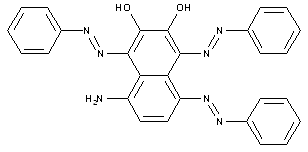
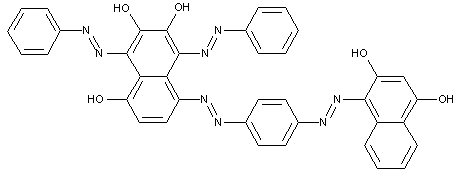
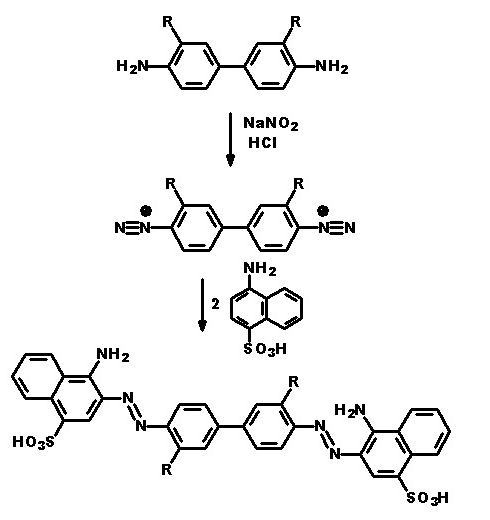
In the following scheme one can derive the diazotising and coupling direction carried out by the synthesis of the disazo dye shown at the bottom of the scheme.
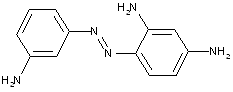
A 4,4'-diamino-biphenyl is twice diazotised, also called 'tetrazotised' with NaNO2 under acid conditions (e.g. HCl), thereby the two amino groups are transferred to two N2+ - groups at the left and at the right. This tetrazo compound is now coupled to two identical coupling components, namely 4-amino-naphthylsulfonic acid. The resulting disazo dye is finally obtained.
This is a typical synthesis for a disazo dye falling under the reaction scheme A<---D--->B.
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
Further details of subgroups:
Disazo dyes characterised by two coupling components of the same type in which the coupling component is a hydroxy or polyhydroxy compound

in which the coupling component is an amine or polyamine
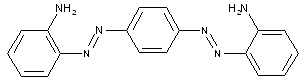
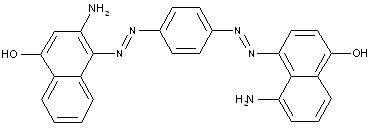
in which the coupling component is a hydroxy-amino compound
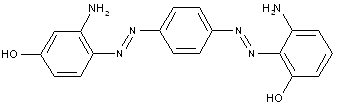
Amino naphtol
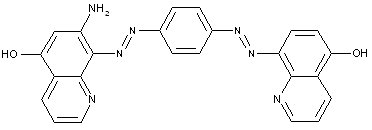
in which the coupling component is a heterocyclic compound
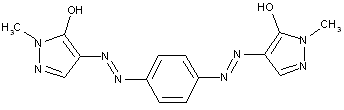
containing a six membered ring with one nitrogen atom as the only ring hetero atom
In which the coupling component is an arylamide of an o-hydroxy-carboxylic acid or of a beta-keto-carboxylic acid
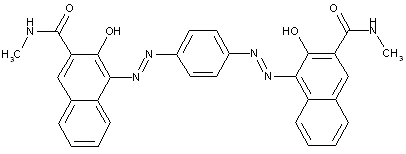
in which the coupling component containing an activated methylene group
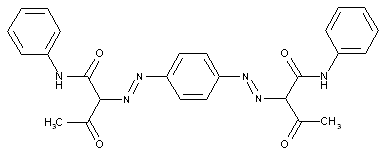
characterised by two coupling components of different types
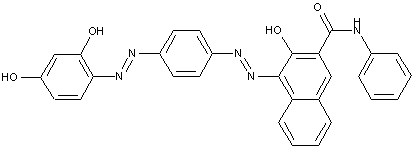
the tetrazo component being a benzene derivative
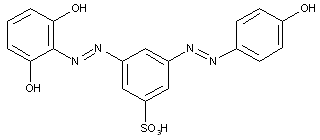
the tetrazo component being a naphthalene derivative
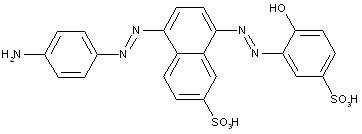
the tetrazo component being a derivative of biphenyl
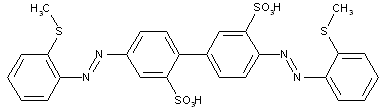
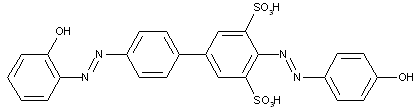
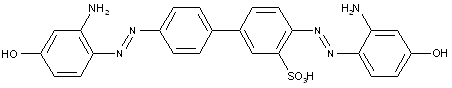
from two coupling components of the same type

from amines

from hydroxy compounds
from hydroxy-amines
from heterocyclic compounds
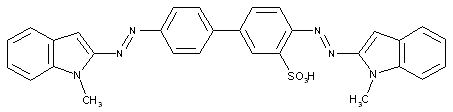
from two coupling compounds of different types
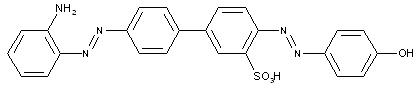
the tetrazo component being a derivative of diarylmethane or triarylmethane
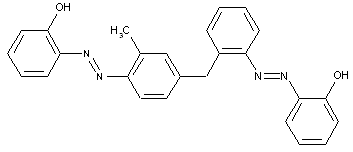
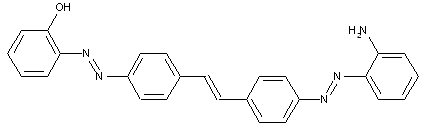
of diarylethane or diarylethene other stilbene-azo dyes, C09B 56/04, C09B 56/06
the tetrazo component being a derivative of a diaryl ether
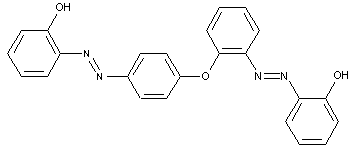
the tetrazo component being a derivative of a diaryl sulfide or a diaryl polysulfide
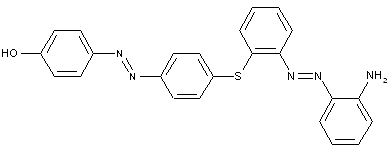
the tetrazo component being a derivative of a diaryl ketone or benzil
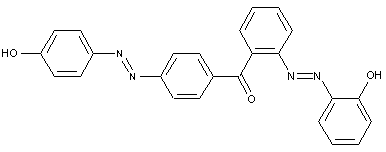
the tetrazo component being a derivative of a diaryl amine
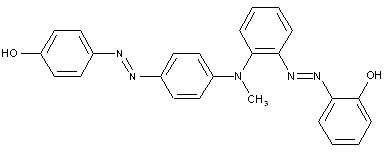
the tetrazo component being a derivative of a diaryl urea
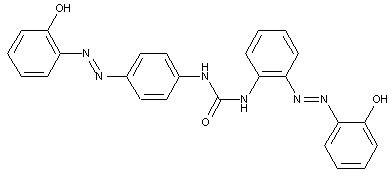
from two identical coupling components
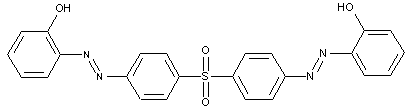
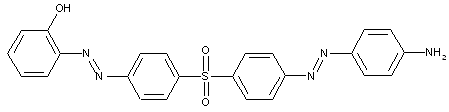
from two different coupling components
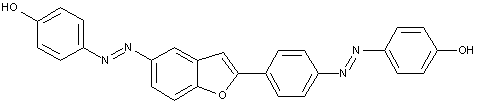
the tetrazo component being heterocyclic
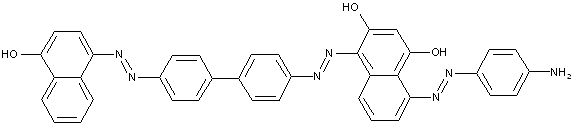
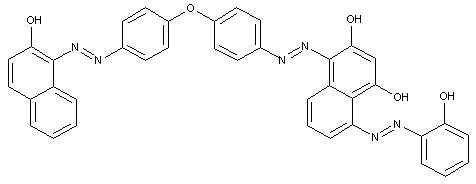
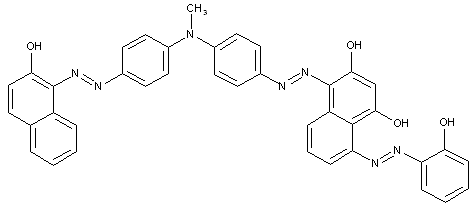
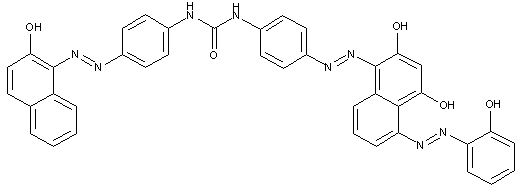
Trisazo dyes in which the tetrazo component is a diamino-azo-aryl compound
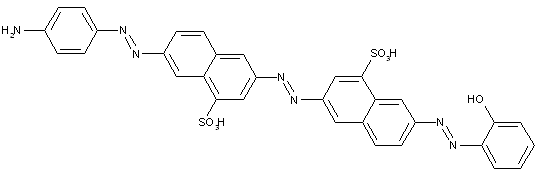
Trisazo dyes of the type
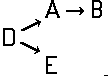
D is benzene
D is naphthalene
D is diphenyl
D is diarylether, a diarylsulfide or a diarylpolysulfide
D is diarylamine
D is diarylurea
D contains two aryl nuclei linked by at least one of the groups -CON, -SO2N, -SO2-, or -SO2-O-
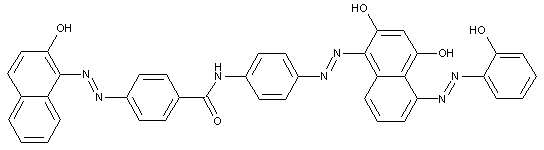
D is a heterocyclic compound
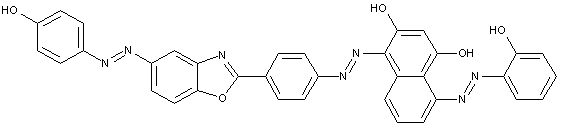
Trisazo dyes of the type
· Trisazo dyes ot the types 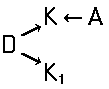
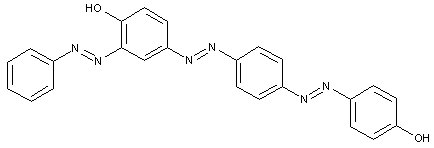
the component K being a dihydroxy or polyhydroxy compound
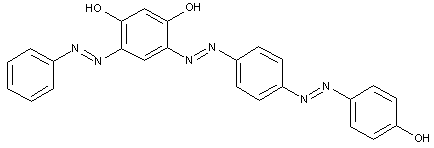
the component K being a diamine or polyamine
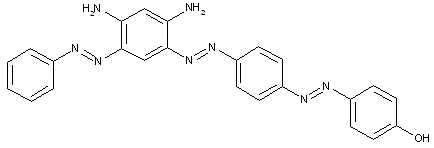
the component K being a hydroxy amine
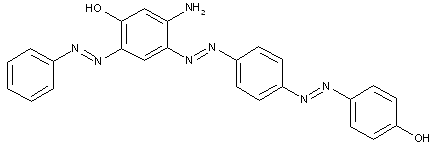
the component K being an amino naphthol
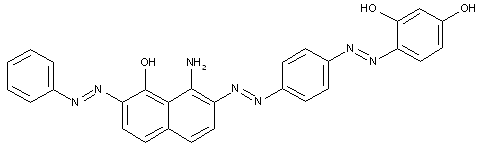
the component K being heterocyclic
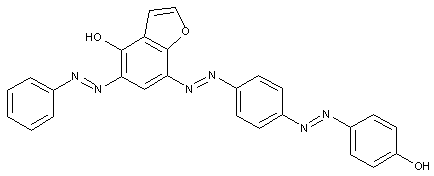
Tetrazo dyes of the type
of the type
of the type
of the type
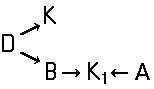
of the type
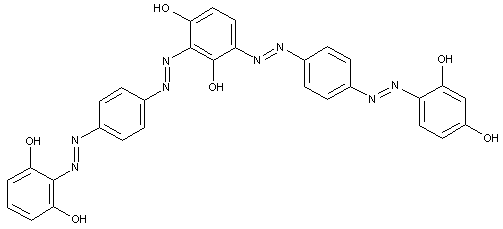
of the type
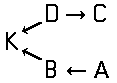
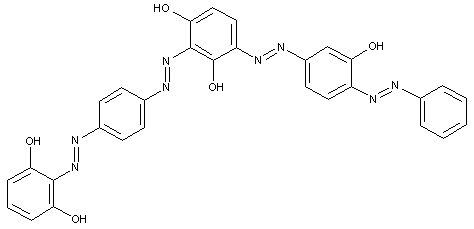
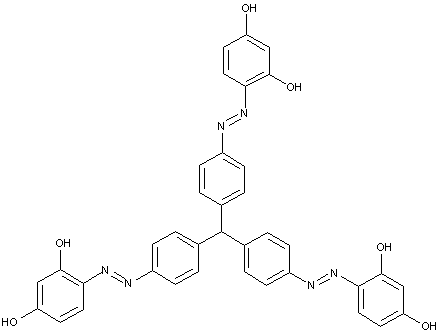
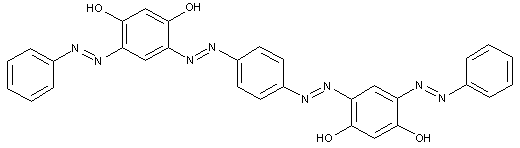
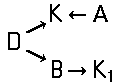
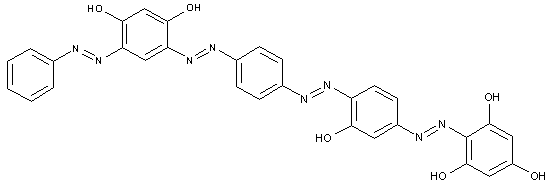
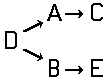
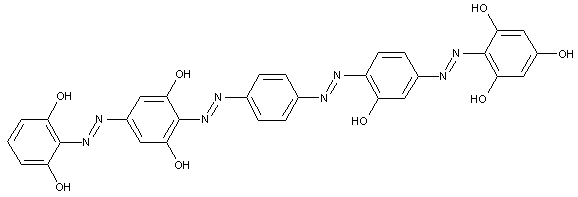
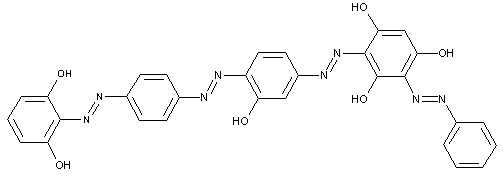
Higher polyazo dyes, e.g. of the types
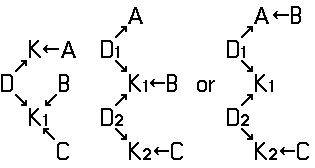 [
[
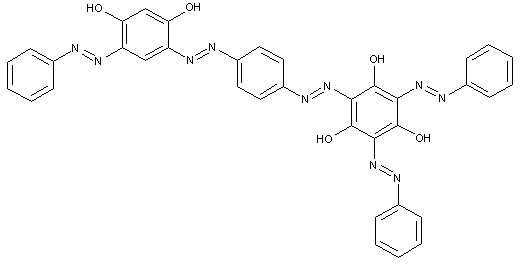
This place covers:
Illustrative example of subject matter classified in this group.
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
This place covers:
Generally documents disclosing dyes which can not be classified in the foregoing groups C09B 29/00 through C09B 37/00.
For instance the reaction product of at least one diazonium salt with a carbon black will be introduced in C09B 39/00 (here a diazo compound is coupled on the solid surface of a carbon black particle); as an other example, obtaining tanning dyestuffs by coupling a diazotised aromatic compound with a water-soluble tanning agent and reacting the product with a heavy-metal salt is classified here as well; [further illustrations can be derived from documents present in this group].
Note(s): In these main groups (Azo dyes C09B 29/00 - C09B 39/00) and related sub groups, arrows in the formulae of the various types of azo dyes indicate which part of an azo dye, prepared by diazotising and coupling, is derived from the diazo component and which part is derived from the coupling component. The arrow is pointing to the part derived from the coupling component.
This place covers:
Coupling reactions e.g. carried out in specific solvents, in the presence of specific reaction assistants [e.g. urea, dispersing agents etc.]; automatically controlled processes, stepwise coupling, the help of mechanical resp. physical means are covered here as well.
This place covers:
Here the azo dye as produced is generally included already basically in the starting product; e.g. functional groups attached in the starting dye could be transfered into other functional groups leading to the final product [acylation of amino groups, acylation of hydroxyl groups etc.].
This place covers:
To the dye e.g. ammonium, phosphonium, sulfonium or other 'onium' groups (see examples below) are covalently attached:
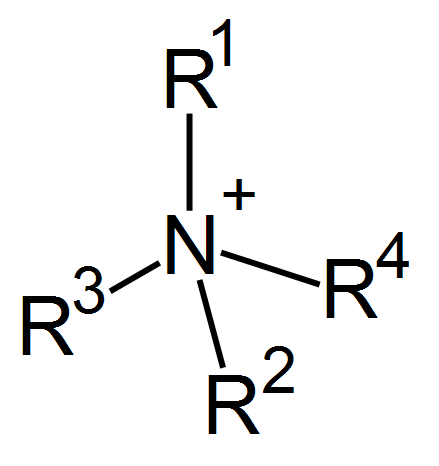
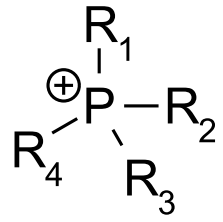
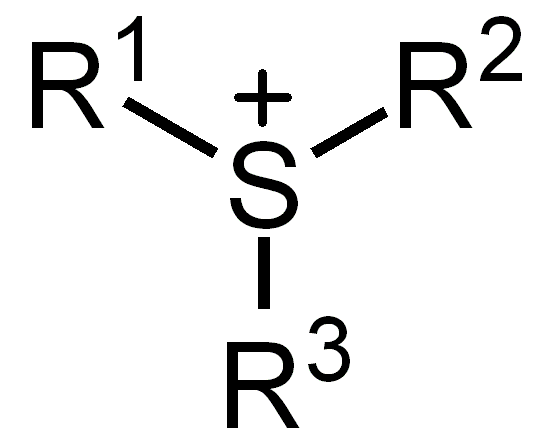
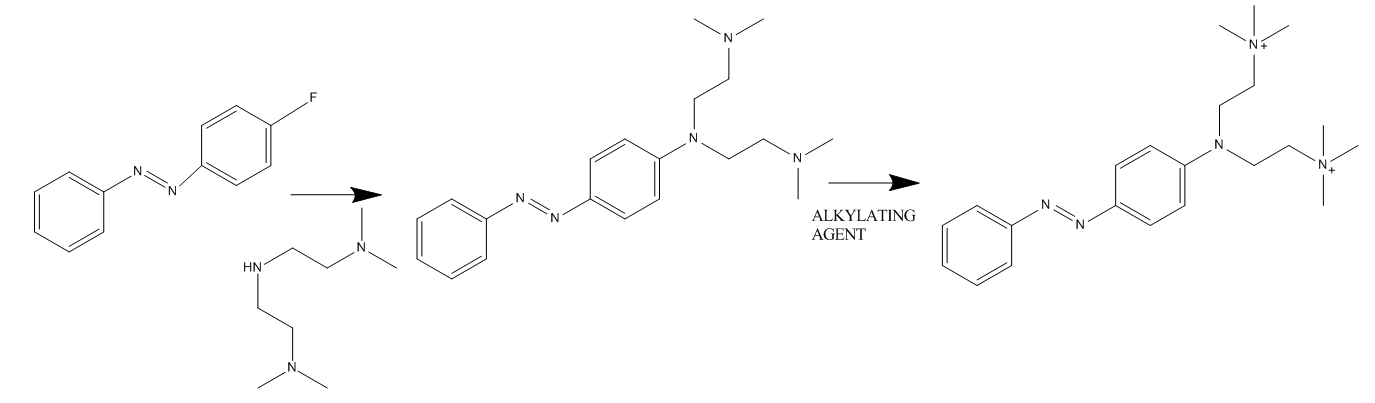
Further details of subgroups:
Special process features in the quaternization reaction
e.g. by treating an aromatic heterocycle which contains at least one nitrogen atom with an alkylating agent or e.g. by treating a benzothiazole azo compound with a dilakylsulfate or by a reaction below:
containing ammonium groups not directly attached to an azo group
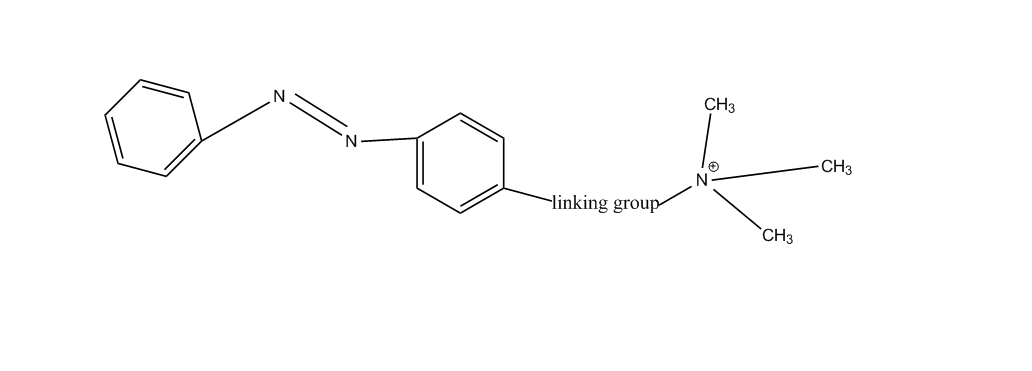
from coupling components containing amino as the only directing group
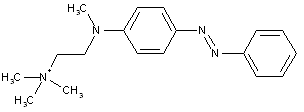
from coupling components containing hydroxyl as the only directing group
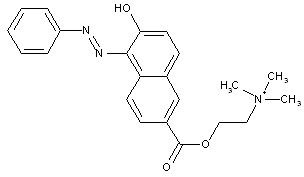
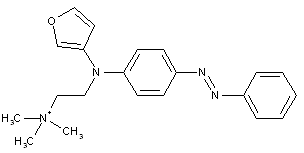
from coupling components containing heterocyclic rings
containing cyclammonium groups attached to an azo group by a carbon atom of the ring system
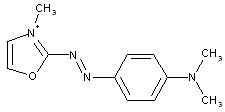
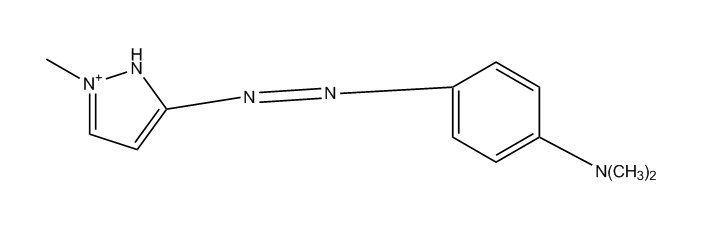
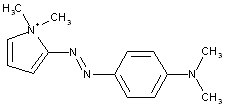
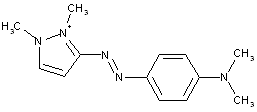
derived from pyrazoles (left), pyrazolones (right)
having one nitrogen atom as the only ring hetero atom
1, 2-Diazoles or hydrogenated 1 ,2-diazoles Pyrazolium; Indazolium
3-Diazoles
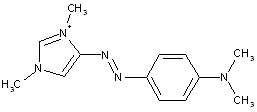
1, 3-Diazoles or hydrogenated 1,3-diazoles (Benz)imidazolium
having three nitrogen atoms as the only ring hetero atoms
Thiazoles or hydrogenated thiazoles
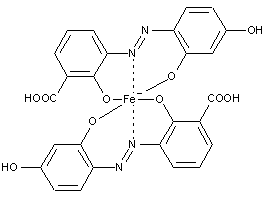
This place covers:
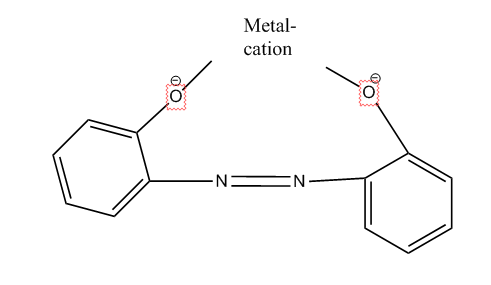
Here the azo dye functions as a ligand linked to a metal cation in a coordinative nature. Thereby the electron donating group in the dye can be the azo group, a hydroxyl group or an acid group (e.g. COO-) etc.
The sub-groups C09B 45/04, C09B 45/14 and C09B 45/24 cover documents where the metal can vary, in this case don't use the respective lower sub-groups (e.g. C09B 45/06, C09B 45/08, C09B 45/10). The sub-groups C09B 45/12, C09B 45/22 and C09B 45/32 should contain documents where the metal is not Cr, Cu or Co, i.e. for example Ni or Fe (maybe both together also).
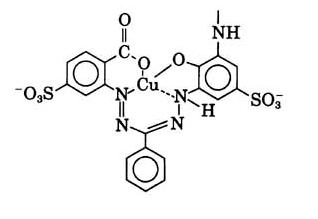
A typical monoazo dye-metal complex is shown below:
Further details of subgroups:
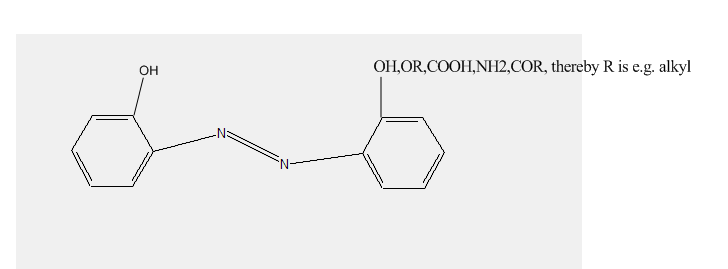
Preparationfromdyescontainingino-postionahydroxylgroupandino'-position hydroxyl, alkoxy, carboxyl, amino or keto groups
[e.g. the dye below could be a possible ligand for metallisation]
containing chromium
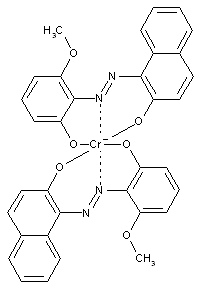
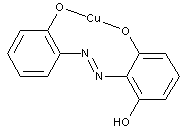
containing copper
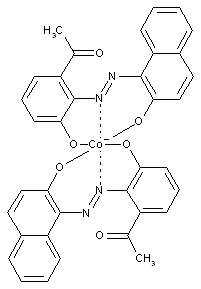
containing cobalt
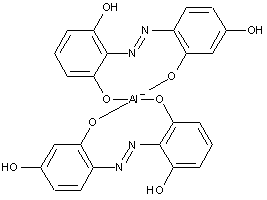
containing other metals
Disazo or poly azo compounds
containing chromium
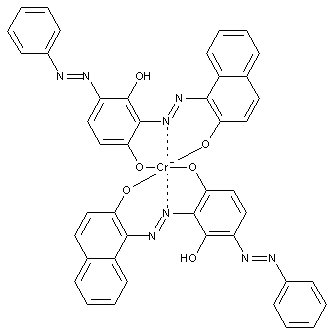
containing copper
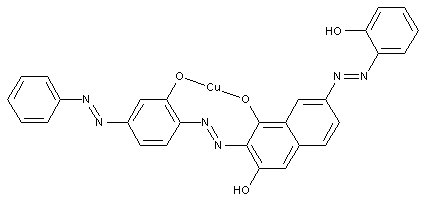
containing cobalt
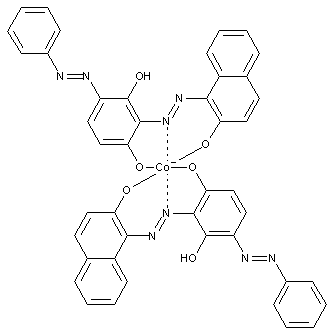
containing other metals
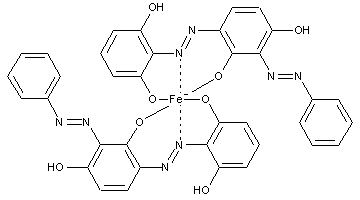
Preparation from o-monohydroxy azo compounds having in the o-position an atom or functional group other than hydroxyl, alkoxy, carboxyl, amino or keto groups
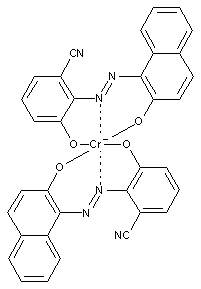
containing chromium
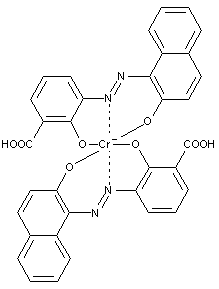
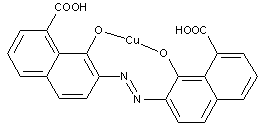
containing copper
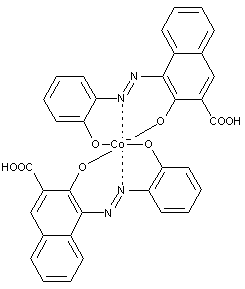
containing cobalt
containing other metals
This place covers:
Illustrative example of subject matter classified in this group.
In case the preparation process of the disclosed phthalocyanine resp. naphthalocyanine is not described, all possible synthesis processes should be classified
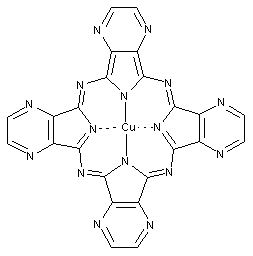
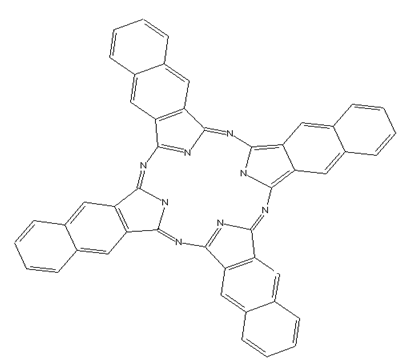
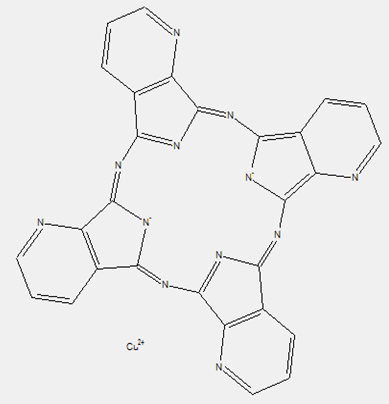
This place covers:
Phthalocyanine compounds, e.g. naphthalocyanines, azaphthalocyanines and subphthalocyanines as illustrated by the examples below.
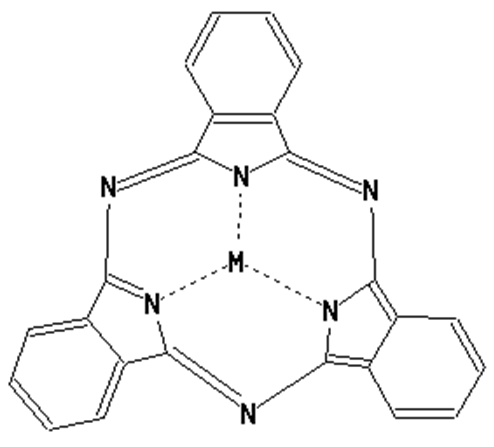
This place covers:
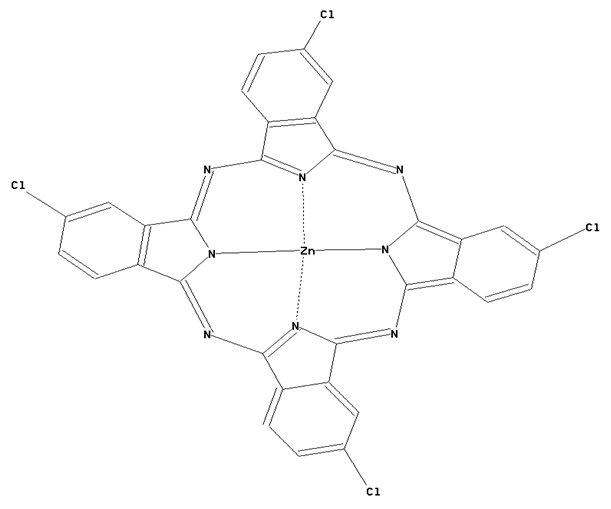
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing halogen atoms directly bound to the phthalocyanine skeleton as illustrated by the example below.
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing alkyl radicals, or alkyl radicals substituted by hetero atoms, bound to the phthalocyanine skeleton as illustrated by the example below.
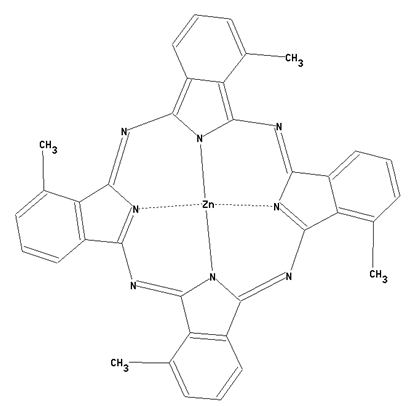
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing alkyl radicals substituted by halogen atoms, bound to the phthalocyanine skeleton as illustrated by the example below.
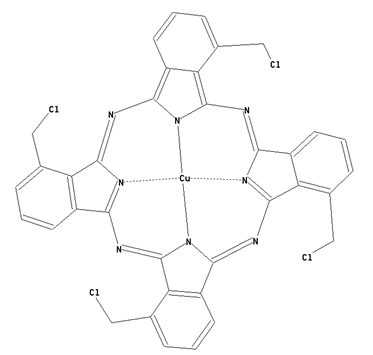
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing alkyl radicals substituted by nitrogen atoms, bound to the phthalocyanine skeleton as illustrated by the example below.
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing oxygen atoms directly bound to the phthalocyanine skeleton as illustrated by the example below.
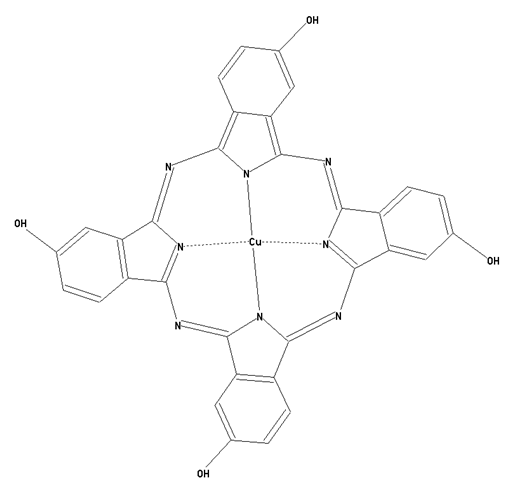
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing sulfur atoms directly bound to the phthalocyanine skeleton as illustrated by the example below.
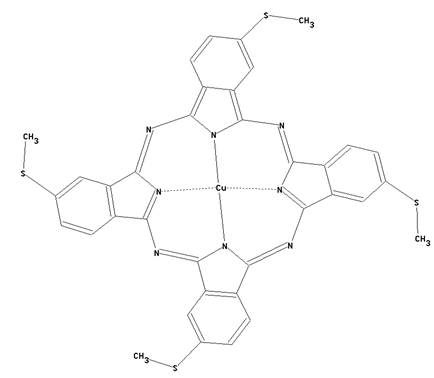
This place covers:
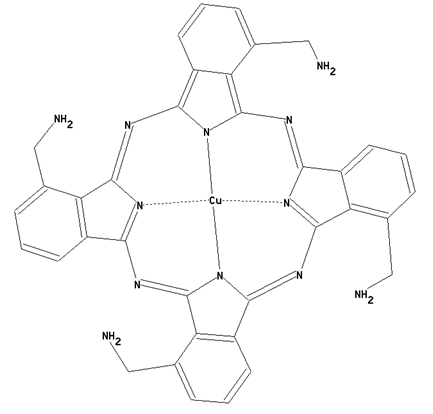
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing nitrogen atoms directly bound to the phthalocyanine skeleton as illustrated by the example below.
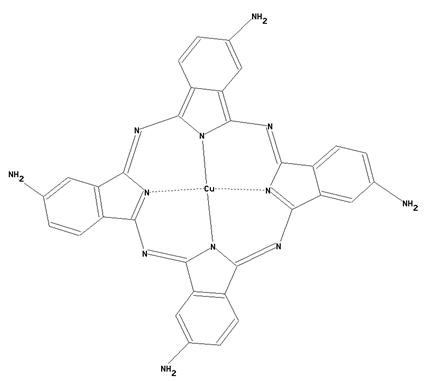
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing -COOH or -SO3H radicals, or derivatives thereof, directly bound to the phthalocyanine skeleton as illustrated by the example below.
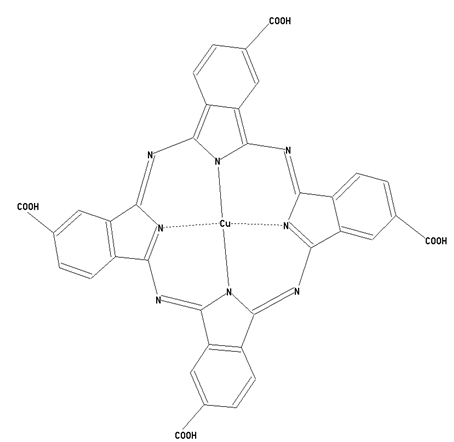
This place covers:
Preparation from other phthalocyanine compounds, phthalocyanine compounds containing amide radicals, directly bound to the phthalocyanine skeleton as illustrated by the example below
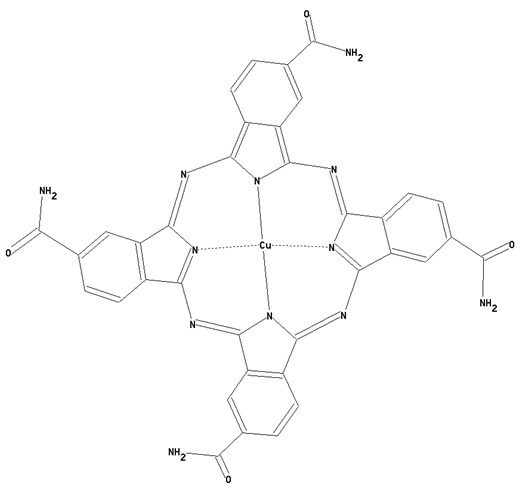
This place covers:
Phthalocyanine dyes containing -S-SO3H radicals as illustrated by the example below.
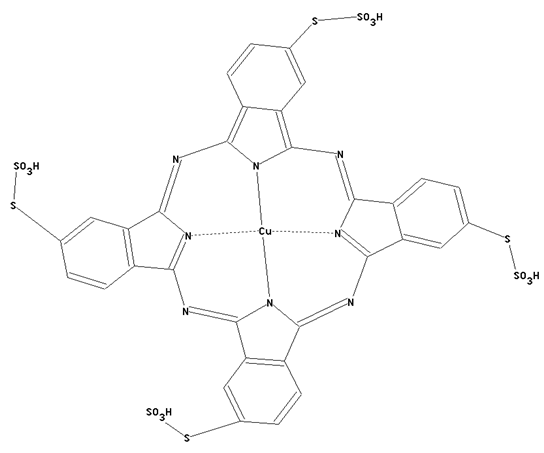
This place covers:
Metal-free phthalocyanines as illustrated by the example below.
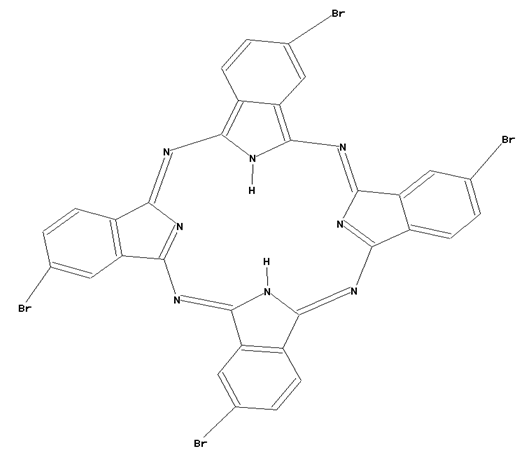
This place covers:
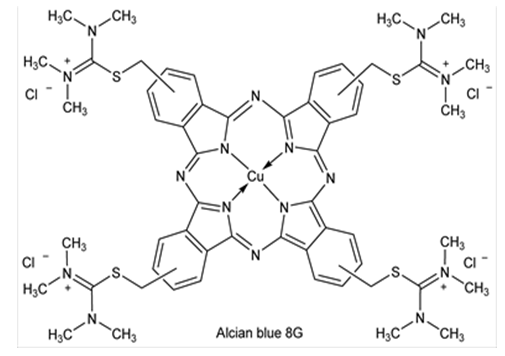
Cationic phthalocyanine dyes as illustrated by the example below.
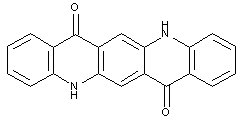
This place covers:
Illustrative example of subject matter classified in this group.
This place covers:
Dyes/Colorants obtained by heating a variety of organic compounds with sulfur or alkali polysulfides. Also colorants bearing a disulfid bridge (-S-S-) or a terminal thiol group (or derivatives thereof) might be classified here.
This place covers:
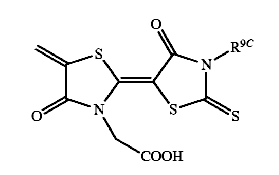
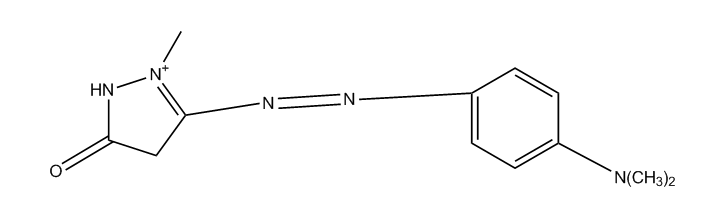
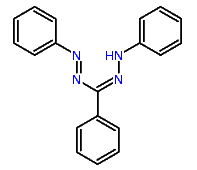
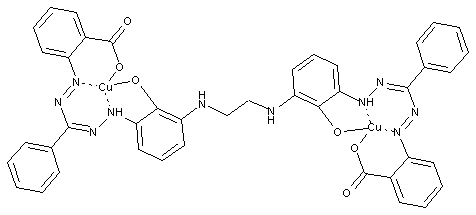
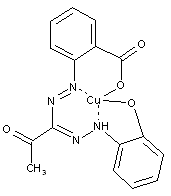
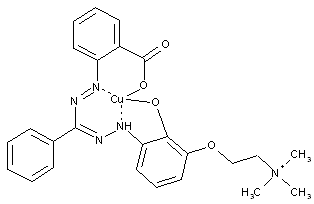
Formazane dyes (left);Tetrazolium dyes (right)
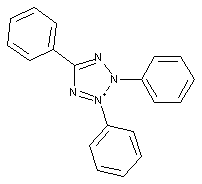
The two structures above are merely examples of the two dye classes covered by C09B 50/00 and sub groups; generally, formazane dyes comprise the basic structure as shown below, left, thereby tetrazolium dyes (below, right) show a heterocyclic ring comprising four N-atoms:
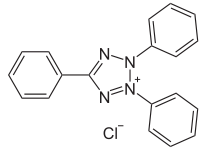
A few documents classified in C09B 50/00 disclose the transfer of the (water soluble) tertazolium precursor into its (water insoluble) formazane derivative (see scheme below):
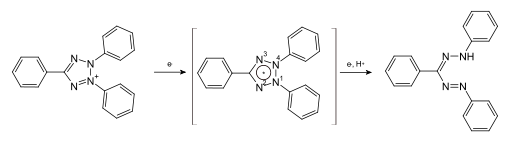
Further details of subgroups:
Tetrazolium dyes:

Metal-free Formazane dyes:
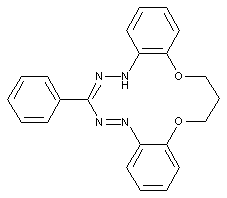
Bis-formazan dyes
Meso-acylformazan dyes
Cationicformazandyes
This place covers:
Illustrative example of subject matter classified in this group.
This place covers:
Illustrative example of subject matter classified in this group.
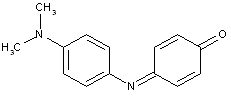
This place covers:
Illustrative example of subject matter classified in this group.
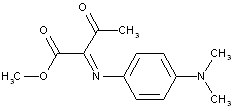
This place covers:
The dyes classified here show an azo-type chromophore which is covalently linked (in most cases by a common chemical linker) to a chromophore being of a different nature than the azo-type; examples might be here: azo - [chemical linker] - anthaquinone or azo - [chemical linker] - phthalocyanine etc.
Further details of subgroups:
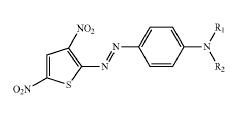
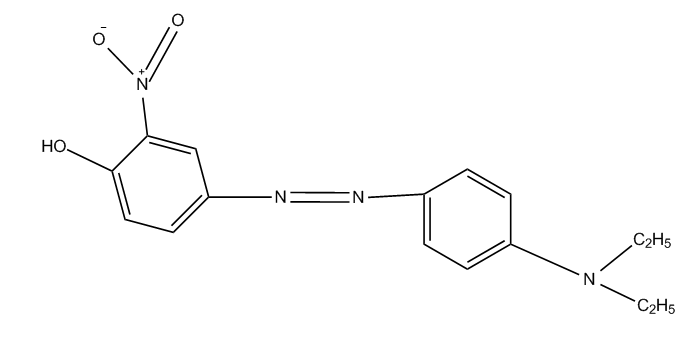
Azo-nitro dyes
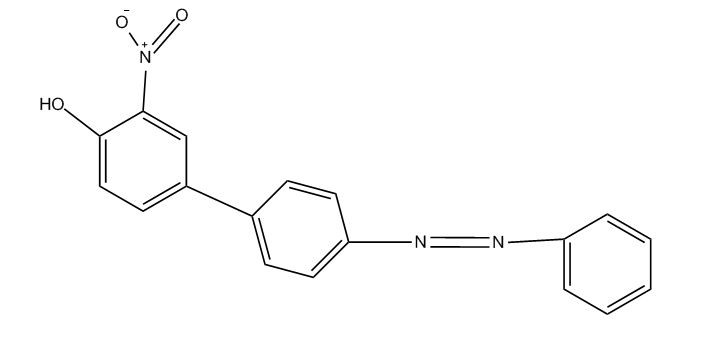
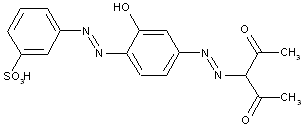
thereby, the following structure should be put into C09B 29/081:
Azomethine-azo dyes 1,2-Complex dyes of AZOMETHINE and AZO dyes, C09B 55/001
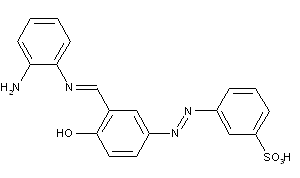
Stilbene-azo dyes disazo dyes from diaminostilbene, C09B 35/215
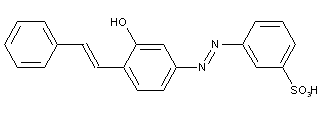
Stilbene-azo dyes disazo dyes from diaminostilbene, C09B 35/215
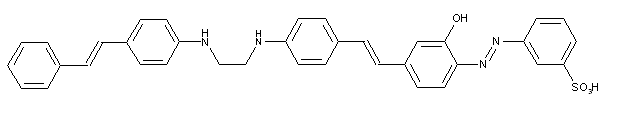
Styryl-azo dyes
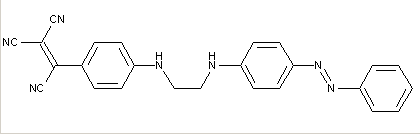
Formazane-azo dyes
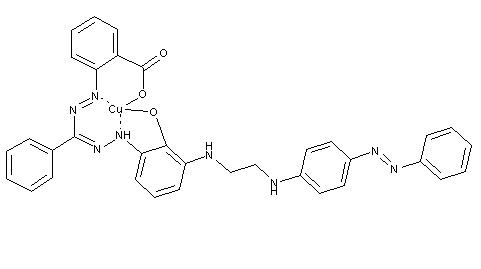
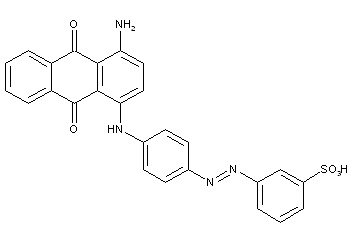
Anthraquinone-azo dyes from diazotised amino anthracene C09B 29/0022, azo dyes containing hydroxyl groups acylated with polyfunctional anthraquinone derivatives C09B 43/26
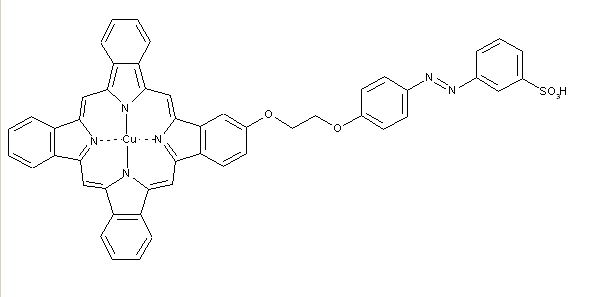
Phthalocyanine-azo dyes
Methine- or polymethine-azo dyes
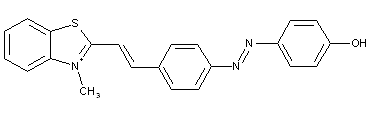
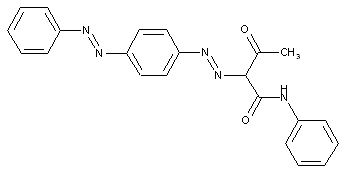
Hydrazone-azo dyes, e.g.
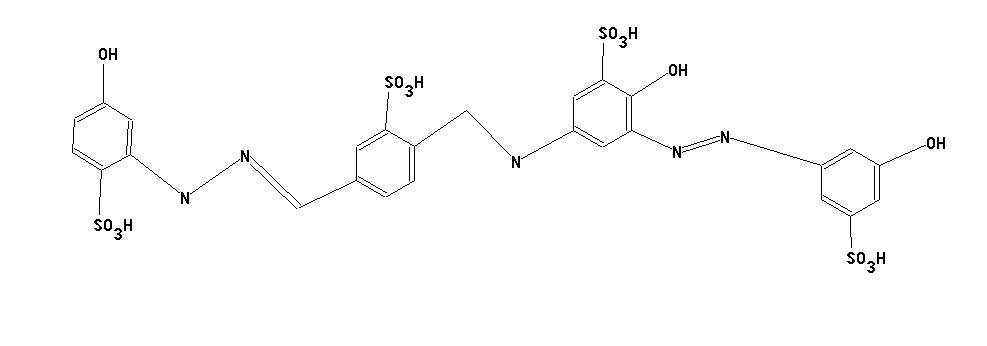
Triazene-azo dyes
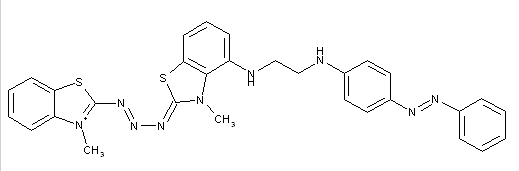
This place covers:
Documents dealing with colorants not belonging to the groups C09B 1/00 through C09B 56/00.
See here also the main group C09B 59/00
Further details of subgroups:
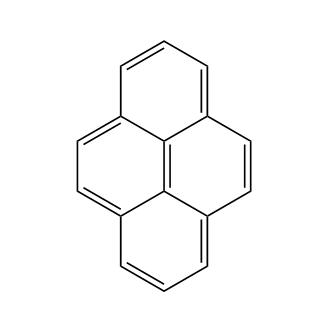
Pyrene dyes
Aminoketone dyes, e.g. arylaminoketone dyes (C09B 13/00 takes precedence)
Diketo-pyrrolo-pyrrole dyes
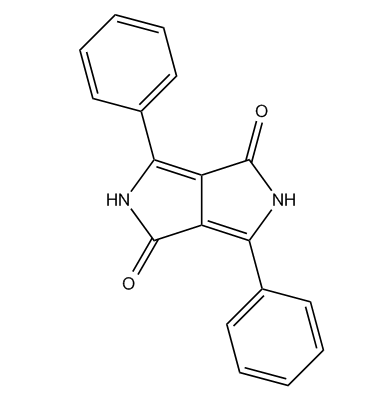
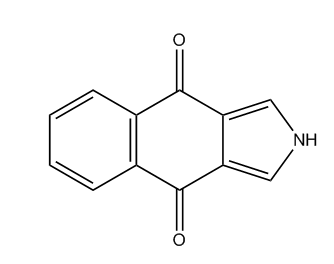
N: Pyrocolline; Phthalcoylpyrrocolline dyes
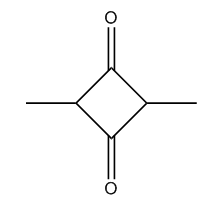
Squaraine dyes
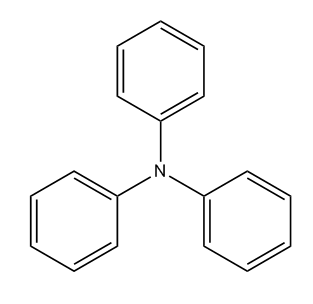
Triarylamine dyes containing no other chromophores [N1006]
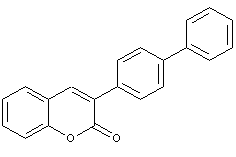
Coumarine dyes
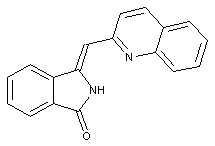
Isoindoline dyes
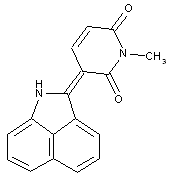
Naphtholactam dyes
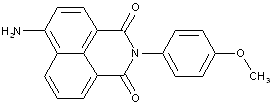
Naphthalimide dyes; Phthalimide dyes
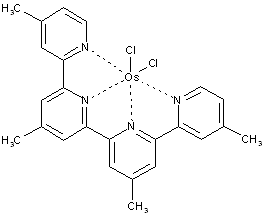
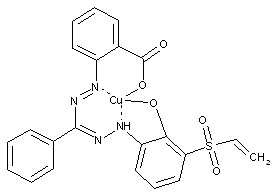
Metal complexes of organic compounds not being dyes in uncomplexed form
in the formula below, the ligand itself is not coloured, the complex as such is coloured
This place covers:
In some cases it is not possible to analyse a well defined chemical structure of a dye or pigment. To this class of dyes belong e.g. the nigrosine dyes (a mixture of benzo-quinoxaline type chromophores), melanin-type colorants, some aniline condensed dyes, some vat dyes etc. Documents dealing with such colorant matters should be classified herein.
See here also the main group C09B 57/00
This place covers:
Dyes resp. pigments of natural origin being found e.g. in plants or animals (e.g. madder, brazilwood, logwood, weld, woad, indigo etc). Some natural dyes, such as cochineal, come from insects, or from mineral sources; they have to be isolated from the natural materials by common techniques like crushing, extracting, boiling with certain solvents, filtering, to mention only a few techniques. Documents dealing with those colorants should be classified here.
This place covers:
A reactive dye comprises a chromophore as the color giving moiety and a substituent which is suitable to react directly with functional groups of the substrate to be coloured or printed. Such reactive dyes normally have good fastness properties, especially washing, wet or sweat fastnesses; most commonly they are used in dyeing of cellulose materials like cotton or flax, but also wool is dyeable with reactive dyes. Documents dealing with such reactive dyes should be classified here.
Further details of subgroups:
with two or more reactive groups at least one of them being directly attached to a heterocyclic system and at least one of them being directly attached to a non-heterocyclic system
the reactive group being an acryloyl group, a quaternised or non-quaternised
aminoalkyl carbonyl group or a (-N)n-CO-A-O-X or (-N)n-CO-A-Hal group, wherein
A is an alkylene or alkylidene group, X is hydrogen or an acyl radical of an organic
or inorganic acid, Hal is a halogen atom, and n is 0 or 1
the reactive group being an esterified or non-esterified hydroxyalkyl sulfonyl or
mercaptoalkyl sulfonyl group, a quaternised or non-quaternised aminoalkyl sulfonyl group, a heterylmercapto alkyl sulfonyl group, a vinyl sulfonyl or a substituted vinyl sulfonyl group, or a thiophene-dioxide group;
examples for reactive dyes with a vinyl sulfonyl group or an esterified hydroxyalkyl sulfonyl group (sulfatoethyl) see below:
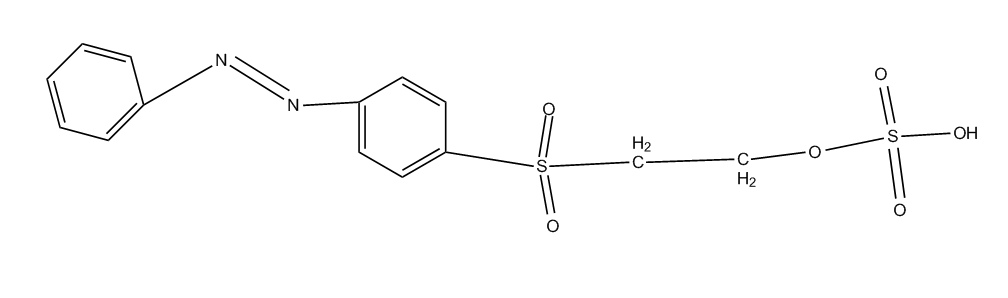
Porphines; Azaporphines (C09B 62/5033, C09B 62/5036 take precedence)
the reactive group being an esterified or non-esterified hydroxyalkyl sulfonyl amido or hydroxyalkyl amino sulfonyl group, a quaternised or non-quaternised amino alkyl sulfonyl amido group, or a substituted alkyl amino sulfonyl group, or a halogen alkyl sulfonyl amido or halogen alkyl amino sulfonyl group or a vinyl sulfonylamido or a substituted vinyl sulfonamido group
the reactive group being an ethylenimino or N-acylated ethylenimino group or a -CO-NH-CH2-CH2-X group, wherein X is a halogen atom, a quaternary ammonium group or O-acyl and acyl is derived from an organic or inorganic acid, or a beta-substituted ethylamine group
This place covers:
Lakes generally are insoluble colorants which are obtained by transferring soluble colorants by certain binders/metal salts into insoluble ones, which have then the properties of pigments.
They can be manufactured by precipitating a dye with an inert binder or dyes made immobilised by e.g. adsorbing it on silica surfaces or concrete.
Water-soluble functional dyes could e.g. be incorporated in a silica matrix with a specified low bleeding rate (' immobilised ').
Further, a water-soluble dye comprising acid groups could be transferred into a pigment/lake by precipitating it with certain metal salts (e.g. earth alcaline metals like Ca2+, Ba2+) into an insoluble salt (this is called 'laking' a dye), see here C09B 63/005 below.
Further details of subgroups:
Metal lakes of dyes; e.g. azo dyes with SO3H groups made insoluble by salting out with cations of alkaline earth metals like Ca2+, Mg2+ or also Al3+; metal complexes of azo dyes see main group C09B 45/00 and sub groups; complexes of metals with ligands being colourless in uncomplexed form see C09B 57/10
This place covers:
Compositions containing mordants; thereby dyes are reacted with so-called mordants; mordants are in most cases metal salts already comprised in the material to be colored (textiles, fibres etc.) by pre-treatment; the dyes build complexes with these metals and are by this way fixed to the material
This place covers:
Post-treatment of organic pigments, crystal modifications thereof, their preparation, blends of dyes and pigments, process features in the making of dyestuff/pigment preparations; solution of dyes/pigments; dispersions of dyes/pigments; dyes in solid form; purification, precipitation, filtration of dyes/pigments; dye/pigment preparation of special physical nature; organic pigments exhibiting interference colours (e.g. nacrous pigments). C09B 67/00 does generally not enclose the chemical modification of pigments (see here: C09B 68/00 and sub groups)
Further details of subgroups:
After the synthesis the obtained organic pigment, the so-called raw-pigment does not have sufficient pigment properties (e.g. with view to colour strength, average particle size, dispersability, crystal modification etc.) to be applied to a material. Therefore further conditioning of the pigment is necessary, which can consist of milling, solvent treatment, acid treatment, tempering, combining with dispersants etc. and combinations thereof. Such conditioning processes ('post-treatment') are a major part of the this subgroup C09B 67/0001.
e.g. membrane processes like ultra-, micro-, nanofiltration; ultra-centrifugation; solvent extraction; combining precipitation and dissolution steps etc., for instance carried out in microreactors
This place covers:
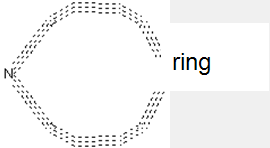
Illustrative example of subject matter classified in this group.
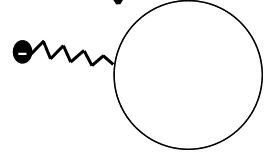
This drawing illustrates e.g. the covalent attachment of a chemical group (might be an alkylene chain) with a terminal negative charge (might be an anionic SO3- group) to the surface of a pigment, e.g. to improve dispersibility. The organic pigment particle is here represented by the ring at the right.
In this main group, in order to emphasise the difference to the main group C09B 67/00, which deals with the treatment of dyes/pigments without chemical reactions, the surface modification of pigments with chemical reactions is covered here.
Thereby in the sub groups of C09B 68/20 until C09B 68/28 the point of view is focussed on the kind of the treating process (e.g. oxidation, azo-coupling etc.), thereby the chemical nature of the introduced/attached groups (e.g. ionic, non-ionic, cyclic, aromatic etc.) is treated in the sub groups C09B 68/40 until C09B 68/485.
This place covers:
All dyes not falling under the preceding main groups
Further details of subgroups:
Dyes containing a substituent, which contains a silicium atom (see formula below):
this sub group takes precedence vis à vis the sub group for the chromophore at which the Si-containing substituent is attached (here: C09B 5/002)
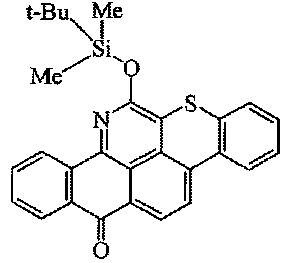
This sub group covers the following compounds:
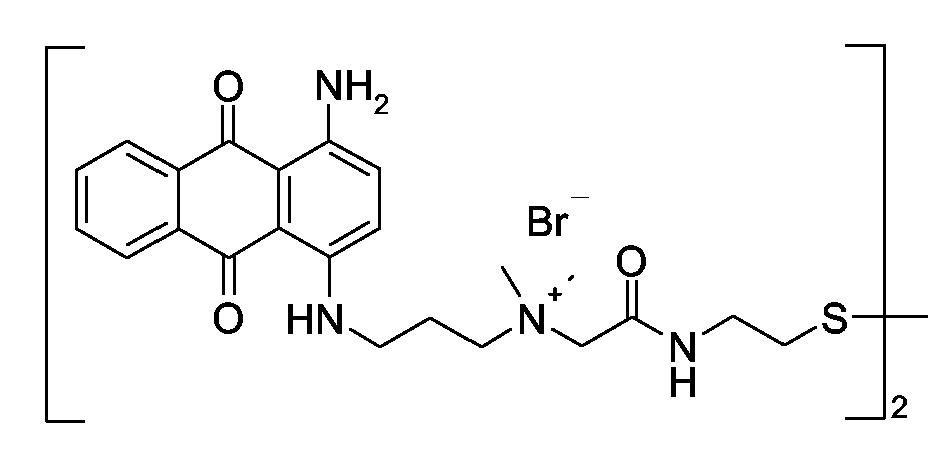
1) Polymers with at least one chromophoric system covalently linked to the polymer chain (could be attached as a pending group in the middle of the chain or as a terminal group at its end)
2) Polymers which comprise a chromophoric system as a monomer being part of the chain.
In case a polymeric polyalkylene oxid chain (e.g. -CH2CH2O- or -CH2CH2CH2O-) is linked to a dye chromophore, please put the document into the group C09B 69/00, as already indicated in this subgroup C09B 69/10.